Departmental Performance Report
2012-13
Departmental Performance Report
Patented Medicine Prices Review Board
The Honourable Rona Ambrose
Minister of Health
Table of Contents
Chairperson's Message
Section I: Organizational Overview
Section II: Analysis of Programs and Sub-Programs by Strategic Outcome(s)
Section III: Supplementary Information
Section IV: Other Items Of Interest
Endnotes
Chairperson's Message
I am pleased to present the 2012–13 Departmental Performance Report for the Patented Medicine Prices Review Board (PMPRB).
The PMPRB's objective to ensure that Canadians do not pay excessive prices for patented medicines is an important one which contributes to the government-wide goal of healthy Canadians.
In 2012, following a comprehensive program evaluation to assess the PMPRB's performance and relevance, we initiated an action plan based on the results. The main objectives of the evaluation were to determine whether we are achieving our outcomes and whether our allocated resources are appropriate for the effective delivery of our mandate.
The Program Evaluation Report found the PMPRB programs appropriate for delivery by a federal agency and well-aligned with both government-wide priorities and our Strategic Outcome. It also found that we are achieving our expected outcomes. According to the evaluation, the incremental funding that was received in 2008–09 was effectively used and has achieved the results for which it was approved.
In our Management Action Plan, we addressed the recommendations outlined in the Evaluation Report by examining ways to (i) expedite its processes and further simplify the Guidelines, (ii) decrease regulatory burden, and (iii) make effective use of our resources, all without compromising the PMPRB's consumer protection role.
In 2012–13, our priorities were to enhance compliance, to explore possible ways to decrease regulatory burden for patentees and to make the most effective use of our resources. To that end, we initiated consultations with stakeholders on proposed regulatory burden reduction initiatives. Also, our Monitoring and Evaluation Plan for the Major Changes in the Guidelines provides opportunities for continued dialogue with stakeholders and allows for timely adjustments to our Guidelines. The Board was presented with the second annual assessment under this Plan in December 2012, and a table summarizing the results was published in January 2013. It is our intention that the Guidelines be responsive to changes in the drug distribution and pricing environment, in an appropriate timeframe.
We also made a commitment to ensuring that our studies and reports are available to policy decision makers in the timeliest manner. Through the National Prescription Drug Utilization Information System (NPDUIS) program, we have expanded our exchanges with stakeholders and increased our participation in discussions and conferences. In addition, in consultation with the NPDUIS Steering Committee, the Canadian Institute for Health Information (CIHI) and Health Canada, the PMPRB has been focusing on updating key NPDUIS framework documents (the CIHI/PMPRB data sharing agreement, Terms of Reference for the NPDUIS Advisory Committee and the Memorandum of Understanding between Health Canada and the PMPRB) to better position the NPDUIS initiative for the future. Furthermore, in 2013, we released the following studies:
- The Use of Blood Glucose Test Strips in Select Public Drug Plans, 2008
(Revised April 2013)
- New Drug Pipeline Monitor - April 2013
- Analytical Snapshot: International Generic Price Comparison, Early 2011, August 2013
As Chairperson of the PMPRB, it is my objective to ensure that our framework continues to have a positive impact for consumers while recognizing the value that innovative medicines offer to patients. The PMPRB remains committed to effectively meeting challenges, serving Canadians, and contributing to the health care system.
Mary Catherine Lindberg
Chairperson
Section I: Organizational Overview
Raison d'être
The Patented Medicine Prices Review Board (PMPRB) is an independent, quasi-judicial body created by Parliament in 1987 under the Patent Act (Act). Its mandate is two-fold:
- Regulatory — to ensure that prices charged by patentees for patented medicines sold in Canada are not excessive; and
- Reporting — to report on pharmaceutical trends of all medicines and on research and development spending by pharmaceutical patentees.
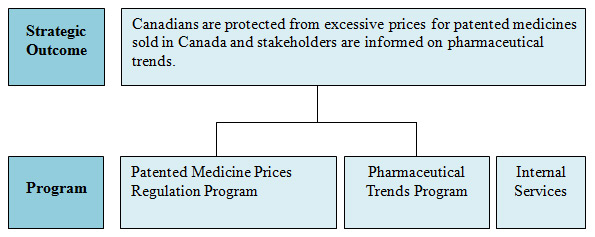
In carrying out its mandate, the PMPRB endeavours to ensure that Canadians are protected from excessive prices for patented medicines sold in Canada, and stakeholders are informed on pharmaceutical trends.
Responsibilities
The PMPRB has two responsibilities:
Regulatory Responsibility
The PMPRB is responsible for regulating the factory-gate prices that patentees charge for prescription and non-prescription patented medicines sold in Canada to ensure that they are not excessive. It includes sales for human and veterinary use to wholesalers, hospitals, pharmacies or others. The PMPRB regulates the price of each patented medicine. This includes each strength of an individual, final dosage form of a medicine. Normally this is the drug product for which Health Canada assigns a Drug Identification Number (DIN) as part of the Notice of Compliance process. The PMPRB's mandate also includes medicines that are available under the Special Access Programme; through a Clinical Trial Application; and as Investigational New Drugs.
The PMPRB's jurisdiction is not limited to medicines with a patent on the active ingredient. It also covers drugs which have patents related to, but not limited to, the manufacturing process, the delivery system or dosage form, the indication or therapeutic use and any formulations. Patented medicines are not limited to brand-name products.
A number of generic companies fall under the PMPRB's jurisdiction by virtue of being licensees selling the same medicines as the brand company or because various generic companies hold manufacturing or processing patents.
The PMPRB has no authority to regulate the prices of non-patented medicines and does not have jurisdiction over prices subsequently charged by wholesalers or pharmacies, or over pharmacists' professional fees. Also, matters such as whether medicines are reimbursed by public drug plans, their distribution and prescribing are outside the purview of the PMPRB.
Under the Act, patentees are required to inform the PMPRB of their intention to sell a new patented medicine. Upon the sale of a patented medicine, patentees are required, as per the Patented Medicines Regulations (the Regulations) to file price and sales information for the first day's sales and, thereafter, twice a year for six-month periods (January to June and July to December) for each strength of each dosage form of each patented medicine sold in Canada for price review purposes, for the duration of the patent(s).
Although patentees are not required to obtain approval of the price before a patented medicine is sold, they are required to comply with the Act to ensure that prices of patented medicines sold in Canada are not excessive. In the event that the Board finds, after a public hearing, that a price is or was excessive in any market, it may order the patentee to reduce the price and take measures to offset any excess revenues it may have received.
Reporting Responsibility
The PMPRB reports annually to Parliament through the Minister of Health on its activities, on pharmaceutical trends of all medicines, and on the R&D spending by pharmaceutical patentees.
Through the National Prescription Drug Utilization Information System (NPDUIS)i, the PMPRB provides critical analyses of price, utilization and cost trends of patented and non-patented prescription drugs sold in Canada to support decision making by participating federal, provincial and territorial public drug plans.
Strategic Outcome and Program Activity Architecture1
[D]
Organizational Priorities
| Priority |
Type2 |
Strategic Outcome and/or program(s) |
| Enhance compliance with Board's Guidelines |
New |
The PMPRB has only one Strategic Outcome (SO) and all priorities are linked to that SO.
- Priority linked to Program 1
|
| Summary of Progress |
|
What progress has been made towards this priority?
The Guidelines are intended to assist pharmaceutical patentees in establishing non–excessive prices by providing transparent and predictable information on how the price review will be undertaken. Enhanced compliance with the Guidelines will help ensure that Canadians are protected from excessive prices for patented medicines sold in Canada.
- In June 2012, the PMPRB published the Monitoring and Evaluation Plan for the Major Changes to the Guidelines (GMEP)ii on its website. Board Staff presented the second annual assessment under the Plan to the Board in December 2012. The results show that patentees are taking the new Guidelines into consideration when determining the appropriate price at which to sell their patented medicines in Canada. The results of the GMEP for two years can be found on the PMPRB website under Legislation, Regulations and Guidelines.
- In the April 2012 issue of its NEWSletter, the Board provided clarification on the Guideline on "Any Market" price reviews.iii The Guideline on "Any Market" price reviews is intended to ensure that no class of customer in any market in Canada will be charged a price which exceeds the maximum average potential price (MAPP) for any patented medicines introduced after January 1, 2010.
- In September 2012, the Chairperson accepted the Report on the findings of the 2011-12 Program Evaluation. In response to the Evaluation Report iv, a Management Response and Action Plan v was developed. The Management Response and Action Plan addresses the four considerations set out in the Evaluation Report. The Action Plan provides details on the initiatives and activities the PMPRB has undertaken, will be undertaking or has completed to address these considerations. It is anticipated that these activities and initiatives will address concerns raised by patentees, which in turn will lead to enhanced compliance with the Guidelines and non–excessive prices for patented medicines sold in Canada. The Evaluation Report along with the Management Response and Action Plan are posted on the PMPRB website under Accountability.
- At the March 27, 2013 Meeting of the Board, Board Staff presented options and a recommendation for changes to the Consumer Price Index (CPI)–Adjustment Methodology. vi The recommendation is intended to provide greater transparency and predictability to the CPI–Adjustment Methodology. This is expected to lead to enhanced compliance with the Guidelines, resulting in non–excessive pricing for patented medicines sold in Canada. The Board began consulting stakeholders on the proposed amendments via a Notice and Comment posted on the PMPRB website in May 2013.
- In April 2012, the DIP Technical Working Group completed its assessment of the pilot application of the DIP Methodology, proposed options to address application–related issues, and recommended that the Board adopt the DIP Methodology on a permanent basis.
|
| Priority |
Type |
Strategic Outcome(s) and/or Program Activity(ies) |
| Decrease regulatory burden and make effective use of Board Staff resources. |
New |
The PMPRB has only one SO and all priorities are linked to that SO.
- Priority linked to Program 1 and Internal Services.
|
| Summary of Progress |
|
What progress has been made towards this priority?
While the irritants identified by the pharmaceutical industry as part to the Red Tape Reduction Commission were ultimately determined to be outside of the scope of this initiative, the PMPRB identified possible ways to reduce the regulatory burden on patentees without adversely affecting its mandate to protect consumers from excessive prices for patented medicines sold in Canada.
- The Board has approved moving forward to consultations with stakeholders on two initiatives intended to reduce the regulatory burden of patentees:
- the Consumer Price Index (CPI)–Adjustment Methodology, namely replacing the use of the forecast CPI with actual CPI in calculating the CPI–Adjustment Factor for the forecast period; and
- the Regulations, namely moving from two to one annual filing of price and sales information for existing patented medicines by patentees, and modifying the requirement for patentees to submit information for the first day of sales of new patented medicines.
- The Notice and Commentvii was posted on the PMPRB website in May 2013. Stakeholder submissions are published on the website under Consultations and Notice and Comments. Based on stakeholder feedback, further consultation will be undertaken on the proposed text in the Compendium of Policies, Guidelines and Procedures and in the Patented Medicines Regulations, as well as on operational/transitional details, prior to final adoption and implementation. In addition, the proposed amendments to the Regulations will follow a formal consultation (Federal Regulatory Development Process) through Cabinet and publication in the Canada Gazette.
- In April 2012, the Board confirmed that the "Any Market" Price Reviews will generally apply only to new and existing patented drug products first sold on or after January 1, 2010. The Board believes that retroactively applying this new policy to all existing drugs would represent a significant administrative burden to patentees and to Board Staff.
- In March 2013, the PMPRB published the following service standardsviii on its website:
- Service Standard for the scientific review of new patented drug products;
- Service Standard for the price review of new patented drug products; and
- Service Standards for the price review of existing patented drug products.
- In 2012–13, the PMPRB introduced a new Web–based electronic database capable of receiving secure direct entry of data from Form 1 and Form 2 regulatory filings. This system enables the PMPRB to process filings more efficiently and brings direct filing by patentees via the Internet a step closer.
|
| Priority |
Type |
Strategic Outcome(s) and/or Program Activity(ies) |
| Transparency and communications. |
New |
The PMPRB has only one SO and all priorities are linked to that SO.
– Priority linked to Programs 1 and 2.
|
| Summary of Progress |
|
What progress has been made towards this priority?
Stakeholders must understand and appreciate the PMPRB's role as a contributor to the health care system. The PMPRB undertook the following initiatives to ensure that stakeholders are both informed and engaged.
- The PMPRB announced the launch of a Twitter account to further enhance communication with its stakeholders.
- The Patentee's Guide to Reportingix was updated and posted on the PMPRB website. The updates clarify the process for completing and filing Forms 1 and 2 in electronic format, which became mandatory under the amended Regulations. The updated Patentee's Guide to Reporting also reflects changes in reporting over–the counter and veterinary drug products, as well as the new schedule for reporting the first day of sales.
- In the October 2012 issue of the NEWSletter, the PMPRB announced the availability of the Foreign Price Verification Formulas back to 2002. The yearly listings outline the sources and the methodology used by Board Staff to derive, where applicable, ex–factory prices from national formulary prices.
- On December 6, 2012, the Regulatory Affairs and Outreach Branch held its very first PMPRB 101 session. The session was developed for participants from pharmaceutical companies who had little experience in working with the PMPRB.
- The results of the Monitoring and Evaluation Plan for the Major Changes in the Guidelines (GMEP 2012) were published on the PMPRB website under Act, Regulations and Guidelines.
- In April 2013, two new analytical reports were released by the PMPRB: The Use of Blood Glucose Test Strips in Select Public Drug Plans, 2008x and the latest edition of the New Drug Pipeline Monitor.xi
- In May 2013, the PMPRB began consultations on initiatives to amend:
- the Consumer Price Index (CPI)–Adjustment Methodology; and
- the Patented Medicines Regulations by moving to one annual filing of price and sales information for existing patented medicines by patentees, and to modify the requirement for patentees to submit information for the first day of sales of new patented products.
|
Risk Analysis
| Risk |
Risk Response Strategy |
Link to Program Alignment Architecture |
Link to Organizational Priorities |
| Non–compliance with the Board's new Guidelines |
- The PMPRB approved a Guidelines Monitoring and Evaluation Plan (GMEP).
- The Board continues to address challenges in the operationalization of the new Guidelines. The Board has been quick to clarify the interpretation and application of its Guidelines and to adopt approaches to facilitate efficient implementation of the new elements. This process is ongoing.
|
The PMPRB has only one SO and all risks are linked to that SO. |
This risk is linked to priority 1 – Enhance compliance with the Guidelines |
| The evolution of the nature and distribution of patented medicines into more complex and innovative areas may hinder the effectiveness of the PMPRB's ability to deliver its mandate |
- The Board is committed in its work to assess and consider potential modifications to its Guidelines so that they remain effective both in facilitating Board Staff's review of patented drug prices and in promoting voluntary compliance on the part of patentees.
- The Board continues to focus on transparency and communications.
- PMPRB funds continue to be committed to holding face–to–face outreach sessions with patentees and to improving the accessibility and usefulness of material on the Board's website.
|
The PMPRB has only one SO and all risks are linked to that SO. |
This risk is linked to priority 1 – Enhance compliance with the Guidelines; and
priority 3 – Transparency and communications |
| Changes respecting the pricing and reimbursement of patented medicines in the seven comparator countries listed in the Regulations may impact the PMPRB's legislative and regulatory framework |
- The PMPRB continues to monitor and assess changes respecting the pricing and reimbursement of patented medicines occurring in the seven foreign comparator countries it considers in conducting its price reviews.
- The PMPRB monitors for new publicly available ex–factory prices and the impact of rebates on the accuracy of these prices.
|
The PMPRB has only one SO and all risks are linked to that SO. |
This risk is linked to priority 1 – Enhance compliance with the Guidelines |
| Greater use of Product Listing Agreements by public payers and, in the future, perhaps private payers, could challenge the PMPRB's impact and relevance |
- The PMPRB monitors and assesses changes respecting the pricing and reimbursement to public payers for patented medicines.
|
The PMPRB has only one SO and all risks are linked to that SO. |
This risk is linked to priority 1 – Enhance compliance with the Guidelines |
The PMPRB is responsible for ensuring that the factory–gate prices patentees charge for patented medicines sold in Canada for human or veterinary use are not excessive. Like many regulators, the PMPRB promotes and relies on voluntary compliance as a means to effectively use resources and control its costs and the costs for patentees. The PMPRB has largely been able to carry out its mandate with limited recourse to public hearings due, at least in part, to the rate of compliance with the Board's Excessive Price Guidelines (Guidelines) and the use of Voluntary Compliance Undertakings (VCUs).
In 2012–13 the prices of 1,328 new and existing patented medicines were reported to the PMPRB. Of that number, 1,090 patented medicines (82.1%) were priced within the Guidelines, 139 patented medicines (10.5 %) were priced at a price which did not trigger the investigation criteria and the prices of 31 patented medicines (2.3%) were considered non–excessive following the approval of a Voluntary Compliance Undertaking (VCU). Consequently, the overall compliance rate for 2012–13 was 94.9%.
The Board continues to address challenges in the operationalization of the new Guidelines. In 2012–13 the Board provided clarification on the Guideline on “Any Market” price reviews. In 2013–14, the Board will consider options for changes to the Consumer Price Index (CPI)–Adjustment Methodology. The changes are intended to provide greater transparency and predictability to the CPI–Adjustment Methodology.
Early indications continue to show that two of the most significant changes to the Guidelines: a) the recognition of incremental innovation, and b) the recognition of patentees' of offering benefits to consumers and payers as part of their business and distribution models have improved compliance and assisted in the effective management of the PMPRB's resources.
Since the implementation of the new Guidelines, the PMPRB has been monitoring and evaluating the application and impact of the changes to the Guidelines on an ongoing basis. In June 2011, the PMPRB published the Monitoring and Evaluation Plan for the Major Changes to the Guidelines. The Board was presented with the second annual assessment under this Plan in December 2012, and a table summarizing the results was published in January 2013.
As patentees and Board Staff gain experience working with the new Guidelines, and as the monitoring and evaluation process proceeds, new issues will continue to be identified. In such cases, clarifications will be promptly communicated through the quarterly NEWSletter, and stakeholders will be consulted on proposed amendments to the Guidelines through the Notice and Comment process. An updated version of the Guidelines, reflecting changes and clarifications, is released annually in June.
The pricing and reimbursement environment in Canada is one that evolves over time and which has changed significantly over recent years. The PMPRB continues to examine its price review process for ways to improve efficiency and to ensure the process remains relevant in an ever–changing environment. The Board is currently consulting on a proposal to replace the semi–annual regulatory filing of Form 2 – Information on the Identity and Prices of the Medicine with an annual filing.
To be responsive to the changing environment, the Board continues to focus on transparency and communications. To this end, the PMPRB announced the launch of a Twitter account to further enhance communication with its stakeholders. In addition, in December 2012, the Regulatory Affairs and Outreach Branch held its very first PMPRB 101 session. The session was developed for participants from pharmaceutical companies who have little experience in working with the PMPRB.
Internationally, the PMPRB continues to monitor and assess changes respecting the pricing and reimbursement of patented medicines occurring in the seven foreign comparator countries it considers in conducting its price reviews.3 In particular, changes in Germany and the United Kingdom are being closely monitored to assess any potential impact they may have on the PMPRB's application of its legislative and regulatory framework and on the Board's Guidelines.
A Management Response and Action Plan was developed in response to the Evaluation Report and was posted on the PMPRB website in February 2013. The Management Response and Action Plan addresses the considerations outlined in the Evaluation Report through examining ways to (i) expedite its processes and further simplify the Guidelines, (ii) decrease regulatory burden, and (iii) make effective use of its resources, all without compromising its consumer protection role. To that end, the PMPRB recently concluded a Notice and Comment and anticipates further consultation with its stakeholders in the fall of 2013 on proposed regulatory burden reduction initiatives.
In April 2013, through the National Prescription Drug Utilization Information System (NPDUIS) initiative, the PMPRB released two new analytical reports: The Use of Blood Glucose Test Strips in Select Public Drug Plans, 2008 and the latest edition of the New Drug Pipeline Monitor. Additional studies are planned for 2013–14 as listed in the NPDUIS Research Agenda available on the PMPRB website under NPDUIS.
The PMPRB also continues to work on its integrated human resources and business planning model. A Strategic Planning Session will be held in September.
Summary of Performance
Financial Resources 1 – Total Departmental ($ thousands)
Total Budgetary Expenditures (Main Estimates)
2012–13 |
Planned Spending
2012–13 |
Total Authorities (available for use)
2012–13 |
Actual Spending (authorities used)
2012–13 |
Difference (Planned vs. Actual Spending) |
|
The difference between Planned Spending and Total Authorities is approx. $888.4 K which is made up of a $464K refund of non–controllable salaries and wages, collective agreement adjustments of $39.9K and a carry forward of $384 K. Total Authorities includes a Special Purpose Allotment (SPA) that is used to conduct Public Hearings in the amount of $3,100K. The SPA can only be used to cover the costs of Public Hearings such as, external legal counsel, expert witnesses, etc. Any unspent amount is returned to the Consolidated Revenue Fund (CRF). Due to challenges related to forecasting the number and complexity of hearings, for the purposes of forecasting Planned Spending, it is assumed that the entire SPA will be spent in a given fiscal year. In 2012–13, the difference between Planned Spending and Actual Spending is primarily the result of a lapse of approx. $3 million in the SPA, and $774K of savings gained through operational efficiencies and streamlining processes.
|
| 11,832.4 |
11,832.4 |
12,720.7 |
8,056.8 |
3,775.6 |
Human Resources (Full–Time Equivalents – FTEs)
Planned
2012–13 |
Actual
2012–13 |
Difference
2012–13 |
|
The PMPRB determined that as a result of efficiencies gained through other initiatives, it was in a position not to staff 8 to 10 vacant positions with minimal impact to its operations.
|
| 76.0 |
60.4 |
15.6 |
| Strategic Outcome: Canadians are protected from excessive prices for patented medicines sold in Canada and stakeholders are informed on pharmaceutical trends. |
| Program |
Total Budgetary Expenditures (Main Estimates 2012–13) |
Planned Spending |
Total Authorities (available for use) 2012–13 |
Actual Spending (authorities used) |
Alignment to Government of Canada Outcomes |
| 2012-13 |
2013–14 |
2014–15 |
2012–134 |
2011–12 |
2010–11 |
|
* In 2011-12, the Federal Court quashed a Board Order issued in 2008-09 and directed that the sum of $2,512,878 be returned promptly to the patentee with appropriate interest and specified costs which totalled $46.9 thousand. Actual Spending for 2011-12 includes the refund of $2,559.8 thousand paid to the patentee.
|
| Program 1 Patented Medicine Price Regulation |
7,508.1 |
7,508.1 |
6,781.3 |
6,781.3 |
7,837.1 |
3,888.8 |
7,346.7 |
4,357.3 |
Healthy Canadians |
| Program 2 Pharmaceutical Trends |
1,265.4 |
1,265.4 |
1,328.8 |
1,287.4 |
1,325.1 |
983.3 |
1,010.5 |
890.4 |
Healthy Canadians |
| Strategic Outcome 1 Sub-Total |
8,773.5 |
8,773.5 |
8,110.1 |
8,068.7 |
9,162.2 |
4,872.1 |
*8,357.3 |
5,122.4 |
|
Performance Summary Table for Internal Services ($ thousands)
| Internal Services |
Total Budgetary Expenditures
(Main Estimates
2012–13) |
Planned Spending |
Total Authorities
(available for use)
2012–13 |
Actual Spending (authorities used) |
| 2012-13 |
2013–14 |
2014–15 |
2012–13 |
2011–12 |
2010–11 |
| |
3,058.9 |
3,058.9 |
3,068.4 |
2,833.9 |
3,558.5 |
3,184.7 |
3,397.1 |
4,348.3 |
| Sub-Total |
3,058.9 |
3,058.9 |
3,068.4 |
2,833.9 |
3,558.5 |
3,184.7 |
3,397.1 |
4,348.3 |
Total Performance Summary Table ($ thousands)
| Strategic Outcome(s) and Internal Services |
Total Budgetary Expenditures
(Main Estimates
2012–13) |
Planned Spending |
Total Authorities
(available for use)
2012–13 |
Actual Spending (authorities used) |
| 2012-13 |
2013–14 |
2014–15 |
2012–13 |
2011–12 |
2010–11 |
| |
11,832.4 |
11,832.4 |
11,178.6 |
10,902.6 |
12,720.7 |
8,056.8 |
11,754.4 |
9,470.7 |
| Total |
11,832.4 |
11,832.4 |
11,178.6 |
10,902.6 |
12,720.7 |
8,056.8 |
11,754.4 |
9,470.7 |
Planned Spending for 2012–13 is based on the assumption that the PMPRB will spend the full $3.1 million held in a Special Purpose Allotment (SPA) reserved for conducting public hearings. This is done because these expenditures are completely dependent on the number, length and complexity of each hearing held, which are very difficult to predict. For this reason, any SPA funds not required for hearings are returned to the Consolidated Revenue Fund. In addition, Planned Spending for 2012–13 was not reduced by the savings the PMPRB gained through operational efficiencies and streamlining processes. However, the savings gained have been deducted from Planned Spending amounts in future years.
Planned Spending for 2013–14 includes an estimated carry–forward of $234.5 thousand.
Actual Spending for 2012–13 is significantly less than planned spending as a result of a lapse of approximately $3 million in the SPA funding and $776 thousand of operating funds. These lapses include the $774 thousand of savings gained through operational efficiencies and streamlining processes.
Actual Spending for 2011-12 includes a refund to a patentee of $2,559.8 thousand.5
Expenditure Profile
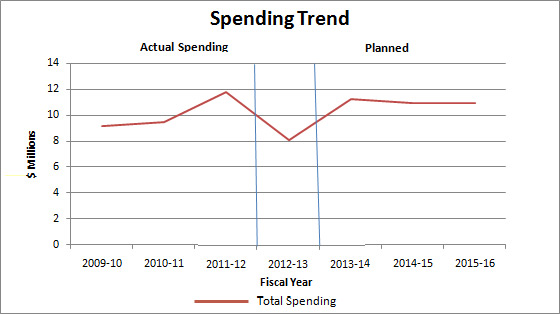
Departmental Spending Trend
[D]
Estimates by Vote
In September 2008, the PMPRB received $4.7 million (excluding EBP), in addition to its core A–base of $5.8 million to meet workload pressures and continue ongoing initiatives related to the delivery of its mandate. Vote 40 (Program expenditures) was increased by $5.6 million for 2009–10, $6.2 million for 2010–11, and $5.8 million for 2011–12 and future years (including EBP and excluding Public Works and Government Services Canada accommodation charges). The PMPRB's total funding includes a Special Purpose Allotment (SPA) of $3.1 million which is used to conduct Public Hearings. The SPA can only be used to cover the costs of public hearings, such as external legal counsel, expert witnesses, etc. Any unspent amount is returned to the Consolidated Revenue Fund (CRF).
In 2011–12, the PMPRB reported an increase in Actual Spending as a result of having to refund $2,559.8 thousand to a patentee as a result of a Federal Court order.
In 2012–13, the PMPRB lapsed approximately $3 million of funding for its SPA and $776 thousand of its operating budget.
Planned Spending for subsequent years is based on the assumption that the entire SPA funding will be spent each fiscal year.
For information on the PMPRB's organizational Votes and/or statutory expenditures, please see the Public Accounts of Canada 2013 (Volume II)xii. An electronic version of the Public Accounts 2013 is available on the Public Works and Government Services Canada website.
Strategic Environmental Assessment [for non–FSDS departments]
During 2012–13 the Patented Medicine Prices Review Board considered the environmental effects of initiatives subject to the Cabinet Directive on the Environmental Assessment of Policy, Plan and Program Proposals. None of the initiatives undertaken by the PMPRB were subject to this directive.
Section II: Analysis of Programs and Sub–Programs by Strategic Outcome(s)
Strategic Outcome
The PMPRB has only one Strategic Outcome:
Canadians are protected from excessive prices for patented medicines sold in Canada and stakeholders are informed on pharmaceutical trends.
This Strategic Outcome is supported by two programs and Internal Services.
| Strategic Outcome: Canadians are protected from excessive prices for patented medicines sold in Canada and stakeholders are informed on pharmaceutical trends. |
| Performance Indicator |
Target |
Actual Results |
| Canada's prices on average are in line with the seven comparator countries listed in the Regulations. |
Canada's prices on average are at or below the median of international prices. |
As in previous years, Canadian prices were typically within the range of prices observed among the comparator countries. |
The PMPRB's Annual Report provides detailed statistics comparing the foreign prices of patented medicines to their Canadian prices. For many years, the PMPRB has reported average foreign–to–Canadian price ratios with foreign prices converted to their Canadian dollar equivalents by means of market exchange rates. (Specifically, the 36–month moving averages of market rates the PMPRB normally used in applying its Guidelines.) Focusing on the results with currency conversion at market exchange rates, it appears that, as in previous years, Canadian prices were typically within the range of prices observed among the comparator countries. Canadian prices were roughly in line with Swiss prices. Prices in France, Italy, the United Kingdom and Sweden were appreciably lower than Canadian prices, while those in Germany were substantially higher. As in previous years, prices reported for the United States were much higher than prices in Canada or any other comparator country.
Programs
The Patented Medicine Price Regulation Program
The PMPRB is responsible for regulating the non–excessive average prices for patented drug products sold in Canada for human and veterinary use. Through this program activity, the PMPRB reviews the prices that patentees charge for patented drug products, based on the price review factors in the Act, to ensure that these prices are not excessive. In the event that the Board finds, following a public hearing, that a price is excessive in any market, it may order the patentee to reduce the price and take measures to offset any excess revenues it may have received as a result of excessive prices.
Financial Resources – For Program ($ thousands)
Total Budgetary Expenditures
(Main Estimates)
2012–13 |
Planned Spending
2012–13 |
Total Authorities
(available for use)
2012–13 |
Actual Spending
(authorities used)
2012–13 |
Difference
2012–13 |
| 7,508.1 |
7,508.1 |
7,837.1 |
3,888.8 |
3,619.3 |
Human Resources (FTEs) – For Program
Planned
2012–13 |
Actual
2012–13 |
Difference
2012–13 |
| 44.0 |
30.3 |
13.7 |
Performance Results – For Program
| Expected Results |
Performance Indicators |
Targets |
Actual Results |
| Patentees comply with the Patent Act, the Regulations and the Guidelines |
Percentage of patented medicines that are priced, as a result of voluntary compliance, within the Guidelines or at a price which does not trigger the investigation criteria |
95% of patented medicines are voluntarily priced within the Guidelines or at a price which does not trigger the investigation criteria |
92.5% of patented medicines are voluntarily priced within the Guidelines or at a price which does not trigger the investigation criteria
|
| Percentage of patented medicines that are subject to Board Orders |
100% of Board Orders are complied with |
100% of VCUs and Board Orders were complied with |
Performance Summary and Analysis of Program Activity
In 2012, Board Staff completed price reviews of 1,321 of the 1,328 new and existing patented medicines sold in Canada for human use reported to the PMPRB. As at March 31, 2013, seven patented medicines were still under review. Of these, 1,090 were priced within the Guidelines and 139 were priced at a price which did not trigger the investigation criteria – 92.5% of the patented medicines were voluntarily priced within the Guidelines or at a price which did not trigger the investigation criteria. This is slightly below the 94.6% reported last year in the DPR.
Since the implementation of the new Guidelines in January 2010, the percentage of patented medicines voluntarily priced within in the Guidelines or at a price which does not trigger the investigation criteria has been over 90%: 91.1% in 2010; 94.6% in 2011; and 92.5% in 2012.
Through its monitoring of short– and long–term impacts of the major changes to the Guidelines, the Board has been able to provide clarification on the Guidelines and, where necessary, make adjustments rapidly. The Board continues to monitor the impact of its Guidelines through the GMEP. The Board is currently consulting with its stakeholders on two initiatives; the CPI–Adjustment Methodology and the number of annual price and sales data filings by patentees.
By eliminating the use of Forecast CPI and committing to the use of an actual CPI, patentees would have greater predictability and certainty when they consider price increases. It is expected that this will result in enhanced compliance. In addition, the proposed Form 1 addition coupled with the elimination of the Form 2 filing for the first day of sales would reduce the regulatory burden of patentees. The Board anticipates that less burdensome reporting requirements will support and perhaps enhance patentee compliance.
The Pharmaceutical Trends Program
The PMPRB conducts research and analysis on pharmaceutical trends and reports annually to Parliament through the Minister of Health on pharmaceutical trends and research and development spending by pharmaceutical patentees. Through the National Prescription Drug Utilization Information System (NPDUIS), the PMPRB also conducts research and provides critical analyses of price, utilization and cost trends for both patented and non–patented prescription drugs.
Financial Resources – For Program Level ($ thousands)
Total Budgetary Expenditures
(Main Estimates)
2012–13 |
Planned Spending
2012–13 |
Total Authorities
(available for use)
2012–13 |
Actual Spending
(authorities used)
2012–13 |
Difference
2012–13 |
| 1,265.4 |
1,265.4 |
1,325.1 |
983.3 |
282.1 |
Human Resources (FTEs) – For Program Level
Planned
2012–13 |
Actual
2012–13 |
Difference
2012–13 |
| 13.0 |
7.8 |
5.2 |
Performance Results – For Program Level
| Expected Results |
Performance Indicators |
Targets |
Actual Results |
| Stakeholders are more aware of pharmaceutical trends and cost drivers |
Number of website hits |
5% increase in the number of hits from previous year |
|
| Number of presentations by the PMPRB at external meetings |
10 events per year |
11 events |
Performance Analysis and Lessons Learned
All PMPRB publications, including studies, Board decisions and reference documents, are available on the PMPRB website. The PMPRB continues its work initiated in 2011 to streamline content to provide added context and create a more user–friendly environment. The revamped website will enhance the PMPRB's external communication activities, increasing general awareness of its role and overall activities, including pharmaceutical trends and information on pharmaceutical cost drivers.
In 2012–13, in addition to numerous meetings with various stakeholders, the PMPRB participated in 11 external events and addressed a variety of audiences, including the PMPRB's main stakeholders: patentees, provinces, third–party payers and patient advocacy groups. The PMPRB continues to use new mediums, such as videoconference and webinar, to reach a greater number of stakeholders.
In 2012–13, through the NPDUIS initiative6, the PMPRB completed two new analytical studies:
- The Use of Blood Glucose Test Strips in Select Public Drug Plans, 2008
(Revised April 2013)
- New Drug Pipeline Monitor (April 2013)
These reports were published and posted on the PMPRB website in April 2013.
Internal Services
Internal Services are groups of related activities and resources that are administered to support the needs of programs and other corporate obligations of an organization. These groups are: Management and Oversight Services; Communications Services; Legal Services; Human Resources Management Services; Financial Management Services; Information Management Services; Information Technology Services; Real Property Services; Materiel Services; Acquisition Services; and Travel and Other Administrative Services. Internal Services include only those activities and resources that apply across an organization and not to those provided specifically to a program.
Financial Resources – For Program Level ($ thousands)
Total Budgetary Expenditures
(Main Estimates)
2012–13 |
Planned Spending
2012–13 |
Total Authorities
(available for use)
2012–13 |
Actual Spending
(authorities used)
2012–13 |
Difference
2012–13 |
| 3,058.9 |
3,058.9 |
3,558.5 |
3,397.1 |
(125.8) |
Human Resources (FTEs) – For Program Level
Planned
2012–13 |
Actual
2012–13 |
Difference
2012–13 |
|
* Two resources were re–allocated from program activities to support the implementation of the mission–critical database.
|
| 19.0 |
22.3 |
(3.3)* |
Section III: Supplementary Information
Financial Statements Highlights
Condensed Statement of Operations and Departmental Net Financial Position
Patented Medicine Prices Review Board
Condensed Statement of Operations and Departmental Net Financial Position (Unaudited)
For the Year Ended March 31, 2013
($ thousands) |
| |
2012–13
Planned
Results |
2012–13
Actual |
2011–12
Actual |
$ Change
(2012–13 Planned vs. Actual) |
$ Change
(2012–13 Actual vs. 2011–12 Actual) |
|
* Revenues that are non–respendable are not available to discharge the Board's liabilities. Non–respendable revenues are earned on behalf of the Government of Canada. The PMPRB collects non–respendable revenues as a result of payments made by patentees to the Government of Canada through Voluntary Compliance Undertakings (VCUs) or Board Orders to offset excess revenues. In 2012–13, the PMPRB collected non–respendable revenues in the amount of $ 19,670.4 thousand. In 2011–12, the non–respendable revenues were $11,195.5 thousand. However, this amount was offset by the establishment of a contingent liability to be paid on behalf of the Government in the amount of $2,801.3 thousand; the net non–respendable revenues were $8,394.2 thousand.
|
| Total expenses |
13,138.5 |
8,338.8 |
10,506.7 |
(4,799.7) |
(2,167.8) |
| Total revenues* |
|
|
|
|
|
| Net cost of operations before government funding and transfers |
13,138.5 |
8,338.8 |
10,506.7 |
(4,799.7) |
(2,167.8) |
| Departmental net financial position |
|
(636.8) |
(1,505.1) |
|
868.3 |
Condensed Statement of Financial Position
Patented Medicine Prices Review Board
Condensed Statement of Financial Position (Unaudited)
As at March 31, 2013
($ thousands) |
| |
2012–13 |
2011–12 |
$ Change |
| Total net liabilities |
1,228.6 |
2,120.2 |
(891.6) |
| Total net financial assets |
525.9 |
615.1 |
(89.2) |
| Departmental net debt |
702.7 |
1,505.1 |
(802.4) |
| Total non–financial assets |
65.9 |
0 |
65.9 |
| Departmental net financial position |
(636.8) |
(1,505.1) |
868.3 |
Net Liabilities
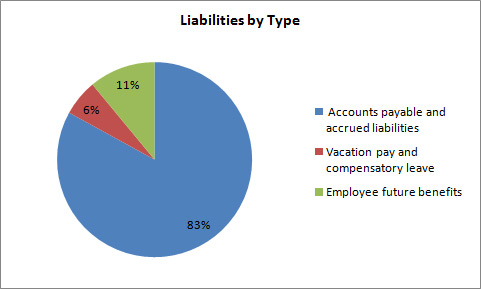
Total liabilities were $1,228.6 thousand at the end of 2012–13, a decrease of $891.6 thousand from the previous year. The decrease in liabilities was as follows:
- Accounts payable and accrued liabilities decreased by $404.2.
- Vacation pay and compensatory leave decreased by $1.3.
- Employee future benefits decreased by $486.0.
[D]
Net Financial Assets
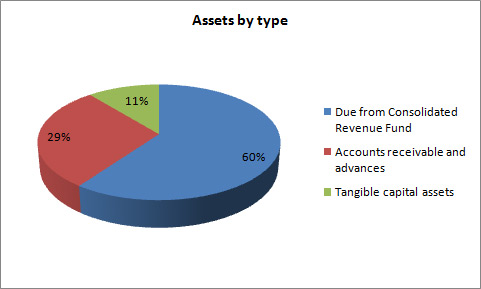
Total assets were $591.8 thousand at the end of 2012–13, a decrease of $23.3 thousand from the previous year. The variances in assets were as follows:
- Decrease in Due from the Consolidated Revenue Fund of $107.5.
- Increase in Accounts receivable and advances of $18.3.
- Increase in Tangible capital assets of $66.
[D]
Financial Statements
The financial highlights presented within this Departmental Performance Report are intended to serve as a general overview of the PMPRB's financial position and operations. The PMPRB's Financial Statementsxiii can be found on its website.
Supplementary Information Tables
- Greening Government Operations
- Internal Audits and Evaluations
- Sources of Respendable and Non–Respendable Revenue
All electronic supplementary information tables listed in the 2012–13 Departmental Performance Report can be found on the PMPRB website.xiv
Tax Expenditures and Evaluations Report
The tax system can be used to achieve public policy objectives through the application of special measures such as low tax rates, exemptions, deductions, deferrals and credits. The Department of Finance publishes cost estimates and projections for these measures annually in the Tax Expenditures and Evaluationsxv publication. The tax measures presented in the Tax Expenditures and Evaluations publication are the sole responsibility of the Minister of Finance.
Section IV: Other Items of Interest
Organizational Contact Information
The Patented Medicine Prices Review Board
Box L40
Standard Life Centre
333 Laurier Avenue West
Suite 1400
Ottawa, Ontario K1P 1C1
Telephone: (613) 952-7360
Toll–free no.: 1-877-861-2350
Facsimilie: (613) 952-7626
TTY: (613) 957-4373
Email: pmprb@pmprb-cepmb.gc.ca
Website: www.pmprb-cepmb.gc.ca
Additional Information
PMPRB Annual Report 2012 – http://www.pmprb-cepmb.gc.ca/english/view.asp?x=9
Quarterly NEWSletter – http://www.pmprb-cepmb.gc.ca/english/View.asp?x=287
Patentee's Guide to Reporting – http://www.pmprb-cepmb.gc.ca/english/view.asp?x=1731
Compendium of Policies, Guidelines and Procedures – Reissued June 2013
http://www.pmprb-cepmb.gc.ca/english/View.asp?x=1733
Patent Act (http://laws.justice.gc.ca/en/P-4/index.html)
Patented Medicines Regulations (http://laws.justice.gc.ca/en/P-4/SOR-94-688/index.html)
Endnotes
1 The Policy on MRRS recently underwent changes that came into effect on April 1, 2013. As a result, updates have been made to the MRRS nomenclature. Specifically, Program Activity Architecture is now Program Alignment Architecture and Program Activity is now simply Program.
2 Type is defined as follows: previously committed to—committed to in the first or second fiscal year prior to the subject year of the report; ongoing—committed to at least three fiscal years prior to the subject year of the report; and new—newly committed to in the reporting year of the RPP or DPR.
3 France, Germany, Italy, Sweden, Switzerland, the United Kingdom and the United States
4 In order to align with departmental authorities by Program, as presented in Vol. II of the Public Accounts, services provided without charge amounts for employer's contribution to employee insurance plans, such as the Public Service Health Care Plan and the Public Service Dental Plan provided by the Treasury Board of Canada Secretariat, accommodations provided by Public Works and Government Services Canada, Workers' compensation provided by Human Resources and Skills Development Canada and legal services provided by the Department of Justice are not to be included in this figure. This information is presented in Departmental Financial Statements only.
5 In 2011-12, the PMPRB is reporting an increase in Total Authorities as a result of receiving additional funds in the amount of $2,559.8 thousand to cover a court awarded refund of a Board Order. Following a hearing of the Board conducted in 2008-09 pursuant to the Patent Act, the Board concluded that the patentee had sold two patented medicines in Canada at excessive prices. The patentee was ordered by the Board to pay the amount of $2,512,878 to the Crown. In 2011-12, the Federal Court quashed the Board Order and directed that the sum of $2,512,878 be returned promptly to the patentee with appropriate interest and specified costs which totalled $46.9 thousand.
6 The National Prescription Drug Utilization Information System (NPDUIS) is a research initiative established by federal, provincial, and territorial Ministers of Health in September 2001. The PMPRB's authority to conduct work under the NPDUIS initiative is based on a formal request by the federal Minister of Health under section 90 of the Patent Act, and is consistent with the PMPRB's mandate to report on pharmaceutical trends. Additional information on the NPDUIS initiative can be found on the PMPRB website: www.pmprb-cepmb.gc.ca under NPDUIS.
i Information on the National Prescription Drug Utilization Information System (NPDUIS) program can be found on the PMPRB website: www.pmprb-cepmb.gc.ca/NPDUIS
ii The Monitoring and Evaluation Plan for the Major Changes to the Guidelines 2012 can be found on the PMPRB website: www.pmprb-cepmb.gc.ca/Act, Regulations and Guidelines/Guidelines
iii For clarification of the Guideline on “Any Market” price reviews see the April 2012 issue of the NEWSletter on the PMPRB website: www.pmprb-cempb.gc.ca/Publications/NEWSletter
iv The Evaluation Report entitled Evaluation of the Patented Medicine Prices Review Board – Final Report – June 30, 2012 can be found on the PMPRB website:
www.pmprb-cepmb.gc.ca/Accountability /Program Evaluation
v The Management Response to the 2011-12 PMPRB Program Evaluation can be found on the PMPRB website: www.pmprb-cepmb.gc.ca/Accountability/Program Evaluation
vi The options and a recommendation for changes to the Consumer Price Index (CPI)–Adjustment Methodology – Final Report –Follow–up Recommendations on the Implementation of the DIP Methodology can be on the PMPRB website: www.pmprb-cepmb.gc.ca/Consultations/DIP Methodology Technical Working Group
vii The Notice and Comment on the proposed changes to the Consumer Price Index (CPI) Methodology and the filing requirements can be found on the PMPRB website: www.pmprb-cepmb.gc.ca/ Consultation/Notice and Comment/Regulatory Burden Reduction Initiative
viii The PMPRB Service Standards can be found on the PMPRB website: www.pmprb-cepmb.gc.ca/Act, Regulations and Guidelines/Regulatory Management/Service Standards
ix The Patentee's Guide to Reporting – Updated February 2012 can be found on the PMPRB website: www.pmprb-cepmb.gc.ca/Publications/Guides and Guidelines
x The analytical report entitled, The Use of Blood Glucose Test Strips in Select Public Drug Plans, 2008 can be found on the PMPRB website; www.pmprb-cepmb.gc.ca/Publications/Analytica Studies
xThe analytical report entitled, New Drug Pipeline Monitor can be found on the PMPRB website: www.pmprb-cepmb.gc.ca/Publications/Analytical Studies
xii The Public Accounts of Canada 2013 (Volume II) can be found on the Public Works and Government Services website: www.tpsgc-pwgsc.gc.ca/recgen/cpc-pac/index-eng.html
xiii The Financial Statements can be found on the PMPRB's website: www.pmprb-cepmb.gc.ca/Reports to Parliament.
xivAll electronic supplementary information tables listed in the 2012–13 Departmental Performance Report can be found on the PMPRB website: www.pmprb-cepmb.gc.ca/Reports to Parliament/Departmental Performance Report
xv The Department of Finance's annual Tax Expenditures and Evaluations publications can be found on the Department of Finance website: http://www.fin.gc.ca/purl/taxexp-eng.asp