Compendium of Policies, Guidelines and Procedures
Table Of Contents
- Introduction and Purpose of the Stakeholder Consultations
- Board Positions on Proposed Revisions to the Compendium of Policies, Guidelines and Procedures
- Preamble
- Part I – Legal Framework
- Part II – Policies
- Part III – Guidelines and Procedures
- Chapter 1 – The Scientific Review Process
- Chapter 2 – The Price Review Process
- Chapter 3 – Investigations
- Schedules
- Schedule 1 – Submissions by Patentees on Therapeutic Improvement
- Schedule 2 – Comparable Dosage Forms
- Schedule 3 – Therapeutic Class Comparison Test
- Schedule 4 – Reasonable Relationship Test
- Schedule 5 – Median International Price Comparison Test
- Schedule 6 – Highest International Price Comparison Test
- Schedule 7 – International Therapeutic Class Comparison Test
- Schedule 8 – Application of Price Tests for New Drug Products
- Schedule 9 – CPI-Adjustment Methodology
- Schedule 10 – DIP Methodology
- Schedule 11 – Criteria for Commencing an Investigation
- Schedule 12 – “Any Market” Price Reviews
- Schedule 13 – Offset of Excess Revenues
Introduction and Purpose of the Stakeholder Consultations
The Patented Medicine Prices Review Board has developed a further draft revised Compendium of Policies, Guidelines and Procedures (Compendium) as part of the next phase of the Guidelines review exercise. This draft document builds on all previous consultation activities that have taken place since the launch of the Guidelines review in 2005, particularly the August 20, 2008, Notice and Comment document.
Pursuant to subsection 96(5) of the Patent Act, the Board is consulting with all stakeholders on this draft revised Compendium in order to seek final feedback on proposed modifications to its various policies, guidelines and procedures. This consultation package is organized into two parts:
- Part A – A summary of the issues that have been under review, along with stakeholder views provided since the last Notice and Comment document released in August 2008, and Board positions; and
- Part B – The draft revised Compendium of Policies, Guidelines and Procedures.
The specific purpose of the consultation is to seek final comments on those sections of the draft revised Compendium that were amended, or added to, since the version released on August 20, 2008. Other sections of the Compendium, as identified in Part A, have already been approved by the Board following previous consultations, and are not the subject of this consultation.
The Board will review all submissions and publish the final revised Compendium in early June 2009, with implementation planned to take effect on July 1, 2009.
Your written feedback on the draft Compendium should be sent directly to Ms. Sylvie Dupont, Secretary of the Board, no later than April 27, 2009, but earlier submissions are encouraged. Submissions should be sent electronically at:
sylvie.dupont@pmprb-cepmb.gc.ca
Or at the following address:
Box L40
Standard Life Centre
333 Laurier Avenue West
Suite 1400
Ottawa, Ontario, K1P 1C1
If your comments are significant in length, please include a summary highlighting the key points of your submission. As with previous consultations, all submissions received by the Board will be posted on its Web site as part of its commitment to openness and transparency.
Board Positions on Proposed Revisions to the Compendium of Policies, Guidelines and Procedures
The following provides an overview of the major issues under review, including those that were raised in the August 2008 Notice and Comment document and those that are being raised for the first time in this consultation package. The background of each issue is discussed, along with stakeholder views (where available) and the Board´s position and/or proposed direction.
Overall Changes to the Board´s Compendium
In the draft revised Compendium released for consultation in August 2008, the Board put forward several overall changes to the existing document, including: modifications to the general structure to improve information flow, a new “Legal Framework” section to provide greater clarity to stakeholders, and wording changes in the mandate statement to clarify the underlying purpose of the PMPRB.
Stakeholder Views
In general, stakeholders were supportive of the changes to the overall structure of the Compendium and additional clarity provided with respect to the PMPRB´s legal framework. Stakeholders had a divergence of opinion as to the adjusted wording of the mandate. Some felt that the PMPRB´s purpose should be to ensure that the prices charged by patentees for patented medicines sold in Canada are not excessive, thereby protecting consumers and contributing to Canadian health care; while others felt that the purpose should be to maintain an appropriate balance between the incentives for research and development and protection against excessive prices.
Board Position
The Board believes that the interests of Canadians and the Canadian health care system are the key reasons the PMPRB was created in 1987. The Board has decided to include language to this effect in its revised mandate statement.
Furthermore, in the August 2008 Notice and Comment document, the Board noted in section 8 of the Legal Framework that any information submitted by a patentee to the PMPRB that is in the public domain will not be considered privileged under subsection 87(1) of the Patent Act (Act). Although it has been the Board´s practice to not publish the publicly available ex-factory prices of a patented medicine in Canada and other countries listed in the Patented Medicines Regulations (Form 2, Block 5) without the patentee´s consent, the Board is of the view that since the Form 2, Block 5 information is by definition required to be publicly available, it will no longer be considered privileged information under subsection 87(1) of the Act.
| Summary of Major Changes for Consultation |
Location |
| The PMPRB´s regulatory mandate statement has been updated as follows: “To ensure that the prices charged by patentees for patented drug products sold in Canada are not excessive, consistent with the interests of consumers and the Canadian health care system.” |
Part I, Subsection 2.1 |
| The Legal Framework of the Compendium has been updated to expressly include, as publicly available information, the publicly available international prices of a patented drug product that are filed with the PMPRB (Form 2 – Block 5). |
Part I, Subsection 9.2 |
Modification of Terminology Regarding the “Maximum Non-Excessive Price”
During recent discussions with industry stakeholders, a concern was raised that the existing terminology describing the regulated maximum price is confusing and potentially misleading to some stakeholders.
Stakeholder Views
Industry stakeholders are concerned that the term “Maximum Non-Excessive (MNE) Price” implies a price ceiling which no one can exceed. However, the PMPRB actually reviews theaverage price of patented drug products in a particular market. Given the possibility of different pricing in different markets, some variability around the National Average Transaction Price is to be expected. As long as the price in each market does not exceed the introductory maximum non-excessive price and over time does not increase by more than what is allowed under the CPI-Adjustment Methodology or become the highest of all the comparator countries, its price would not be presumed to be excessive. Nevertheless, when that market average price is compared to the aggregate National Average Transaction Price and to the national maximum non-excessive price, it may appear excessive.
Board Position
The Board agrees that clear terminology is important in ensuring transparency for all stakeholders. The Board believes that revised terminology is also needed to better assist stakeholders in understanding price reviews at the level of the individual market. The Board is seeking stakeholder feedback on the following revised terms:
- The term “Introductory Maximum Non-Excessive Price” would be replaced by the “Maximum Average Potential Price”, since it is intended that this would set the upper price threshold for the introductory average price in any and all markets (national, class of customer, province/territory).
- For existing patented drug products, the term “Maximum Non-Excessive Price” would be replaced by “Non-Excessive Average Price”. Each market (national, class of customer, province/territory) would have its own unique Non-Excessive Average Price, calculated based on the previous years´ actual non-excessive Average Transaction Prices for that market.
| Summary of Major Changes for Consultation |
Location |
| “Maximum Average Potential Price” used in lieu of “Introductory Maximum Non-Excessive (MNE) Price” for new drug products. |
Throughout Guidelines |
| “Non-Excessive Average Price” used in lieu of “Maximum Non-Excessive (MNE) Price” for existing drug products. |
Throughout Guidelines |
Publication of the CPI-Inflated Maximum Average Potential Price
Industry stakeholders have asked the Board to publish both the Maximum Average Potential Price (which is the price at introduction that no market may exceed), and this price grown by CPI on a cumulative basis every year
The Board is prepared to consider acting on this proposal and is seeking stakeholder comments as part of its final round of consultations.
It should be noted that the CPI-Inflated Maximum Average Potential Price could not be relied upon for regulatory purposes. One should not assume that the CPI-Inflated Maximum Average Potential Price represents a non-excessive average price for a particular market. The Non-Excessive Average Price for existing drug products will continue to be determined on the basis of actual Average Transaction Prices in each market and allowable CPI increases, and the highest international price.
It should also be noted that published CPI-Inflated Maximum Average Potential Prices will not be public prices that can be used for the purposes of the PMPRB´s price review.
Finally, the confidentiality provisions of the Act require that any published price be based on a public source and not the commercially confidential prices filed by patentees with the Board. It may not always be possible for the PMPRB to find public prices suitable for reporting purposes.
Levels of Therapeutic Improvement
During consultations undertaken in 2006, stakeholders expressed concern that the PMPRB´s approach to assessing the level of therapeutic improvement of a drug product did not recognize the nature of incremental pharmaceutical innovation. Additional stakeholder consultations were held, and a multi-stakeholder Working Group on Therapeutic Improvement was formed to provide recommendations on how to improve the PMPRB´s method of assessing a drug product´s level of therapeutic improvement. In the draft revised Guidelines released for consultation in August 2008, the PMPRB proposed four new levels of therapeutic improvement (with the addition of “moderate improvement” as a level), as well as revised primary and secondary factors for determining levels of therapeutic improvement.
Stakeholder Views
Stakeholder comments on the August 2008 Notice and Comment document were supportive of creating a new “moderate” level of therapeutic improvement, which was considered an improvement over the existing categories. Stakeholder comments were mixed on primary and secondary factors, with some favouring inclusion of additional factors on economic impacts, while others supported those selected by the Board.
Board Position
The Board has decided to adopt the proposed changes regarding levels of therapeutic improvement as put forward in the August 2008 Notice and Comment document. The Board has also decided to adopt the proposed changes regarding the primary and secondary factors for determining levels of therapeutic improvement, with the exception that secondary factors can only result in the level of therapeutic improvement being assessed at up to the level of moderate therapeutic improvement. The Board´s position is that secondary factors do not carry sufficient weight to move the level of therapeutic improvement from “moderate” to “substantial improvement”, which should only be possible through consideration of primary factors.
Introductory Price Tests
The development of new levels of therapeutic improvement necessitated a reconsideration of the associated price tests. In 2008, the multi-stakeholder Working Group on Price Tests was formed to consider how the introductory price tests should be revised in light of the newly proposed levels of therapeutic improvement.
i) The Reasonable Relationship (RR) Test
In the draft revised Guidelines released for consultation in August 2008, the Board adopted many of the recommendations of the Working Group, including maintaining the Reasonable Relationship test for line extensions (where no therapeutic improvement is proposed by the patentee or, if proposed, not recommended by the Human Drug Advisory Panel).
Stakeholder Views
Industry stakeholders reacted to a modification in the third part of the RR test put forward in the August 2008 Notice and Comment document. The Board had proposed that the price of a new lower strength drug product would be proportional to that of a higher strength product already being sold in Canada, rather than allowing the lower strength to be priced as high as the higher strength. Industry stakeholders commented that this would create a disincentive to making available drug products used in “titration dosing”.
Board Position
The Board acknowledges that the original RR test did not create disincentives to making available a “titration dose”, whereas the revised test may discourage such a practice. Therefore, the Board proposes to revert back to the original intent and methodology of this test.
In reviewing all of the aspects of the RR test, the Board has determined that modifications are needed to test number two of the RR test. The purpose of these modifications is twofold. First, the proposed changes recognize the possibility of level pricing per unit for multiple strengths of a patented drug product. Second, the proposed changes address the issue of a possible negative price due to a negative Y-intercept. The Board is putting forward the following for notice and comment:
- RR test number two has been modified to allow for the possibility of a zero slope in the calculation of the reasonable relationship. This has been done to rectify a problem with the existing Guidelines that move drug products with a clear reasonable relationship under test two (e.g., level pricing per unit for multiple strengths of a patented drug) to test three by virtue of the zero slope linear relationship.
- Given the possibility that the line representing the highest positive linear relationship between the prices of existing strengths may result in a Y-intercept that is negative, implying that for some new strengths the allowable price would be less than zero, the Board proposes that in such cases the negative Y-intercept would be replaced by zero and the linear relationship would be established by drawing a line between the origin (0) and the per unit price of the highest priced comparable drug product.
The Board has also generally added to the description of the RR test in order to better assist stakeholders in their understanding of when and how this test would be conducted.
| Summary of Major Changes for Consultation |
Location |
| Additional clarity provided on when and how the Reasonable Relationship test would be conducted. |
Schedule 4 |
| Reverting back to existing Reasonable Relationship test number three. |
Schedule 4, Test 3 |
| RR test number two has been modified to recognize level pricing per unit for multiple strengths of a patented drug product. |
Schedule 4, Test 2, Bullet 2 |
| Reasonable Relationship test number two has been modified so that if the Y-intercept is less than zero, the negative Y-intercept would be replaced by drawing the reasonable relationship line between the origin (0) and the per unit price of the highest priced comparable drug product. |
Schedule 4, Test 2, Bullet 5 |
ii) Modified Guidelines for Certain Patented Generic Drug Products
A) Limited Therapeutic Class Comparison
In the August 2008 Notice and Comment document, the Board put forward for consultation a proposal for modified Guidelines for patented generic drug products, which would limit the comparators used in a therapeutic class comparison to only the reference (bioequivalent) or licensing brand´s drug product. This was a proposal that was championed by representatives of the generic pharmaceutical industry and considered a reasonable approach by the Board.
Stakeholder Views
Stakeholders from the generic pharmaceutical industry supported the proposals in the draft revised Guidelines of August 2008. No significant concerns were expressed by other stakeholders about limiting the therapeutic class comparison to the reference/licensing brand drug product.
Board Position
The Board considers a streamlined therapeutic class comparison, where a “bioequivalent” or “licensed” generic drug product would have as its sole comparator either the referenced or licensing brand´s drug product to be reasonable. In fact, this limitation involves comparing products with the same chemical entity, the same indication or use, the same or comparable dosage form and the same dosage regimen. Therefore it is appropriate to place text regarding this limited comparison in the Reasonable Relationship section (see chapter 2, paragraph 2.11(c)) and Schedule 4 of the draft revised Guidelines. This is a proposal that has been accepted by the regulated parties, would be less demanding of Board Staff time and would not require the resources of the Human Drug Advisory Panel. This change will be retained.
ii) Modified Guidelines for Certain Patented Generic Drug Products
B) Highest International Price Comparison (HIPC) Test
In 2008, consultations involving the generic pharmaceutical industry identified a number of issues that were argued to be unique to the market dynamics and competitive forces faced by generic drug products. In the draft revised Guidelines released in August 2008, the Board put forward for consultation a proposal whereby, for a bioequivalent patented generic drug product, the result of the domestic Therapeutic Class Comparison test would prevail even if the new patented generic drug product´s price in Canada exceeded the same drug product´s price in the comparator countries i.e., effectively exempting the generic drug product from the Board´s Highest International Price Comparison (HIPC) test.
Stakeholder Views
Stakeholders from the generic pharmaceutical industry supported the proposal in the draft revised Guidelines of August 2008. Nearly all other stakeholders that commented on the issue indicated that there should be no special category of patentee based on the type of company selling the drug product (i.e., generic versus brand/innovative), and that patentees should be treated equally. The Board was urged not to allow the exemption from the HIPC test.
Board Position
The Board met with representatives of the generic pharmaceutical industry and invited any further submissions on this point. The Board agrees that fairness is a key principle in price regulation and therefore all patentees should face the same Highest International Price Comparison constraint.
| Summary of Major Changes for Consultation |
Location |
| Removed the proposed exception for patented “bioequivalent” generic drug products to the HIPC rule in the draft revised Compendium. |
Removed from: Part III, Chapter 2, Subsection 2.11, Paragraph C, Bullet 1 |
iii) International Therapeutic Class Comparison (ITCC) Test
In 2007, the Board tasked the multi-stakeholder Working Group on International Therapeutic Class Comparison (ITCCWG) to make recommendations on the selection of comparators in the ITCC test. Following many of the recommendations of the ITCCWG, the August 2008 Notice and Comment document proposed that the ITCC test not be a primary price test, but that it be used to provide information in the context of an investigation into apparent excessive prices. The Board also proposed that two variations may be used to calculate the ITCC test: 1) the “ratio approach” and 2) the “straight class approach”.
Stakeholder Views
Industry stakeholders expressed concern that the PMPRB was not adopting the particular recommendation of the ITCCWG, which specified that generic drug products should not be included in the ITCC if the Board decides that the relevant result is established by any measure below the “top” of the ITCC test.
Board Position
The Board has re-examined this test and is proposing some further changes:
- When using both the “straight class” and “ratio” approaches of the ITCC test, the PMPRB proposes to use the resulting median international price as the measure of an excessive price.
- When undertaking the ITCC test, the comparable generic drug products used in the ITCC test will only be those sold by companies that also sell the same generic drug product in Canada.
The first modification is being put forward to provide clarity to stakeholders as to what measure will be used in applying the ITCC test. The Board considers that the previous reference to developing a “series of statistical values” is not very helpful. Stakeholders will benefit from knowing in advance what statistical measure will be predominantly considered when undertaking the ITCC test.
The second modification addresses industry´s concerns over the inclusion of generics in the ITCC test. The Board does not support the complete exclusion of generic drug products from the test, but given the vast number of potential generic drug product comparators in foreign countries, it acknowledges that the inclusion of all available generic drug products could unfairly skew the results of the test. In order to ensure that the comparators used in the test are both relevant and appropriate, the patented drug product under review will only be compared against those generic drug products that are being sold by a company that also sells the same generic drug product in Canada.
| Summary of Major Changes for Consultation |
Location |
| When using both the “straight class” and “ratio” approaches of the ITCC test, the resulting median international price will be compared against the patentee´s National Average Transaction Price. |
Schedule 7, Section 3 |
| The comparable generic drug products used in the ITCC test will only be those sold by companies that sell the same generic drug product in Canada. |
Schedule 7, Section 2 |
Any Market Price Reviews
In earlier consultations, some stakeholders expressed concerns that the ability of some classes of customers or provinces to obtain favourable prices might result in other customers/provinces paying a price that would be excessive. In response to this concern, pursuant to sections 83 and 85 of the Act, the August 2008 Notice and Comment document put forward proposals for price review in “any market”, including: ensuring that the introductory prices of particular markets are not excessive; and undertaking price reviews at the level of any market for existing drug products on a case-by-case basis where price variability in different markets appears to be an issue. The Board also sought feedback from stakeholders on the appropriate methodology for calculating excess revenues arising from a price that is excessive in a particular market.
Stakeholder Views
Feedback from stakeholders indicated that support for price review at the level of any market was mixed. In general, industry stakeholders felt that the need for such analysis has not been sufficiently demonstrated, while non-industry stakeholders clearly saw a need to undertake such price reviews. Common among many of the submissions received was that the language used to describe the proposals for price review at the level of any market was vague and unclear.
Board Position
In principle, the Board believes that some level of market-specific price review is part of its statutory mandate. The fact that some markets at introduction can pay as much as 25% or more above the Maximum Average Potential Price (formerly known as the Introductory Maximum Non-Excessive Price) was clearly demonstrated in the Board´s Discussion Guide released for consultation in May 2006.
The Board acknowledges that the approach to Any Market price reviews should be clarified. The new version of the draft revised Guidelines has more clear and detailed language with respect to Any Market price reviews, including a new schedule that clearly explains the methodology.
In addition to reviewing the price nationally, and for each of the three classes of customers, the Board is proposing to review the price in each province and territory at introduction.
For existing drug products, the Board will continue to review the price at the national level and only conduct reviews at the level of class of customer and/or province/territory if the national Average Transaction Price appears excessive or in the case of a complaint.
With respect to the appropriate methodology for the calculation of excessive revenues, stakeholders indicated that the excess revenue should be based on the average price across all markets in Canada (i.e., the National Average Transaction Price) to recognize and also take into account lower prices in other markets, rather than being limited to the market where the price was excessive. The Board supports this position and has included language to this effect in the draft revised Guidelines.
| Summary of Major Changes for Consultation |
Location |
| A new Schedule 12 was added to clarify the approach to any market price review. |
Schedule 12 |
| The markets that will undergo any market price review at introduction have been expanded to include: each province and territory in addition to each of the three classes of customers (hospital, pharmacy and wholesaler) and the national level. |
Part III, Chapter 2, Subsection 2.13 |
| Excess revenue will be calculated on the basis of the average price across all markets in Canada (i.e., the National Average Transaction Price). |
Schedule 12, Bullet 7, Sub-bullet 2 |
Re-Setting the Non-Excessive Average Price After Introduction
In the Discussion Paper released in January 2008, the PMPRB proposed three possible circumstances when it may be appropriate to “re-set” a Non-Excessive Average Price: 1) in the case of new scientific information; 2) when the new drug product was sold in too few countries and the Median International Price test was the pivotal introductory price test; and 3) because of potential cost of making and marketing arguments.
In the August 2008 Notice and Comment document, the Board took the position that:
- With respect to new scientific information, the Human Drug Advisory Panel would be asked to identify any important weaknesses or gaps in scientific evidence, but a specific Guideline was not needed on the subject.
- When a new patented drug product is sold in too few countries and the Median International Price (MIP) test is the pivotal introductory price test, the status quo approach is appropriate (i.e., reviewing the MIP within three years or once the patented drug product is sold in five comparator countries, whichever is earlier).
- Reviewing a price in regard to the costs of making and marketing may be appropriate, but this is not a mandatory price factor (under ss. 85(1)) which the Board must consider. Flexibility on the part of Board Staff was permitted but no specific Guideline was proposed.
Stakeholder Views
Feedback received on the August 2008 Notice and Comment document indicated that, in general, stakeholders were supportive of the direction taken by the Board. One concern identified by stakeholders was the omission of the following clause, which is in the existing Guidelines, but did not appear in the draft revised Guidelines of August 2008:
“The PMPRB recognizes that once a Notice of Compliance (NOC) has been obtained, it may be appropriate to adjust the benchmark price of a drug product first sold as an Investigational New Drug or under the Special Access Programme. In these cases, the average transaction price of the drug product following receipt of the NOC may be reviewed to determine if it appears to be excessive, based on the Guidelines applicable to new drug products.”
Board Position
The Board understands the concerns of stakeholders seeking to potentially re-set the Non-Excessive Average Price of a patented drug product once it receives an NOC, but takes the position that simply receiving an NOC in and of itself is not sufficient cause to trigger consideration of re-setting. The Board recognizes that it may be appropriate to adjust the Non-Excessive Average Price of a drug product based on cost arguments, and such arguments could arise in situations where the patented drug product was first sold on a compassionate basis as an Investigational New Drug or through the Special Access Programme and then, having obtained an NOC, other costs are incurred in bringing the drug to market in Canada. Language to this effect is included in the new draft revised Guidelines for Notice and Comment.
| Summary of Major Changes for Consultation |
Location |
| Addition of the following paragraph to the Guidelines: “The Board recognizes that there may be cost of making and marketing arguments, whereby it may be appropriate to adjust the Non-Excessive Average Price of a patented drug product (e.g., once a Notice of Compliance has been obtained and the drug product was first sold on a compassionate basis as an Investigational New Drug, through a Clinical Trial Application or under the Special Access Programme).” |
Part III, Chapter 2, Subsection 3.5 |
Recognizing Benefits (DIP Methodology)
In the August 2008 Notice and Comment document, the Board highlighted that it did not wish to unduly create disincentives to the offering of benefits to customers, and proposed what has come to be known as the “DIP Methodology” as a possible alternative to the application of the CPI-Adjustment Methodology in certain specific circumstances. The proposed methodology was based on the principle that in a circumstance where an apparent excessive price was due solely to the termination or reduction in a benefit, and the patentee provides evidence in this regard, the resulting average transaction price (ATP) should not be viewed as excessive if it simply “rebounds” to the pre-benefit price.
Stakeholder Views
Industry stakeholders shared many common concerns with the DIP Methodology, including: that the methodology is overly complex; that it will increase the workload of patentees and Board Staff; and that offering benefits and keeping track of them is not as straightforward as the methodology assumes. It was also recommended that a committee of representatives from the PMPRB and Rx&D be struck to discuss the challenges the proposal posed for the industry. An ad hoc bilateral Rx&D/PMPRB Board-level committee was formed and met on three occasions to discuss various issues of concern to industry.
Board Position
The Board´s position is that a methodology to address potential disincentives stemming from the mandatory reporting of benefits is appropriate. In essence the DIP Methodology allows flexibility for patentees whose prices, net of such benefits, would otherwise be constrained by the CPI-Adjustment Methodology even when such benefits end. The new draft revised Guidelines provide further clarification on the DIP Methodology, including:
- Clearer descriptions of how the DIP Methodology would work in practice;
- Flexibility to allow the ATP within a specific market to potentially “re-bound” up to the pre-benefit price in that market if evidence is provided;
- Flexibility to allow the price in a market that, from the date of first sale and in every period thereafter, received a benefit to potentially “rebound” up to the non-excessive price in another market that never received a benefit, again following receipt of evidence of the introductory benefit; and
- Guidelines on the minimum expectations from patentees in terms of the evidence of benefits that would be required in order to qualify for the DIP Methodology.
| Summary of Major Changes for Consultation |
Location |
| A new Schedule was added to provide greater clarity on the DIP Methodology. |
Schedule 10 |
Use of Patented and Non-Patented Drug Products in the Price Tests
During the price review of a new patented drug product, it is the current practice of Board Staff to examine the price of all pivotal comparator drug products, both patented and non-patented, to determine if they are priced excessively according to the Excessive Price Guidelines. While this has been the practice of Board Staff for some time, no consolidated policy statement exists.
Board Position
The Board is including a Policy on the Use of Patented and Non-Patented Drug Products in the Price Tests, in order to provide greater clarity to stakeholders on the current practices of Board Staff. The Board has also added details in the Guidelines as to how the prices of patented and non-patented drug products will be assessed to determine whether or not they would appear excessive.
| Summary of Major Changes for Consultation |
Location |
| Addition of Policy on the Use of Patented and Non-Patented Drug Products in the Price Tests |
Part II, Section 6 |
| Additional details have been added as to how the prices of patented and non-patented drug products will be assessed to determine whether or not they would appear excessive. |
Schedule 3, Section 2, Paragraphs 3-5 Schedule 4, Paragraphs 5-8 |
Offset of Excess Revenues
Under the existing Guidelines, when the Average Transaction Price of a drug product exceeds its Maximum Non-Excessive Price, excess revenues are generated. While the current Guidelines provide some guidance to patentees and Board Staff regarding how such excess revenues may be offset, the Board believes it is timely as part of the Guidelines review exercise to provide clarification and direction with respect to the measures patentees may take to offset excess revenues.
Board Position
As part of this consultation, the Board is putting forward a policy statement and a consolidated Schedule on the Offset of Excess Revenues. Stakeholders should note the following proposed modifications:
- To offset excess revenues via a price reduction, the average price of a patented drug product will only be considered to have been reduced if it is below the previous year´s Non-Excessive Average Price; not taking an allowable price increase will not be considered for purposes of offsetting excess revenues.
The rationale is that excess revenues generated by a patentee reflect the fact that customers on average were paying a price that appeared excessive, and that in accordance with Section 83 of the Act an actual price reduction is necessary, as described above, to offset the revenue.
- Excess revenue balances below the amount sufficient to trigger the investigation criteria that are carried for six consecutive six-month reporting periods (3 years) will be expected to be offset through a Voluntary Compliance Undertaking. Failing this, Board Staff will refer the matter to the Chairperson.
The rationale here is that under the existing Guidelines, patentees with excess revenue balances at levels below the investigation criteria can effectively maintain these balances indefinitely. Internal research by Board Staff indicates that it is not an uncommon occurrence that such excess revenues can exist over multiple years.
- The following exception to the CPI-Adjustment Methodology applies when a price reduction below the Non-Excessive Average Price is taken in one or more markets specifically to offset excess revenues. Following the repayment of excess revenues, the Average Transaction Prices in those markets may return in the next reporting period up to the Market-Specific Non-Excessive Average Prices prior to the price reduction.
The rationale here is that the Board does not wish to create a disincentive to taking a price reduction to offset excess revenues.
| Summary of Major Changes for Consultation |
Location |
| Addition of a Policy on the Offset of Excess Revenues |
Part II, Section 7 |
| A new Schedule was added for stakeholder comment on the proposed Policy on the Offset of Excess Revenues |
Schedule 13 |
Preamble
The Patented Medicine Prices Review Board (PMPRB) is committed to making the price review process more open and transparent to all stakeholders.
One of the primary objectives of the Compendium of Policies, Guidelines and Procedures (Compendium) is to ensure that patentees are aware of the policies, guidelines and procedures under which Board Staff reviews the prices of patented drug products sold in Canada, and the procedures normally undertaken in the scientific and price review processes and when a price appears to be excessive.
From time to time, the PMPRB finds it necessary to update the Guidelines under which it operates to ensure that they remain relevant and appropriate, as well as uphold the principles of fairness, transparency, openness and predictability. When considering Guidelines amendments, the PMPRB consults with its stakeholders through its Notice and Comment process.
The Compendium is organized as follows:
Part I – Legal Framework
Part II – Policies
Part III – Guidelines and Procedures
Chapter 1 – The Scientific Review Process
Chapter 2 – The Price Review Process
Chapter 3 – Investigations
Schedules
Part I – Legal Framework
1. Origin of the PMPRB
1.1 The PMPRB was established pursuant to amendments to the Patent Act (the Act) that came into force on December 7, 1987. Prior to 1987, Canada sought to moderate the prices of patented medicines by means of compulsory licenses to increase competition. Under the 1987 amendments, Canada strengthened patent protection of medicines to provide patentees with an incentive to invest in more pharmaceutical research and development (R&D) in Canada. These amendments also established the PMPRB as the consumer protection pillar of the pharmaceutical patent law reform to ensure that the prices of patented medicines are not excessive.
1.2 Further amendments to the Act, which came into force on February 15, 1993, abolished the granting of compulsory licenses for patented medicines so that patentees have a statutory monopoly for the entire term of their patents. In order to fill the vacuum created by the abolition of compulsory licenses, these amendments also expanded the PMPRB´s remedial powers so that it could now order payment of excess revenues derived by patentees while selling a medicine at an excessive price, in addition to ordering price reductions.
2. Mandate of the PMPRB
2.1 The PMPRB has the following dual mandate:
- Regulatory – To ensure that the prices charged by patentees for patented medicines sold in Canada are not excessive, consistent with the interests of consumers and the Canadian health care system; and
- Reporting – To report annually to Parliament on its activities, on the ratios of R&D expenditures to sales by the patented pharmaceutical industry and by individual patentees, and on pricing trends within the pharmaceutical industry, relating to all medicines, thereby contributing to informed decisions and policy making in health care; other reporting activities include inquiring into and reporting on any matter that the Minister of Health refers to it, pursuant to section 90 of the Act.
3. Structure and Operation of the PMPRB
3.1 The PMPRB is an independent and autonomous quasi-judicial body. To ensure this independence and autonomy, the Act provides no power, either expressly or implicitly, to the government to direct the PMPRB or to review its decisions and orders. As well, the PMPRB has no involvement in federal policy-making.
3.2 Decisions of the PMPRB are subject to judicial review by the Federal Court on substantive or procedural grounds in accordance with administrative law principles.
3.3 The PMPRB is composed of Board members, appointed pursuant to subsection 91(1) of the Act, and staff (Board Staff), appointed pursuant to subsection 94(1) of the Act.
3.4 The PMPRB has the authority to develop policies and procedures as to how it will carry out its statutory duties in a fair and effective manner. Part of the process by which the PMPRB has determined to carry out its statutory obligations is by the administrative separation of its review and prosecutorial functions, performed by Board Staff, and its adjudicative function performed by Board members.
3.5 Board Staff carries out the day-to-day work of the PMPRB including the administration of the Patented Medicines Regulations (the Regulations) to ensure compliance with the prescribed filing requirements. The review of prices of patented medicines is carried out in accordance with the Guidelines, which are approved by the Board.
3.6 If the Chairperson of the Board decides that it is in the public interest that a hearing be held, pursuant to subsection 83(6) of the Act, to determine whether a patented medicine is being or has been sold at an excessive price in any market in Canada, the Chairperson will issue a Notice of Hearing and will appoint a panel of Board members to preside at the hearing (Hearing Panel).
3.7 To preserve the impartiality of Board members, until a matter is brought before a Hearing Panel at a public hearing, no Board member is informed of the results of Board Staff´s review into an instance of possible excessive pricing, other than the Chairperson in his management capacity as the Chief Executive Officer of the PMPRB, pursuant to subsection 93(2) of the Act, which is done solely for the purpose of determining whether a hearing is in the public interest.
4. Jurisdiction of the PMPRB Pertaining to Price Regulation
4.1 The Act gives the PMPRB jurisdiction to determine whether a patentee or former patentee of an invention pertaining to a medicine is selling or has sold the medicine at an excessive price in any market in Canada if the following criteria are satisfied:1
4.1.1 Patentee or Former Patentee
- Subsection 79(1) of the Act defines a “patentee” as a person for the time being entitled to the benefit of a patent for an invention, including any other person entitled to exercise rights in relation to the patent, with the exception of a person granted a compulsory license by the Commissioner of Patents before December 20, 1991 that was not terminated before the day amendments to the Act came into force on February 15, 1993.
- The PMPRB also has jurisdiction over a former patentee of an invention, while it was a patentee.
4.1.2 Patent pertains to the medicine
Medicine
- The term “medicine” is not defined in the Act. Please refer to the Board´s Policy with respect to the Meaning of Medicine.
Patent
- Subsection 79(2) of the Act provides that a patent for an invention pertains to a medicine if the invention is intended or capable of being used for medicine or for the preparation or production of medicine.
- The PMPRB considers a patent to include any Canadian patent of invention that pertains to a medicine. This includes, but is not restricted or limited to:
- Patents for active ingredients;
- Patents for processes of manufacture;
- Patents for a particular delivery system or dosage form that are integral to the delivery of the medicine;
- Patents for indications/uses; and
- Patents for formulations.
- A patent pertains to a medicine if it is capable of being used, whether or not it is being worked.
- On the face of the patent, there must be a rational connection or nexus between the invention described in the patent and the medicine, which can be one of the merest slender thread.2
4.1.3 Sale in any market in Canada
- The patentee or former patentee must be selling or have sold the patented medicine in any market in Canada.
- With the exception of medicines sold under compulsory licenses granted by the Commissioner of Patents before December 20, 1991 that were not terminated before the day amendments to the Act came into force on February 15, 1993, all patented medicines sold in any market in Canada for human or veterinary use are covered by the PMPRB´s price review jurisdiction, including patented medicines sold pursuant to Notices of Compliance, under the Special Access Programme, through Clinical Trial Applications, or as Investigational New Drugs.
- The PMPRB reviews the prices of the first sale of a patented medicine at arm´s-length by the patentee, directly to a class of customer, namely a wholesaler, hospital, pharmacy or other. The PMPRB has no authority over prices charged by wholesalers or retailers or over pharmacists´ professional fees.
- Prices do not need to be approved by the PMPRB before patented medicines are sold in Canada. At the request of the patentee, Board Staff may provide pre-sale advisory assistance on whether a price would appear to be excessive.
- The PMPRB does not set the prices at which patented drug products can be sold but determines the Maximum Average Potential Price and the Non-Excessive Average Prices at which these drug products can be sold in Canada.
5. Price Regulation Factors
5.1 Subsection 85(1) of the Act stipulates those factors that the Board, during the course of a hearing, must take into consideration when determining whether a patented medicine is being sold or has been sold at an excessive price in any market in Canada by a patentee or former patentee. These factors are:
- The prices at which the medicine has been sold in the relevant market;
- The prices at which other medicines in the same therapeutic class have been sold in the relevant market;
- The prices at which the medicine and other medicines in the same therapeutic class have been sold in countries other than Canada;
- Changes in the Consumer Price Index; and
- Such other factors as may be specified in any regulations made for the purposes of this subsection.
5.2 If after considering the above factors, the Board is unable to determine if a price is excessive, subsection 85(2) of the Act stipulates that it may consider the costs of making and marketing the medicine, as well as other factors which can be specified by regulations or that the Board considers relevant in the circumstances.
5.3 The Board, following considerable deliberation and consultation with all stakeholders, pursuant to subsection 96(5) of the Act, published the PMPRB´s Guidelines pursuant to subsection 96(4) of the Act. Although the Guidelines are not binding on the Board or the patentee, they establish an approach and methodology in applying the factors set out in subsection 85(1) of the Act.
6. Remedies
6.1 Where the Board finds that a patentee is selling a patented medicine in any market in Canada at an excessive price, the Board may order the patentee to reduce the maximum price at which the patentee sells the medicine in that market.
6.2 In addition, where the Board finds that a patentee or former patentee, while a patentee, has sold a patented medicine in any market in Canada at an excessive price, the Board may order the patentee to offset the amount of excess revenues estimated by it to have been derived by the patentee or former patentee from the sale of the medicine at an excessive price.
6.3 Where the Board finds that the patentee or former patentee has engaged in a policy of selling the medicine at an excessive price, the Board may order the patentee to offset up to twice the amount of excess revenues estimated by it to have been derived by the patentee or former patentee from the sale of the medicine at an excessive price.
6.4 In order to offset excess revenues, the Board may order a patentee or former patentee to:
- reduce the price at which the patentee sells the medicine in any market in Canada;
- reduce the price at which the patentee sells one other patented medicine in any market in Canada; or
- make a payment to Her Majesty in right of Canada.
7. Filing Requirements Pertaining to Price Regulation
7.1 The PMPRB must have timely and accurate information to fulfill its regulatory mandate.
7.2 The Act and the Regulations set out the filing requirements pertaining to price regulation for a patentee or former patentee of an invention pertaining to a patented medicine that falls under the jurisdiction of the PMPRB. Further details on each element of information to be reported, and how and when the information is to be submitted to the PMPRB, can be found in the Patentee´s Guide to Reporting.
Notification of Intention to Sell a Patented Medicine
- Section 82 of the Act requires a patentee to notify the PMPRB of its intention to offer a patented medicine for sale in a market in Canada in which it has not previously been sold, and of the date on which sales are expected to begin, as soon as it is practicable to do so.
- The Board may order a patentee to provide information relating to the price at which it intends to sell the patented medicine. However, information relating to the price need not be provided earlier than 60 days before the date on which the product is intended to be sold.
Form 1 (Medicine Identification Sheet)
- Subsection 3(1) of the Regulations requires a patentee or former patentee of an invention pertaining to a medicine to report to the PMPRB prescribed information identifying the patented medicine (Form 1). Form 1 is required for all patented medicines for human or veterinary use and shall be accompanied by the product monograph for the patented medicine or, if an NOC has not been issued in respect of the patented medicine, by information similar to that contained in a product monograph.
- Subsections 3(2) and 3(3) of the Regulations require that Form 1 information must be reported if an NOC has been issued in respect of the medicine or if the medicine is being offered for sale in Canada, within seven days after the day on which the first NOC is issued in respect of the medicine, or within seven days after the day on which the medicine is first offered for sale in Canada, whichever comes first.
- If a patentee or former patentee begins selling a medicine in Canada during the pre-grant period, once the patent is issued the patentee or former patentee is required to file Form 1 information with the PMPRB.
Form 2 (Information on the Identity and Prices of the Medicine)
- Subsection 4(1) of the Regulations requires a patentee or former patentee of an invention pertaining to a medicine, which is selling or has sold the medicine in any market in Canada, to report to the PMPRB prescribed information identifying the medicine and concerning the price of the medicine (Form 2). This includes the date on which the medicine is first sold in Canada, the quantity of medicine sold in final dosage form, and either the average price per package or net revenues from the sales of each dosage form, strength and package size in which the medicine was sold by the patentee or former patentee to each class of customer in each province and territory.
- Subsection 4(4) of the Regulations provides that, in calculating the average price per package or net revenues, the actual price or actual revenue after any reduction including rebates, discounts, refunds, free goods, free services, gifts or any other benefit of a like nature and after the deduction of federal sales taxes shall be used.
- Subsection 4(2) of the Regulations requires that, if the medicine is for human use and contains a controlled substance as defined in the Controlled Drugs and Substances Act, such as opioids, amphetamines, barbiturates and benzodiazepines, or is a substance listed or described in Schedules C or D of the Food and Drugs Act, such as radiopharmaceuticals, vaccines, blood products and insulins, or is listed or described in Schedule F of the Food and Drug Regulations, such as medicines requiring a prescription, the prescribed information under Form 2 must be reported within 30 days after the day on which the medicine is first sold in Canada (for the first day´s sales), and within 30 days after each six month period commencing on January 1 and July 1 of each year, in respect of each of these periods, including the final partial period.
- Subsection 4(3) of the Regulations requires that, for medicines for human use that do not contain a controlled substance or do not contain a substance listed or described in the schedules listed in subsection 4(2), including non-prescription medicines for human use or all medicines for veterinary use, the prescribed information under Form 2 must be reported for all periods of sale, within 30 days after the date on which the PMPRB sends a request in response to a complaint, and for the two years following the request, within 30 days after each reporting period. A patentee or former patentee shall maintain up-to-date Form 2 information from the date of first sale in the event of a request for this information from the PMPRB in response to a complaint.
- A patentee or former patentee who does not voluntarily file Form 2 information for a medicine being sold during the pre-grant infringement period is required to ensure that this information is kept up-to-date for ultimate submission to the PMPRB, upon the issuance of the patent pertaining to the medicine.
7.3 All required information referenced in Paragraph 7.2, must be submitted using the appropriate electronic documents made available on the PMPRB´s Web site, under the heading “Legislation, Regulations and Guidelines – Patentee´s Guide to Reporting”. The completed electronic document, in its original format and file type, must be sent to the e-mail address specified on the PMPRB´s Web site.
7.4 The electronic documents submitted to the PMPRB must bear the electronic signature of an authorized individual, certifying that the information set out in the document is true and complete.
8. Consequences of Failure to File Required Information Pertaining to Price Regulation
8.1 Evidence of failure to file a Notification of Intent to Sell a Patented Medicine, pursuant to subsection 82(1) of the Act, may be brought to the attention of the Chairperson who may issue an order requiring production of this information.
8.2 If a patentee or former patentee, as the case may be, fails to file some or all of its Form 1 or Form 2 information for one or more periods by the regulatory deadlines, it will be advised in writing by Board Staff that it is in failure to file and be given seven days from the date the letter is sent out to file the missing information. If the patentee or former patentee does not comply, Board Staff will bring a motion before the Chairperson seeking a Board Order, pursuant to section 81 of the Act, requiring the patentee or former patentee to file the information within such time as is specified in the order.
8.3 If it appears to the Chairperson or to the Board that the patentee or former patentee failed to file information pursuant to sections 80, 81 or 82 of the Act or pursuant to an Order of the Board, the Board may refer the matter to the Attorney General of Canada to determine if summary conviction proceedings should be commenced under subsection 76.1(1) of the Act.
8.4 Pursuant to section 99 of the Act, any Order of the Board may be made an order of the Federal Court or any superior court of a province, enforceable in the same manner as an order of the court.
9. Protection of Confidential Information Pertaining to Price Regulation
9.1 Pursuant to subsection 87(1) of the Act, apart from the exceptions noted below, any information or document provided to the PMPRB under sections 80, 81 or 82 of the Act, or in any proceeding under section 83, is privileged, and cannot be disclosed without the authorization of the person who provided it, unless it has been disclosed at a public hearing under section 83.
9.2 Any information that a patentee or former patentee submits to the PMPRB that is in the public domain will not be considered privileged under subsection 87(1) of the Act. This information includes the publicly available ex-factory prices of a patented medicine in Canada and other countries listed in the Regulations (Form 2, Block 5), publicly available product monographs, clinical trials and practice guidelines.
9.3 The privilege provided under subsection 87(1) does not extend to information and materials collected by the PMPRB, including any analysis performed by Board Staff of that information.
9.4 Subsection 86(1) of the Act gives the Board discretion to hold a hearing, or any part of it, in private if satisfied on representations from the patentee that specific, direct and substantial harm would be caused to the patentee by the disclosure of information or documents at a public hearing.
9.5 Information on the status of the price review by Board Staff, including the compliance status of patentees, is not protected by privilege under subsection 87(1) and may be made publicly available. As such, the PMPRB will publish summary reports on the results of the price review for all new active substances. The PMPRB may publish the results of other reviews.
9.6 Under subsection 87(2) of the Act, any information or document provided to the PMPRB under sections 80, 81 or 82 of the Act, or in any proceeding under section 83, may be disclosed to any person engaged in the administration of the Act under the direction of the Board; the Minister of Industry or other federal Minister designated by the Regulations; and the provincial ministers of health and their officials, for the purpose of making representations to the Board with respect to a hearing under section 83.
Part II – Policies
Introduction
From time to time, the Board finds it necessary to adopt policies to indicate to stakeholders the principles it applies when interpreting its mandate. The following is a consolidation of the key policies that have been decided on by the Board. While the following policies are not binding on the Board, they help to promote consistency and transparency for stakeholders.
1. Patent Pending Policy
1.1 When a medicine subject to a pending patent is being sold in any market in Canada, the PMPRB will, when the patent is issued, review the price as of the date of first sale or the date on which the patent application was laid open, whichever comes later. Once the patent is granted, the PMPRB´s jurisdiction over the price at which the medicine was sold extends to the pre-grant period, as the party selling the medicine derives the benefit of the patent during this period and so is a “patentee”, pursuant to subsection 79(1) of the Act.
2. Patent Dedication Policy
2.1 The PMPRB will continue to assert jurisdiction over the price at which a patented medicine is sold in any market in Canada after the patent has been dedicated until the cancellation or surrender of the patent pursuant to the express provisions of the Act or the expiry of the term of the patent. The Act, which is the mechanism by which the state grants patents, and which confers rights and benefits for the duration of the term of the patent, does not expressly recognize patent dedication as a mechanism by which patent rights may be terminated before the normal expiry of the patent term.
3. Policy on the Meaning of Medicine
3.1 A medicine is defined as any substance or mixture of substances made by any means – whether produced biologically, chemically or otherwise – that is applied or administered in vivo in humans or in animals to aid in the diagnosis, treatment, mitigation or prevention of disease, symptoms, disorders, abnormal physical states, or in modifying organic functions in humans or animals, however administered.
3.2 For greater certainty, this definition includes vaccines, topical preparations, anaesthetics and diagnostic products used in vivo, regardless of delivery mechanism (e.g., transdermally, capsule form, injectable, inhaler, etc.). This definition excludes medical devices, in vitro diagnostic products and disinfectants that are not used in vivo.
4. Policy on Unit of Price Review
4.1 The PMPRB reviews the average price of each strength of an individual, final dosage form of each patented drug product sold in Canada, including:
- Drug products that have been assigned a Drug Identification Number (DIN) by Health Canada
- Drug products available under the Special Access Programme
- Drug products available through a Clinical Trial Application, and
- Investigational New Drug Products
4.2 Each strength of an individual, final dosage form of a patented drug product is referred to as a “patented drug product” throughout this Compendium.
4.3 The average price of a patented drug product will normally be expressed as the price per unit in which that patented drug product is sold (i.e., tablet, millilitre, inhaler, etc.) rounded to the fourth decimal place.
5. Policy for When a Price May be Considered Excessive
5.1 The price of a patented drug product will be presumed to be excessive in the following cases:
- If the National Average Transaction Price exceeds the Maximum Average Potential Price at introduction, or after introduction it exceeds the National Non-Excessive Average Price;
- If any Market-Specific Average Transaction Price exceeds the Maximum Average Potential Price at introduction, or after introduction it exceeds its respective Market-Specific Non-Excessive Average Price.
5.2 If the National Average Transaction Price exceeds the Maximum Average Potential Price or National Non-Excessive Average Price, but does not trigger the criteria for commencing an investigation (see Schedule 11), the patentee will be notified and the patented drug product will be reported on the PMPRB Web site as “Appears Excessive.” The patentee will be expected to decrease its price and offset any excess revenues (see the PMPRB´s Policy on the Offset of Excess Revenues in section 7).
5.3 If the National Average Transaction Price is found to exceed the Maximum Average Potential Price or the National Non- Excessive Average Price by an amount which triggers the investigation criteria, the patentee will be notified of the commencement of an investigation and the patented drug product will be reported as “Under Investigation” (see Chapter 3, Investigations).
6. Policy on the Use of Patented and Non-Patented Drug Products in the Price Tests
6.1 Board Staff may exclude from the price tests any drug product identified for comparison purposes, both patented and non-patented, if it has reason to believe it is being sold at an excessive price.
6.2 Pivotal drug products used for comparison purposes in a price test will generally be assessed against the price tests described in the Guidelines.
7. Policy on the Offset of Excess Revenues
7.1 As set out in section 6 of the Legal Framework, the Board may allow a patentee to offset any excess revenues estimated by it to have been derived from the sale of the medicine at an excessive price through either: (i) the reduction of the price of the medicine or the price at which the patentee sells another patented medicine in Canada; or (ii) a payment to Her Majesty in right of Canada.
7.2 To offset excess revenues via a price reduction, the average price of a patented drug product will only be considered to have been reduced if it is below the previous year´s Non-Excessive Average Price; not taking an allowable price increase will not be considered for purposes of offsetting excess revenues.
7.3 Cumulative excess revenues cannot fall below zero.
Part III – Guidelines and Procedures
Preface
The following Guidelines and procedures represent direction from the Board, to patentees and Board Staff, in order to provide assistance on how to comply with the Patent Act and the Patented Medicines Regulations. Please note: These Guidelines are not binding on patentees or on the Board in the context of a hearing.
The Guidelines are organized as follows:
Chapter 1 – The Scientific Review Process: An evidence-based process that assesses the level of therapeutic improvement of a patented drug product and recommends, where appropriate, the drug products to be used for comparison purposes and the comparable dosage regimens.
Chapter 2 – The Price Review Process: The level of therapeutic improvement of a patented drug product is used to determine the Maximum Average Potential Price at introduction. Following introduction, the price of an existing patented drug product is reviewed according to the relevant price tests to establish the National and Market-Specific Non-Excessive Average Prices.
Chapter 3 – Investigations: The approach used and procedures undertaken when a price appears to exceed the investigation criteria (see Schedule 11).
Schedules: These Schedules form part of the Guidelines.
Chapter 1 – The Scientific Review Process
1. Introduction
1.1 The PMPRB´s scientific review is an evidence-based process that recommends the level of therapeutic improvement of a patented drug product.
1.2 The scientific review process for all new patented drug products (including those with an NOC or available through the Special Access Programme, Clinical Trial Applications and as Investigational New Drugs) will be undertaken following the Guidelines and procedures in this chapter.
2. Sources of Scientific Information
2.1 The scientific review of a new patented drug product is based on information from a variety of sources:
- Patentee Submission – Patentees may provide Board Staff with a brief submission (see Schedule 1), which clearly explains the rationale for the patentee´s proposals relative to level of improvement, drug products for comparison purposes and comparable dosage regimens.
- Research by a Drug Information Centre (DIC) – Board Staff uses the services of various drug information centres to obtain scientific information, such as clinical trial information, clinical practice guidelines, etc. The basis of the review by the DIC is the product monograph or information similar to that contained in a product monograph if an NOC has not been granted.
- Research by Board Staff – Board Staff may also update research and supplement data and evidence from the patentee and DIC using other sources.
- Research by HDAP Members – Members of the Human Drug Advisory Panel (HDAP) may also undertake their own research and supplement the evidence obtained from the patentee, the DIC and Board Staff for a review.
3. Human Drug Advisory Panel
3.1 The Human Drug Advisory Panel provides expertise and advice to Board Staff in conducting the scientific review. HDAP performs the following functions:
- Reviews and evaluates scientific information provided as described in Paragraph 2;
- Considers advice from other experts (when deemed necessary);
- Recommends the level of therapeutic improvement of the new patented drug product, and identifies drug products for comparison purposes and dosage regimens where possible; and
- Identifies significant uncertainties in the evidence which may affect the analysis on which its recommendations are based.
3.2 In general, new patented drug products are referred to HDAP. However, the following new patented drug products will not be referred to HDAP unless the patentee files a submission claiming therapeutic improvement:
- The new patented drug product represents a new DIN of an existing dosage form of an existing drug product, or a new DIN of another dosage form of the existing drug product that is comparable to the existing dosage form as per Schedule 2 and has the same indication or use as the existing DIN; or
- The new patented drug product is a combination drug product, the individual components of which are sold in Canada and have the same indication or use; or
- The new patented generic drug product is considered by Health Canada to be bioequivalent to the reference brand drug product sold in Canada; or
- The new patented generic drug product is a licensed version of an existing brand drug product sold in Canada.
Procedures:
3.3 HDAP is composed of members with recognized expertise in drug therapy who have experience in clinical research methodology, statistical analysis and the evaluation of new drug products.
3.4 HDAP and its individual members do not meet with patentees.
3.5 The names of the members of HDAP are posted on the PMPRB´s Web site.
3.6 The dates of HDAP meetings are posted on the PMPRB´s Web site.
3.7 At the request of a patentee, a new patented drug product will also be referred to HDAP to provide pre-sale and/or pre-patent advisory assistance.
3.8 For a new patented drug product referred to HDAP, a patentee must file a submission which contains the elements referred in Schedule 1 at least two months prior to an HDAP meeting.
3.9 In the event that a large number of submissions are received for any one HDAP meeting, priority will be determined as follows:
- Drug products that are patented and sold;
- Drug products that are patented and about to be sold;
- Drug products that are patented but not sold;
- Drug products that are not patented but sold;
- Drug products that are not patented and are not sold.
3.10 The patentee will be advised of the date of the HDAP meeting at which its submission will be considered.
3.11 The HDAP report will include recommendations on the level of therapeutic improvement, the drug products to be used for comparison purposes and comparable dosage regimens, as well as an explanation of how the primary and secondary factors (see section 6 below) were applied and a description of the evidence (see section 7 below) relied upon.
3.12 A copy of the HDAP report will be sent to the patentee.
4. Determining the Primary Indication/Use of a New Patented Drug Product
4.1 Determining the primary approved indication (or proposed indication if an NOC is pending), or primary use if not approved for market in Canada, is important for the assessment of the level of therapeutic improvement of a new patented drug product with multiple approved indications/multiple uses.
Procedures:
4.2 The level of therapeutic improvement for new patented drug products with multiple approved indications or multiple uses will be based on the approved indication or use for which the drug product offers the greatest therapeutic advantage in relation to alternative therapies for the same indication/use in a significant patient population. This would exclude rare medical conditions or diseases (i.e., low incidence and prevalence in Canada).
4.3 This approved indication or use will be considered the “primary indication” for the purpose of selecting drug products to be used for comparison purposes.
4.4 Where there is no apparent single approved indication or use for which the new patented drug product offers the greatest therapeutic advantage, the approved indication or use representing, potentially, the greatest proportion of sales will be the basis for recommending its level of therapeutic improvement and selection of drug products to be used for comparison purposes.
4.5 Estimates of potential sales can be based on several sources including actual prescribing patterns (when available), epidemiological data (Canadian incidence and prevalence) and prescribing patterns in other countries.
5. The Level of Therapeutic Improvement
5.1 HDAP utilizes the following set of definitions to recommend the level of therapeutic improvement of a drug product:
Breakthrough: A breakthrough drug product is the first one to be sold in Canada that treats effectively a particular illness or addresses effectively a particular indication.
Substantial Improvement: A drug product offering substantial improvement is one that, relative to other drug products sold in Canada, provides substantial improvement in therapeutic effects.
Moderate Improvement: A drug product offering moderate improvement is one that, relative to other drug products sold in Canada, provides moderate improvement in therapeutic effects.
Slight or No Improvement: A drug product offering slight or no improvement is one that, relative to other drug products sold in Canada, provides slight or no improvement in therapeutic effects.
6. Factors Considered in Recommending the Level of Therapeutic Improvement
6.1 The following factors are to be used in recommending the level of therapeutic improvement of a drug product:
Primary Factors
- Increased efficacy
- Reduction in incidence or grade of important adverse reactions
Secondary Factors
- Route of administration
- Patient convenience
- Compliance improvements leading to improved therapeutic efficacy
- Caregiver convenience
- Time required to achieve the optimal therapeutic effect
- Duration of usual treatment course Success rate
- Percentage of affected population treated effectively
- Disability avoidance/savings
6.2 The primary factors will be given the greatest weight, followed by an assessment of any additional improvement as a result of the secondary factors.
6.3 In recommending the level of therapeutic improvement of new patented drug products, factors such as the following will generally not be taken into consideration, unless the impact of these factors results in either increased efficacy and/or a reduction in the incidence or grade of important adverse reactions:
- The mechanism of action
- A new chemical entity
- A different pharmacokinetic profile
Procedures:
6.4 Primary factors will be considered in order to assess if the new patented drug product is a breakthrough, or represents substantial, moderate or slight/no improvement relative to other drug products available in Canada.
6.5 Secondary factors will then be considered. These factors will be weighed by HDAP based on sound evidence and reasonable clinical judgement. These secondary factors could result in the level of therapeutic improvement being assessed at up to the level of moderate therapeutic improvement.
7. Methodology for the Evaluation of the Level of Therapeutic Improvement
7.1 An evidence-based approach will be used to assess the new patented drug product under review using the hierarchy of evidence from the Oxford Centre for Evidence-Based Medicine (see Schedule 1).
Procedures:
7.2 HDAP will critically appraise the evidence with regards to validity, impact and applicability. Level 1 evidence will be given greater weight compared to other levels of evidence in recommending the level of therapeutic improvement and the selection of drug products to be used for comparison purposes.
7.3 Since uncertainty in the relative efficacy of a new patented drug product is common, level 1 evidence is preferred for new patented drug products to be assessed as having a breakthrough or substantial level of improvement relative to other drug products sold in Canada.
7.4 HDAP may consider other levels of evidence, as required, on a case by case basis in order to assess the secondary factors.
8. Selection of Drug Products to be Used for Comparison Purposes and Comparable Dosage Regimens
Drug Products to be Used for Comparison Purposes
8.1 HDAP uses the World Health Organization (WHO) Collaborating Centre for Drug Statistics Methodology´s Anatomical Therapeutic Chemical (ATC) Classification System in the selection of drug products to be used for comparison purposes.
8.2 The chemical substances to be used for comparison purposes will typically be those identified under the ATC classification system at the sub-class level above the single chemical substance. This will normally be the fourth sub-class level. HDAP may also choose from the next higher sub-class or another sub-class. In some instances, it may be appropriate to select from the fifth or single chemical substance level.
8.3 HDAP may omit from the comparison a chemical substance of the same ATC therapeutic class as the new patented drug product under review if, in HDAP´s opinion, it is unsuitable for comparison. For example, drug products with a primary indication/use other than the primary indication/use of the new patented drug product under review may be omitted from the comparison.
Procedures:
8.4 HDAP will identify all drug products to be used for comparison purposes, which have the same approved indication or use as the new patented drug product under review.
Breakthrough:
8.5 There will be no drug products recommended by HDAP for comparison purposes for a new patented drug product that represents a breakthrough, given that such a drug product is, by definition the first one to be sold in Canada that treats effectively a particular illness or addresses effectively a particular indication.
Substantial Improvement:
8.6 For new patented drug products that represent a substantial therapeutic improvement, HDAP will identify drug products with the same approved indication or use over which the new patented drug product represents a substantial therapeutic improvement.
Moderate Improvement:
8.7 For new patented drug products that represent a moderate therapeutic improvement, HDAP will identify drug products with the same approved indication or use over which the new patented drug product represents a moderate therapeutic improvement.
Slight or No Improvement:
8.8 Any drug product that is not considered a breakthrough and that is not considered to offer substantial or moderate improvement will fall into the category of drug products offering slight or no improvement.
8.9 For new patented drug products that represent slight or no therapeutic improvement, HDAP will first attempt to identify comparable drug products, based on the primary and secondary factors set out in subsection 6.1 of this chapter, with the same approved indication or use as the new patented drug product under review.
8.10 If no comparable drug products are found, HDAP will identify all drug products that are considered superior in treating the approved indication or use, based on primary and secondary factors.
8.11 The comparable drug products for a new patented drug product that is a new presentation of the same chemical entity as another drug product, with the same or comparable dosage form (as per Schedule 2), with the same indication or use, and the same dosage regimen, will be limited to the same chemical entity as the new patented drug product under review in the same or comparable dosage forms (as per Schedule 2), unless the patentee makes a submission claiming therapeutic improvement and HDAP identifies the new patented drug product as providing therapeutic improvement.
8.12 For greater certainty, the comparable drug products for a new patented drug product that is a new presentation of the same chemical entity as another drug product, with the same or comparable dosage form (as per Schedule 2), with the same indication or use, but where the dosage regimen differs materially, will be limited to the same chemical entity in the same or comparable dosage form (as per Schedule 2) as the new patented drug product under review, unless the patentee makes a submission claiming therapeutic improvement and HDAP identifies the new patented drug product as providing therapeutic improvement.
8.13 The comparable drug products for a new patented combination drug product, where each of the elements of the combination drug product are sold in Canada and have the same indication or use, will be limited to the component parts, unless the patentee makes a submission regarding therapeutic improvement and HDAP identifies the new patented drug product as providing therapeutic improvement.
8.14 The comparable drug products for a new patented generic drug product that is bioequivalent to a brand drug product sold in Canada, or that is a licensed version of the same brand drug product sold in Canada, will be limited to that brand drug product.
Comparable Dosage Regimens
8.15 The comparable dosage regimen recommended for comparison purposes will normally not be higher than the maximum of the usual recommended dosage in the Product Monograph (or similar information) taking into account relevant clinical variables. The most appropriate strength of the drug product will be chosen for a particular dosage regimen.
8.16 Generally, a dosage regimen based on a course of treatment will be applicable to acute indications, while a per-day regimen (based on maintenance dose) will be applicable to chronic situations.
9. Provisions for Over-the-Counter (OTC) and Veterinary Drug Products
9.1 As per the regulatory and reporting provisions outlined in Part I – Legal Framework, the scientific review for patented OTC and veterinary drug products will only be undertaken following the PMPRB´s receipt of a complaint regarding the price of the patented drug product.
Procedures:
9.2 Upon receipt of a complaint, the PMPRB will undertake the scientific review of the patented OTC or veterinary drug product in the same manner as is undertaken for all other patented drug products, as outlined in this chapter.
9.3 If a complaint is received for a patented OTC drug product, the required scientific information will be sent to HDAP to recommend the level of therapeutic improvement, the drug products to be used for comparison purposes and comparable dosage regimens.
9.4 If a complaint is received for a patented veterinary drug product, a Veterinary Drug Advisory Panel (VDAP) will be formed to recommend the level of therapeutic improvement, the drug products to be used for comparison purposes and comparable dosage regimens.
Chapter 2 – The Price Review Process
1. Introduction
1.1 The Price Review Process is conducted for the purposes of:
- Establishing the Maximum Average Potential Price at introduction for the new patented drug product; and
- Assessing whether or not the price of an existing patented drug product appears to be excessive.
2. Review of Prices of New Patented Drug Products at Introduction
Introduction
2.1 The test applicable to the introductory price of a new patented drug product is dependant on the level of therapeutic improvement recommended for the new patented drug product during the scientific review process (see Chapter 1). A detailed description of how the price tests will be applied to the levels of therapeutic improvement can be found in Schedule 8.
2.2 The PMPRB may review the price of any new patented drug product in any market in Canada (including for class of customer in a province/territory).
Highest International Price Comparison (“IPC”) Test
2.3 Notwithstanding subsections 2.4 to 2.8, the Maximum Average Potential Price for a new patented drug product during its introductory period shall not exceed the results of the Highest International Price Comparison test (see Schedule 6).
Breakthrough
2.4 Subject to subsection 2.3, the introductory price(s) of a breakthrough new drug product will be presumed to be excessive if the National Average Transaction Price or any Market-Specific Average Transaction Price exceeds the Maximum Average Potential Price at introduction as determined by the Median International Price Comparison test (see Schedule 5).
Substantial Improvement
2.5 Subject to subsection 2.3, the introductory price(s) of a new drug product providing substantial improvement will be presumed to be excessive if the National Average Transaction Price or any Market-Specific Average Transaction Price exceeds the Maximum Average Potential Price at introduction as determined by the higher of:
- The highest non-excessive price of the drug products identified pursuant to subsection 8.6 of Chapter 1, based on a Therapeutic Class Comparison (TCC) test (see Schedule 3), and
- The median international price as determined by the Median International Price Comparison test (see Schedule 5).
Moderate Improvement
2.6 The introductory price(s) of a new drug product providing moderate improvement will be presumed to be excessive if the National Average Transaction Price or any Market-Specific Average Transaction Price exceeds the Maximum Average Potential Price at introduction as determined by the higher of:
- The mid-point between the number obtained in paragraph (b) and the median international price determined by the Median International Price Comparison test (see Schedule 5), and
- The highest non-excessive price of the drug products identified pursuant to subsection 8.7 of Chapter 1 based on a TCC test (see Schedule 3).
2.7 If it is not possible to conduct a TCC test, the introductory price(s) of a new drug product providing moderate improvement will be presumed to be excessive if the National Average Transaction Price or any Market-Specific Average Transaction Price exceeds the median international price determined by the Median International Price Comparison test (see Schedule 5). This could occur where the HDAP is unable to derive comparable dosage regimens for all of the drug products identified pursuant to subsection 8.7 of Chapter 1 or where the prices of these drug products appear to be excessive.
Slight or No Improvement
2.8 The introductory price(s) of a new drug product providing slight or no improvement will be presumed to be excessive if the National Average Transaction Price or any Market-Specific Average Transaction Price exceeds the Maximum Average Potential Price as determined by the highest non-excessive price of the comparable drug products identified pursuant to subsection 8.9 of Chapter 1, based on a TCC test (see Schedule 3).
2.9 It is possible that the HDAP may determine that a new patented drug providing slight or no improvement has no comparable drug products. In such exceptional cases, the introductory price(s) of the new drug product will be presumed to be excessive if the National Average Transaction Price or any Market-Specific Average Transaction Price exceeds the lower of:
- The lowest non-excessive price of the superior drug products identified pursuant to subsection 8.10 of Chapter 1 based on a TCC test (see Schedule 3), and
- The median international price determined by the Median International Price Comparison test (see Schedule 5).
2.10 If it is not possible to conduct a TCC test, the introductory price(s) of a new drug product providing slight or no improvement will be presumed to be excessive if the National Average Transaction Price or any Market-Specific Average Transaction Price exceeds the median international price determined by the Median International Price Comparison test (see Schedule 5). This could occur where the HDAP is unable to derive comparable dosage regimens for all of the drug products identified pursuant to subsection 8.10 of Chapter 1 or where the prices of these drug products appear to be excessive.
Exceptions
2.11 Unless the patentee makes a submission claiming therapeutic improvement and HDAP identifies the new patented drug product as providing moderate or substantial improvement,
- When a new patented drug product is a combination product as defined in subsection 8.13 of Chapter 1, its introductory price(s) will be presumed to be excessive if the National Average Transaction Price or any Market-Specific Average Transaction Price exceeds the sum of the prices of the individual components.
- Subject to paragraph (c), the introductory price(s) of a new patented drug product that is a new presentation of the same chemical entity, with the same or comparable dosage form (as per Schedule 2), the same comparable dosage regimen and the same indication or use, will be presumed to be excessive if the National Average Transaction Price or any Market-Specific Average Transaction Price exceeds the result of the Reasonable Relationship (“RR”) test (see Schedule 4). Where the comparable dosage regimen differs materially, the introductory price(s) of the new patented drug product will be presumed to be excessive if the National Average Transaction Price or any Market-Specific Average Transaction Price exceeds the highest non-excessive price of the drug products identified pursuant to subsection 8.12 of Chapter 1 based on a TCC test (see Schedule 3).
- Exceptions to the price test outlined in paragraph 2.11(b):
- (i) The introductory price(s) of a new patented generic drug product that has been deemed by Health Canada to be bioequivalent to the reference brand drug product sold in Canada will be presumed excessive if the National Average Transaction Price or any Market-Specific Average Transaction Price exceeds the price of the reference brand drug product, based on the RR test (see Schedule 4).
- (ii) The introductory price(s) of a new patented generic drug product that is a licensed version of a patented brand drug product sold in Canada will be presumed to be excessive if the National Average Transaction Price or any Market-Specific Average Transaction Price exceeds the price of the patented brand drug product, based on the RR test (see Schedule 4).
Procedures:
2.12 Based on the application of the appropriate introductory price test(s) to the first day of sale price and sales data, Board Staff will provide interim advice to the patentee as to whether or not the price would appear to be excessive.
2.13 The introductory price(s) of a new patented drug product is determined by calculating the National Average Transaction Price of the drug product across all classes of customers and provinces/territories, as well as the Market-Specific Average Transaction Prices for each of three classes of customers (hospital, pharmacy and wholesaler) and for each province/territory (see Schedule 12) for the introductory period.
2.14 The introductory period is the period from the date of first sale to the end of the six-month regulatory reporting period (June 30 or December 31), as long as the period covered is greater than one month. If the period is less than one month, the following six-month reporting period will be used.
2.15 Board Staff may exclude from the price tests any drug product, both patented and non-patented, if it has reason to believe it is being sold at an excessive price.
2.16 All pivotal drug products used for comparison purposes will be assessed against the price tests described in the Guidelines.
3. Review of Prices of Existing Patented Drug Products
3.1 Subject to subsections 3.2 to 3.6, the price of an existing patented drug product will generally be presumed to be excessive if it:
- Exceeds the change in the CPI as per the CPI-Adjustment Methodology (see Schedule 9), or
- Exceeds the HIPC test (see Schedule 6).
3.2 When the National Average Transaction Price or a Market-Specific Average Transaction Price of a drug product increases from a previous year due to the reduction or end of a benefit(s) and the patentee provides evidence to demonstrate that the price increase was due solely to the reduction or termination of the benefit(s), it may be appropriate to adjust the Non-Excessive Average Prices (national and market-specific) through the DIP Methodology, as described in Schedule 10. Regardless of the application of the DIP Methodology, the price of a drug product will be presumed to be excessive if it exceeds the HIPC test.
3.3 In addition, when a patentee can demonstrate that an increase in the National Average Transaction Price is due solely to a sales-mix shift and none of the Market-Specific Average Transaction Prices for each class of customer and in each province/territory exceed their respective Market-Specific Non-Excessive Average Prices as determined by the CPI-Adjustment Methodology, the National Average Transaction Price will not be presumed to be excessive.
3.4 In the event that the actual change in the CPI is less than the forecast CPI and an apparent excessive price arises solely due to the patentee´s reliance on the forecast CPI, the price will not be presumed to be excessive. The patentee is expected to comply with the actual CPI in all subsequent reporting periods, and the application of the CPI-Adjustment Methodology for the forecasted year will be based on the actual change in the CPI for that year. The result for patentees that took price increases based on the forecast inflation will be that the actual change in the CPI for the forecasted year will be used to calculate the next year´s National and Market-Specific Non-Excessive Average Prices.
3.5 The Board recognizes that there may be cost of making and marketing arguments, whereby it may be appropriate to adjust the Non-Excessive Average Price(s) of a patented drug product (e.g., once a Notice of Compliance has been obtained and the drug product was first sold on a compassionate basis as an Investigational New Drug, through a Clinical Trial Application or under the Special Access Programme).
3.6 The PMPRB may review the price of any existing patented drug product in any market in Canada (e.g., by class of customer in a province/territory).
Procedures:
3.7 Board Staff will actively monitor the National Average Transaction Price in relation to the National Non-Excessive Average Price based on the CPI-Adjustment Methodology and the HIPC test.
3.8 The PMPRB will further investigate the changes in prices at the level of the Market-Specific Average Transaction Prices for specific markets (hospital, pharmacy, wholesaler and each province and territory) when:
- The National Average Transaction Price exceeds the National Non-Excessive Average Price based on the CPI-Adjustment Methodology to such an extent as to trigger the criteria for commencing an investigation (see investigation criteria in Schedule 11);
- A complaint is received; or
- As required as part of the monitoring of compliance with a VCU or Board Order.
3.9 Upon investigation, the price of a patented drug product will be presumed to be excessive if the National Average Transaction Price exceeds the National Non-Excessive Average Price or any of the Market-Specific Average Transaction Prices exceed their respective Market-Specific Non-Excessive Average Prices. Market-specific price review for existing patented drug products is described in Schedule 12.
4. Existing Patented Drug Products Subsequently Sold by Another Patentee
4.1 Where an existing patented drug product is sold in Canada by a patentee other than the original patentee, the National and Market-Specific Average Transaction Prices at introduction of the drug product sold by the new patentee will be presumed to be excessive if they exceed their respective National and Market-Specific Non-Excessive Average Prices established in the last period the patented drug product was sold by the previous patentee.
4.2 For the purpose of calculating the National Non-Excessive Average Price in subsequent periods, the benchmark price, as defined in Schedule 9, for the new patentee is established as the lower of the National Non-Excessive Average Price of the previous patentee and the National Average Transaction Price at introduction of the new patentee.
4.3 Where the new patentee does not have access to the pricing information of the previous patentee, the CPI-Adjustment Methodology would be applied as though the patented drug product is in the first year of sale.
4.4 If the national benchmark price of the subsequent patentee is set by the National Non-Excessive Average Price of the previous patentee, and the subsequent patentee can demonstrate that it has access to the pricing information of the previous patentee, the CPI-Adjustment Methodology would be applied as though the new patented drug product was a continuation of the patented drug product of the original patentee. The subsequent patentee would therefore be allowed to keep any banked CPI price increases accrued by the original patentee, as per the CPI-Adjustment Methodology outlined in Schedule 9.
5. International Therapeutic Class Comparison (ITCC) Test
5.1 The International Therapeutic Class Comparison (ITCC) test compares the price of the patented drug product with the publicly available ex-factory prices in the comparator countries listed in the Regulations of comparable drug products identified in the domestic price test (i.e., the RR or TCC test). The ITCC test will only be conducted on a case-by-case basis if it appears it might add useful information in cases of dispute regarding whether the price of the drug product under review appears to be excessive based on other price tests. It will not be used as a primary price test. This test is described in Schedule 7.
Chapter 3 – Investigations
1. Introduction
1.1 When the price of a patented drug product appears to exceed the Guidelines but not by an amount that triggers the investigation criteria (Schedule 11), the patentee will be notified and the patented drug product will be reported on the PMPRB´s Web site as “Appears Excessive.” The patentee will be expected to reduce its National Average Transaction Price and Market-Specific Average Transaction Prices and to offset any excess revenues that may have accrued (see Schedule 13, Offset of Excess Revenues), but no immediate action will be taken by Board Staff.
1.2 When the National Average Transaction Price of a patented drug product appears to exceed the National Non-Excessive Average Price and the circumstances are within the criteria established by the Board (Schedule 11), the patentee will be notified of the commencement of an investigation and the patented drug product will be reported on the PMPRB´s Web site as “Under Investigation.”
1.3 The examination will include an analysis of the pricing history of the patented drug product from introduction for both the National Average Transaction Price and Market-Specific Average Transaction Prices (i.e., for each class of customer (hospital, pharmacy, wholesaler) and each province/territory).
1.4 The period of time available to the patentee to respond to Board Staff following a notification that an investigation has been commenced is ordinarily brief. For example, if the patentee should have known that a price would appear excessive based on its own filings (e.g., where the price increased by more than would be permitted under the CPI-Adjustment Methodology), the period of time may be as short as seven calendar days. A longer period of time, 30 calendar days, may be available if it is reasonable to believe that the patentee might have been unaware that the National Average Transaction Price or Market- Specific Average Transaction Prices may appear to be excessive (e.g., if HDAP has recommended the use of different drug products for comparison purposes or dosage regimens from those which were proposed by, and may have been reasonably anticipated by, the patentee).
1.5 There are three possible outcomes to an investigation:
- The National Average Transaction Price and/or Market-Specific Average Transaction Prices do not appear to be excessive; or
- The National Average Transaction Price and/or Market-Specific Average Transaction Prices appear to be excessive and the patentee submits an acceptable Voluntary Compliance Undertaking (VCU); or
- The National Average Transaction Price and/or Market-Specific Average Transaction Prices appear to be excessive and the patentee does not submit an acceptable VCU in which case Board Staff will refer the matter to the Chairperson and recommend the issuance of a Notice of Hearing.
2. Where the Price Appears Non-Excessive
2.1 If the investigation concludes that the National Average Transaction Price and/or Market-Specific Average Transaction Prices of the patented drug product do not appear to be excessive, the investigation will be terminated and the patentee will be advised accordingly.
3. Voluntary Compliance Undertaking
3.1 If the investigation confirms that the National Average Transaction Price and/or Market-Specific Average Transaction Prices appears to be excessive, the patentee will be given an opportunity to submit a written proposal in the form of a VCU to reduce its price and offset any excess revenue accrued as a result of sales at a price presumed to be excessive (see Schedule 13, Offset of Excess Revenues).
3.2 The proposal of a VCU does not constitute an admission by the patentee that the National Average Transaction Price and/or Market-Specific Average Transaction Prices of the drug product are or were excessive.
3.3 Board Staff will assist a patentee with the preparation of a VCU, and may provide sample documents or other advice as may be appropriate to the situation.
3.4 If a patentee submits a VCU consistent with the Guidelines, it is the policy of the Board that only the Chairperson (or, if the VCU is submitted after the issuance of a Notice of Hearing, the Board Hearing Panel) may approve the VCU.
3.5 The Chairperson is not authorized to enter into negotiations on the terms of a VCU with a patentee.
3.6 The proposed VCU should include a statement as to the Maximum Average Potential Price at introduction and subsequent National and Market-Specific Non-Excessive Average Prices with which the patentee agrees to comply and the means by which the patentee proposes to offset any excess revenues.
3.7 In most cases, the VCU should specify a payment to Her Majesty in right of Canada as the means to offset excess revenues.
3.8 In deciding whether to accept a VCU, the Chairperson (or Hearing Panel) will be guided by section 83 of the Act.
3.9 The PMPRB will report publicly on all VCUs accepted by the Chairperson or a Hearing Panel. The information reported will ordinarily include the name of the patented drug product and/or the patentee and such other information as is considered appropriate. This information will be included in the PMPRB´s Annual Report and be published on the PMPRB Web site. It may also be published in the NEWSletter or other publications.
Schedules
These Schedules form part of the Guidelines.
- Submissions by Patentees on Therapeutic Improvement
- Comparable Dosage Forms
- Therapeutic Class Comparison Test
- Reasonable Relationship Test
- Median International Price Comparison Test
- Highest International Price Comparison Test
- International Therapeutic Class Comparison Test
- Application of Price Tests for New Drug Products
- CPI-Adjustment Methodology
- DIP Methodology
- Criteria for Commencing an Investigation
- “Any Market” Price Reviews
- Offset of Excess Revenues
Schedule 1 – Submissions by Patentees on Therapeutic Improvement
Each submission should clearly explain the rationale behind the patentee´s proposals for level of therapeutic improvement, drug products for comparison purposes and for comparable dosage regimens.
The patentee should provide ten copies of the submission and all supporting references. Board Staff will verify that the supporting references mentioned or listed in the submission have been included and advise the patentee if any information is missing.
1. Supporting Clinical Evidence
1.1 Drug: name of drug, drug class, brief description of drug mechanism, approved indication(s) or use(s), and approved or proposed dosing.
1.2 Product Monograph (or similar information if no NOC): Submitted with Form 1, Identity of the Medicine.
1.3 Individual Trials/Studies:
- Level 1 Evidence: Published randomized clinical trials (RCTs) of the drug under review versus active comparators, if any; Published RCTs of the drug under review versus placebo; High quality unpublished RCTs, if available.
- Published clinical trials with lower levels of evidence (e.g., outcome studies, systematic reviews of cohort and case-controlled trials) if Level 1 evidence is unavailable. Note: In relation to both Level 1 and other levels of evidence, the patentee is encouraged to focus the submission on key trials that lead to an NOC or to a change in clinical practice, or would be of the highest quality/best evidence the patentee has available.
- Editorials and errata of published clinical trials
- Other clinical evidence, such as ecological studies, case series and community surveys of the drug product under review if the patentee is proposing therapeutic improvements due to secondary factors.
1.4 Summary of trials included in submission in tabular format:
- Study reference(s) (abstracts and publications if published), and study identification assigned by the patentee.
- Brief description of the study and outcomes measures.
- Trial Phase (i.e., Phase II, III or IV); Phase I trials will not be reviewed.
1.5 Brief overview of standards of therapy or accepted clinical practice for which the drug under review is indicated or used:
- For example, class reviews, systematic reviews/meta-analyses.
1.6 Published Clinical Practice Guidelines regarding the indication or use of the drug under review if available:
- Peer reviewed Canadian guidelines are preferred; American, UK, Australian and European guidelines will be considered.
2. Proposal of the Patentee
2.1 Executive Summary:
- Brief description of the drug and its place in therapy, as well as a Summary of the clinical evidence.
2.2 Proposed level of therapeutic improvement.
2.3 Proposed Comparators:
- Evidence of the same approved indication or use as the drug product under review.
2.4 Proposed comparable dosage regimens for the comparator and the drug product under review:
- Approved or proposed doses.
- Doses used in clinical trials.
- Doses recommended in clinical practice guidelines.
3. Hierarchy of Evidence for Recommending Level of Therapeutic Improvement3
3.1 The table below outlines the hierarchy of evidence that will be considered by HDAP in recommending the level of therapeutic improvement of a new patented drug product.
| Level |
Therapy/Prevention |
Economic and decision analyses |
| 1a |
SR (with homogeneity*) of RCTs |
SR (with homogeneity*) of Level 1 economic studies |
| 1b |
Individual RCT (with narrow Confidence Interval) |
Analysis based on clinically sensible costs or alternatives; systematic review(s) of the evidence; and including multi-way sensitivity analyses |
| 1c |
All or none§ |
Absolute better-value or worse-value analyses † |
| 2a |
SR (with homogeneity*) of cohort studies |
SR (with homogeneity*) of Level >2 economic studies |
| 2b |
Individual cohort study (including low quality RCT; e.g., <80% follow-up) |
Analysis based on clinically sensible costs or alternatives; systematic review(s) of the evidence, or single studies; and including multi-way sensitivity analyses |
| 2c |
“Outcomes” Research; Ecological studies |
Audit or outcomes research |
| 3a |
SR (with homogeneity*) of case-control studies |
SR (with homogeneity´) of 3b and better studies |
| 3b |
Individual Case-Control Study |
Analysis based on limited alternatives or costs, poor quality estimates of data, but including sensitivity analyses incorporating clinically sensible variations. |
| 4 |
Case series (and poor quality cohort and case-control studies§§) |
Analysis with no sensitivity analysis |
| 5 |
Expert opinion without explicit critical appraisal, or based on physiology, bench research or “first principles” |
Expert opinion without explicit critical appraisal, or based on economic theory or “first principles” |
* Homogeneity means a systematic review that is free of worrisome variations (heterogeneity) in the directions and degrees of results between individual studies. Not all systematic reviews with statistically significant heterogeneity need be worrisome, and not all worrisome heterogeneity need be statistically significant. Studies displaying worrisome heterogeneity should be tagged with a “-” at the end of their designated level.
§ Met when all patients died before the Rx became available, but some now survive on it; or when some patients died before the Rx became available, but none now die on it.
§§ Poor quality cohort study is defined as a study that failed to clearly define comparison groups and/or failed to measure exposures and outcomes in the same (preferably blinded), objective way in both exposed and non-exposed individuals and/or failed to identify or appropriately control known confounders and/or failed to carry out a sufficiently long and complete follow-up of patients. A poor quality case-control study is defined as a study that failed to clearly define comparison groups and/or failed to measure exposures and outcomes in the same (preferably blinded), objective way in both cases and controls and/or failed to identify or appropriately control known confounders.
† Better-value treatments are clearly as good but cheaper, or better at the same or reduced cost. Worse-value treatments are as good and more expensive, or worse and equally or more expensive.
SR: Systematic Review
RCT: Randomized Clinical Trials
Rx: Therapy
Schedule 2 – Comparable Dosage Forms
This Schedule identifies comparable dosage forms for the purpose of the Reasonable Relationship (RR) price test for new patented drug products. Formulations within each class are considered comparable, but dosage forms in a different class are not.
The PMPRB reviews the list of comparable dosage forms periodically to ensure that it includes those currently used.
| Comparable Dosage Forms |
| Topical |
Nasal/Pulmonary |
Oral Solid |
| Aerosol |
Drops |
Tablet |
| Cream |
Aerosol |
Caplet |
| Gel |
Spray |
Capsule |
| Liquid |
Solution |
Modified release tablets |
| Ointment |
Powder |
Modified release caplets |
| Paste |
Gas |
Modified release capsule |
| Powder |
Metered dose preparations |
Effervescent powder |
| Shampoo |
|
Effervescent tablets |
| Spray |
|
Effervescent granules |
| Patches |
|
|
| Disks |
|
|
| Dressings |
|
|
| Oral Liquid |
Vaginal |
Parenteral |
| Powder for solution |
Suppository |
Solution |
| Powder for suspension |
Cream |
Powder for solution |
| Suspension |
Tablet |
Suspensions or emulsions |
| Drops |
Douche |
Modified release injections |
| Modified release liquid |
Foam |
Implant |
| |
Cone |
|
| |
Ovule |
|
| |
Gel |
|
| |
Tampon |
|
| |
Sponge |
|
| |
Insert |
|
| Optic/Ophthalmic |
Rectal |
Dental/Sublingual Buccal |
| Liquid |
Suppository |
Mouth wash |
| Powder for solution |
Cream |
Solution |
| Drops |
Ointment |
Suspension |
| Suspension |
Enema |
Powder for suspension |
| Ointment |
Suspension |
Lozenge |
| Gel |
Foam |
Gel |
| Modified release ocular devices |
|
Gum |
| |
|
Modified release buccal tablets |
| |
|
Sprays – sublingual |
| |
|
Sprays – buccal |
| |
|
Sublingual tablets |
| |
|
Tooth paste |
| |
|
Tooth powder |
Schedule 3 – Therapeutic Class Comparison Test
1. Approach
The Therapeutic Class Comparison (TCC) test compares a new patented drug product´s National Average Transaction Price and the Market-Specific Average Transaction Prices in each class of customer – hospital, pharmacy, wholesaler and province/territory with the price of drug products identified for comparison purposes that are sold at prices that the PMPRB considers not to be excessive. Drug products are first identified for comparison purposes pursuant to section 8 of chapter 1 and then their prices are compared against those of the new patented drug product under review.
2. Measuring the Price
The PMPRB considers it appropriate to compare the prices of drug products used for comparison purposes taking into consideration the comparable dosage regimens determined pursuant to subsections 8.15 and 8.16 of Chapter 1. The PMPRB will make these price comparisons in terms of the price per course of treatment or price per day, whichever is more applicable. Generally, the price per course of treatment will be applicable to acute indications, whereas price per day (based on maintenance dose) will be applicable to chronic situations.
If the drug product used for comparison purposes is patented and sold by the same patentee introducing the new patented drug product, the price for the drug product used for comparison purposes will be the National Average Transaction Price of that drug product.
Where the drug products used for comparison purposes are themselves patented but sold by a different patentee than the patentee introducing the new patented drug product, Board Staff will use public sources for prices for the drug products used for comparison purposes. Board Staff will find the public price that is sufficiently close to the National Non-Excessive Average Price of the patented drug product used for comparison purposes.
Where the drug products used for comparison purposes are not patented, Board Staff will use public sources for the prices of the drug products used for comparison purposes if they are not excessive. Board Staff will find the public price that is sufficiently close to the National Non-Excessive Average Price determined by the Guidelines.
Board Staff may exclude from the TCC test any drug product it has reason to believe is being sold at an excessive price (see the PMPRB´s Policy on the Use of Patented and Non-Patented Drug Products in the Price Tests in Part II – Policies, under section 6).
Schedule 4 – Reasonable Relationship Test
In order to conduct the Reasonable Relationship (RR) test, the new patented drug product under review must meet four requirements:
- It must be the same chemical entity as the comparable drug product(s);
- It must have the same indication or use as the comparable drug product(s);
- It must be in the same or comparable dosage form as the comparable drug product(s) (see Schedule 2); and
- It must have the same dosage regimen as the comparable drug product(s).
Unless a patentee makes a submission claiming therapeutic improvement and the Human Drug Advisory Panel identifies the new drug product as providing moderate or substantial therapeutic improvement, the RR test will be conducted if the four requirements are met.
Reasonable relationship refers to the association between strength per unit (see the Policy on Unit of Price Review in Part II – Policies, under section 4) and price. The RR test defines a Maximum Average Potential Price for the new strength of the patented drug product.
This schedule describes in general terms the process by which reasonable relationship may be determined.
If the comparable drug product(s) is patented and sold by the same patentee introducing the new patented drug product, the price for the drug product used for comparison purposes will be the National Average Transaction Price of that drug product.
Where the comparable drug product(s) is itself patented but sold by a different patentee than the patentee introducing the new patented drug product, Board Staff will use public sources for price(s) for the comparable drug product(s). Board Staff will find the public price that is sufficiently close to the National Non-Excessive Average Price of the patented comparable drug product(s).
Where the comparable drug product(s) is not patented, Board Staff will use public sources for the price(s) of the comparable drug product(s) if they are not excessive. Board Staff will find the public price that is sufficiently close to the National Non-Excessive Average Price determined by the Guidelines.
Board Staff may exclude from the RR test any drug product it has reason to believe is being sold at an excessive price (see the PMPRB´s Policy on the Use of Patented and Non-Patented Drug Products in the Price Tests in Part II – Policies, under section 6).
The determination of reasonable relationship will be based on one of three possible tests. Only one test will be appropriate for a particular new patented drug product. To determine which test is appropriate for a particular case, the three are considered in descending order.
Test 1: Same Strength Test
If there are one or more comparable drug products of the same strength as the new patented drug product, then the highest priced comparable drug product of the same strength determines the Maximum Average Potential Price for the new patented drug product. Prices above this threshold are considered to be excessive. The result of this test takes precedence over the other two tests.
In Figure 1 below, given three comparable drug products of equal strength but different prices (P1, P2, and P3) a new patented drug product will have a Maximum Average Potential Price (MAPP) equal to that of the highest priced comparable drug product, in this case P1.
Figure 1 – Same Strength Test
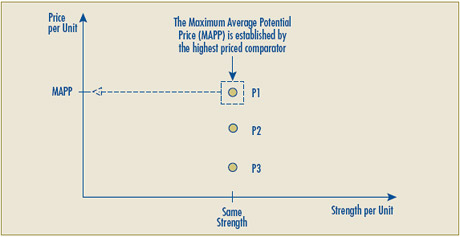
Test 2: Linear Relationship Test
If there are two or more comparable drug products, and none are the same strength as the new patented drug product, this test will be conducted.
The test is conducted in a series of steps:
1. As shown in Figure 2A below, lines are drawn for all possible pairs of comparable drug products (e.g., A to B, A to C, B to C).
2. The pair with a slope that is greater than or equal to zero and with the highest Y-axis intercept determines the Y-intercept for the line which will set the Maximum Average Potential Price. In the example in Figure 2A, the highest Y-intercept results from the line running from A to B.
Figure 2A – Linear Relationship Test – Representing Steps 1-2
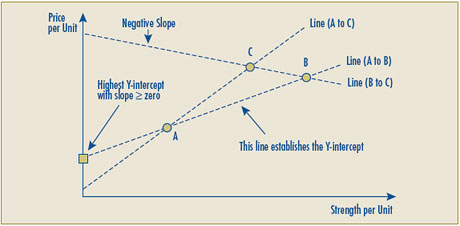
3. A new line joining this Y-intercept with the point representing the per unit price of the highest priced comparable drug product is drawn. In the example in Figure 2B, the comparable drug product C has the highest price per unit.
4. The National and Market-Specific Average Transaction Prices of the new patented drug product will not be presumed to be excessive if they do not exceed the Maximum Average Potential Price (MAPP) Line illustrated in Figure 2B below.
Figure 2B – Linear Relationship Test – Representing Steps 3-4
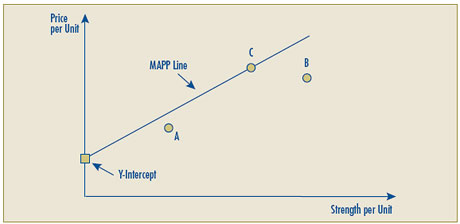
5. It could happen that none of the lines with slopes greater than or equal to zero created by drawing lines through the prices of pairs of comparable drug products produces a Y-intercept greater than or equal to zero (i.e., only negative Y-intercepts, implying the Maximum Average Potential Price for some strengths would also be negative). Should this occur, the Maximum Average Potential Price line will then be established by drawing a line between the origin (0) and the per unit price of the highest priced comparable drug product. In the example in Figure 2C, the original Y-intercept would have been negative (determined using the same methodology as above in step 2). The Y-intercept used to establish the Maximum Average Potential Price (MAPP) Line cannot be less than zero, so the new Y-intercept is established at the origin (0). In this example, the Maximum Average Potential Price Line is drawn from the origin (0) through the point established by drug product C. This line would be used to establish the relationship between the strength of the new product and its Maximum Average Potential Price.
Figure 2C – Linear Relationship Test – Representing Step 5
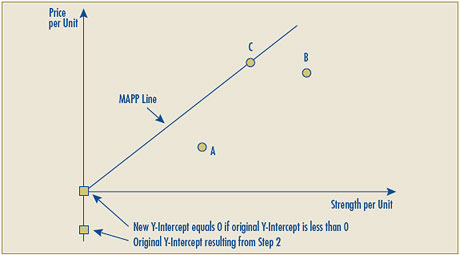
Test 3: Different Strength Test
This test is used when there is only one other (higher or lower) strength of a comparable drug product sold in Canada. Although there is only one other strength sold, there could be several products of this strength sold at different prices. The highest priced comparable drug product of the different strength is used for this test.
When the strength of the new patented drug product is higher than the strength of the comparable drug product, the Maximum Average Potential Price for the new patented drug product will be determined based on the proportional relationship of the strength of the new patented drug product compared to the comparable drug product multiplied by the price of the comparable drug product.
Example 1 (higher strength is introduced):
A 5 mg strength drug product is being sold and the highest price at which it is sold is $10.
A 7.5 mg strength new patented drug product is introduced.
The price of the new 7.5 mg patented drug product will be presumed to be excessive if it exceeds $15.00.
7.5mg 5mg X $10 = $15
When the strength of the new patented drug product is lower than the strength of the comparable drug product, the Maximum Average Potential Price for the patented drug product will be equal to the price of the higher strength comparable drug product.
Example 2 (lower strength is introduced):
A 5 mg strength drug product is being sold and the highest price at which it is sold is $10.
A 2.5 mg strength new patented drug product is introduced.
The price of the new 2.5 mg patented drug product will be presumed to be excessive if it exceeds $10.00.
Schedule 5 – Median International Price Comparison Test
1. Median International Price Comparison (MIPC) Test
1.1 The median of the ex-factory prices of the same strength and dosage form of the same patented drug product for each country listed in the Regulations (France, Germany, Italy, Sweden, Switzerland, the United Kingdom, and the United States) will set the Maximum Average Potential Price for a new patented drug product when the Median International Price Comparison test is the pivotal introductory price test.
1.2 When the Median International Price Comparison test is being conducted and the new patented drug product is sold in an even number of countries, the median will generally be the simple average of the middle two prices.
1.3 When the new patented drug product is sold in fewer than five countries at the time it is first sold in Canada, the median international price will be calculated on an interim basis. At the end of three years or when the same patented drug product, with the same strength and dosage form is sold in at least five countries, whichever occurs first, Board Staff will re-determine the median international price. Whenever this occurs, the drug product´s Non-Excessive Average Price will be the lower of:
(a) the re-determined median international price, and
(b) the Non-Excessive Average Price derived from the ordinary application of the CPI-Adjustment Methodology (see Schedule 9).
1.4 Where the re-determined median international price establishes a drug product´s Non-Excessive Average Price pursuant to subsection 1.3, the patentee is expected to reduce its National Average Transaction Price and Market-Specific Average Transaction Prices to the level of the Non-Excessive Average Price calculated in accordance with subsection 1.3 within the next two six-month reporting periods. If the patentee complies with this timeframe, its price will not be presumed to have been excessive.
2. Indirect International Price Comparison
2.1 When a direct international price comparison of the drug product under review is not possible because the drug product is only sold in Canada, the most similar strengths of comparable dosage forms (as per Schedule 2) of the same patented drug product may be considered.
3. Exchange Rates
3.1 To calculate the MIPC test for a new patented drug product, the exchange rates used are the simple average of the thirty-six monthly average noon spot exchange rates for each country (taken to eight decimal places) as published by the Bank of Canada for the thirty-six months ending four months before the date of the first sale of the drug product. e.g., If the new patented drug product under review was first sold in October 2008, the exchange rates used are for the months of June 2005 through May 2008.
3.2 Exchange rates are published on the PMPRB Web site on a monthly basis, under Frequently Requested Items.
Schedule 6 – Highest International Price Comparison Test
1. Highest International Price Comparison (HIPC) Test
1.1 Subject to subsection 1.2, both at introduction and in future years, the National Average Transaction Price and the Market-Specific Average Transaction Prices (pharmacy, hospital, wholesaler, provinces/territories) will be presumed to be excessive if they exceed the highest price of the same strength and dosage form of the same patented drug product for each country listed in the Regulations (France, Germany, Italy, Sweden, Switzerland, the United Kingdom, and the United States).
1.2 If the National Average Transaction Price and the Market-Specific Average Transaction Prices of a patented drug product are the highest relative to comparator countries, its price will not be presumed to be excessive at or below the level of the price in Canada of a patented drug product of the same or comparable dosage form that is the same or lower strength.
2. Exchange Rates
2.1 To calculate the HIPC test for a new patented drug product, the exchange rates used are the simple average of the thirty-six monthly average noon spot exchange rates for each country (taken to eight decimal places) as published by the Bank of Canada for the thirty-six months ending four months before the date of the first sale of the drug product. e.g., If the new patented drug product under review was first sold in October 2008, the exchange rates used are for the months of June 2005 through May 2008.
2.2 To calculate the HIPC test for an existing patented drug product, the exchange rates used are the simple average of the thirty-six monthly average noon spot exchange rates for each country (taken to eight decimal places), as published by the Bank of Canada for the thirty-six months ending with the last month of the pricing period under review. e.g., The pricing period under review is July to December 2007. The exchange rates used are for the months of January 2005 through December 2007.
2.3 Exchange rates are published on the PMPRB Web site on a monthly basis, under Frequently Requested Items.
3. Existing Drug Products with Unusual Circumstances
3.1 The Guidelines require that patentees take appropriate action when an investigation concludes that the price of its patented drug product appears excessive. There are, however, circumstances where a patented drug product whose price does not appear to be excessive in one review period then appears excessive in a subsequent period, due to the application of the Highest International Price Comparison test. This could be as a result of events beyond the control of the patentee. The following are examples of three such circumstances:
- Exchange rate variations;
- A foreign regulator forcing price reductions; or
- The highest priced drug product is removed from the market.
Under the circumstances identified above, patentees will be notified that the patented drug product´s price appears excessive and will be expected to adjust the National Average Transaction Price by the end of the next two reporting periods, in which case the price will not be presumed to have been excessive. Failing this the patentee would be requested to submit a Voluntary Compliance Undertaking (VCU) and repay any excess revenues dating back to the first period in which the price exceeded the HIPC test. If the patentee declines to submit a VCU, then the matter would be reported to the Chairperson with the recommendation that a Notice of Hearing be issued.
Schedule 7 – International Therapeutic Class Comparison Test
1. Concept and Application
1.1 The International Therapeutic Class Comparison (ITCC) test compares the National Average Transaction Price of the patented drug product under review with the prices of comparable drug products that are sold in the seven comparator countries listed in the Regulations (France, Germany, Italy, Sweden, Switzerland, the United Kingdom, and the United States).
1.2 The ITCC test is not considered a primary price test. However, it may be appropriate to conduct an ITCC test in order to provide information in the context of an investigation into apparent excessive prices.
2. Selection of Comparable Drug Products
2.1 For the purpose of the ITCC test, the comparable drug products identified in the TCC test will be used. For more details on the selection of comparable drug products for the TCC test, please refer to Chapter 1, section 8.
2.2 In terms of comparable generic drug products, only those sold by the same company in the comparator country that sells the generic drug product in Canada will be included. For greater clarity, if a comparable generic drug product is sold by company “X” domestically, but internationally it is sold by companies “X” and “Y”, then only the generic drug product sold by company “X” will be considered in the ITCC test.
3. Derivation of the ITCC Test
3.1 The following two methods may be used to calculate the ITCC test:
- The Straight Class Approach: The prices of all comparable drug products in the seven comparator countries listed in the Regulations are identified. The median international price is determined and compared against the National Average Transaction Prices of the patented drug product in Canada.
- The Ratio Approach: The prices of the drug product under review in the seven countries listed in the Regulations are identified. The prices of all comparable drug products in the seven comparator countries are also identified. The ratios between the price of the drug product under review and the price of comparable drug products is determined for each combination within each comparator country. The median of all the resulting ratios is then applied to the National Average Transaction Price of the patented drug product under review in Canada. 3.2 Where the price of a comparable drug product was excluded from the TCC test because it appeared to be excessive, it will also be excluded from the ITCC test.
4. Exchange Rates
4.1 To calculate the ITCC test for a new patented drug product, the exchange rates used are the simple average of the thirty-six monthly average noon spot exchange rates for each country (taken to eight decimal places), as published by the bank of Canada for the thirty-six months ending four months before the date of the first sale of the drug product. e.g., If the new patented drug product under review was first sold in October 2008, the exchange rates used are for the months of June 2005 through May 2008.
4.2 To calculate the ITCC test for an existing patented drug product, the exchange rates used are the simple average of the thirty-six monthly average noon spot exchange rates for each country (taken to eight decimal places), as published by the Bank of Canada for the thirty-six months ending with the last month of the pricing period under review. e.g., The pricing period under review is July to December 2007. The exchange rates used are for the months of January 2005 through December 2007.
4.3 Exchange rates are published on the PMPRB Web site on a monthly basis, under Frequently Requested Items.
Schedule 8 – Application of Price Tests for New Drug Products
| Level of Therapeutic Improvement |
Price Test Used for New Drug Product |
| Breakthrough |
Median of the IPC test |
| Substantial Improvement |
Higher of:
1) Top of the TCC test comprised of all drug products identified by HDAP pursuant to subsection 8.6 of Chapter 1; and
2) Median of the IPC test. |
| Moderate Improvement |
Higher of:
1) Midpoint of: i) Top of the TCC test comprised of all drug products identified by HDAP pursuant to subsection 8.7 of Chapter 1 and ii) Median of the IPC test; and
2) Top of the TCC test comprised of all drug products identified by HDAP pursuant to subsection 8.7 of Chapter 1.
If it is impossible to conduct a TCC test (i.e., unable to derive comparable dosage regimens or the prices of the only drug products used for comparison purposes appear to be excessive), then use the Median of the IPC test. |
| Slight or No Improvement |
1) Top of the TCC test comprised of all comparable drug products identified by HDAP pursuant to subsection 8.9 of Chapter 1.
2) In the exceptional cases where HDAP does not identify any comparable drug products, use the lower of i) the bottom of the TCC test comprised of all superior drug products identified by HDAP pursuant to subsection 8.10 of Chapter 1 and ii) the Median of the IPC test.
3) If it is impossible to conduct a TCC test (i.e., unable to derive comparable dosage regimens or the prices of the drug products used for comparison purposes appear to be excessive), then use the Median of the IPC test.
Please note that additional Guidelines are applicable to the following drug products, unless the patentee makes a submission claiming therapeutic improvement and HDAP identifies the new patented drug product as providing moderate or substantial improvement:
- a combination drug product;
- a patented generic drug product;
- a drug product that is a new presentation of the same chemical entity, with the same or comparable dosage form (as per Schedule 2), the same comparable dosage regimen and the same indication or use.
The Guidelines specific to these particular drug products are outlined in subsection 2.11 of Chapter 2. |
Schedule 9 – CPI-Adjustment Methodology
1. Consumer Price Index (CPI)-Adjustment Methodology
1.1 Subject to subsection 1.5 and Schedule 10, the National Average Transaction Price and the Market-Specific Average Transaction Prices of an existing patented drug product will be presumed to be excessive if they increase by more than that allowed under the Board´s CPI-Adjustment Methodology.
1.2 The CPI-Adjustment Methodology involves the following calculations:
- Adjusting the benchmark prices of the drug product for the cumulative change in the CPI from the benchmark year to the year under review (CPI-Adjusted Price); and
- Applying a cap on the maximum price increase in any one year, equal to 1.5 times the forecast change in the annual CPI. In periods of high inflation (over 10%), the limit will be five percentage points more than the forecast change in the CPI.
1.3 The lower of the results of both calculations will set the Non-Excessive Average Price for a particular year.
1.4 The calculation of the Non-Excessive Average Price will be performed independently for each market (national, class of customer (pharmacy, hospital, wholesaler), province/territory) based on the price history in that market.
1.5 The following exception to the CPI-Adjustment Methodology applies when a price reduction below the Non-Excessive Average Price is taken in one or more markets specifically to offset excess revenues (see Schedule 13, Offset of Excess Revenues). Following the repayment of excess revenues, the Average Transaction Prices in those markets may increase in the next reporting period up to the level of the Market-Specific Non-Excessive Average Transaction Prices prior to the price reduction.
2. Terminology
2.1 Forecast Period: The forecast period is the year for which prices are being set.
2.2 Introductory Period: The introductory period for new patented drug products is the period from the date of first sale to the end of the six-month regulatory reporting period (i.e., June 30 or December 31) when that period is greater than one month. For example, a patented drug product first sold in March 2007 would have an introductory period of March to June 2007, whereas a patented drug product first sold in December 2007 would have an introductory period of January to June 2008.
2.3 Benchmark Year:
- For patented drug products first sold in Canada more than three years prior to the forecast period, the benchmark year is the calendar year three years preceeding the forecast period.
- For example, for 2009 the corresponding benchmark year is 2006. For patented drug products first sold three years or less prior to the forecast period, the benchmark year is the year in which the patented drug product was first sold in Canada.
2.4 Benchmark Prices:
- For patented drug products first sold in Canada more than three years prior to the forecast period, the national and market-specific benchmark prices of the patented drug product are, respectively, its National Average Transaction Price and Market-Specific Average Transaction Prices in the benchmark year based on the patentee´s Form 2, Block 4 submission, or if those prices appear to be excessive, the National and Market-Specific Non-Excessive Average Prices in the benchmark year.
- For patented drug products first sold three years or less prior to the forecast period, the national and market-specific benchmark prices of the patented drug product are, respectively, its National Average Transaction Price and Market-Specific Average Transaction Prices in the introductory period based on the patentee´s Form 2, Block 4 submission, or if those prices appear to be excessive, the National and Market-Specific Non-Excessive Average Prices, in its introductory period.
2.5 Base CPI: Calculated as the annual average of the monthly increases in the CPI, as published by Statistics Canada, for the benchmark year. The base CPI figures are calculated and published annually by the PMPRB in its April NEWSletter.
2.6 Forecast CPI: The forecast CPI for the forecast period is based on the previous year´s actual CPI published by Statistics Canada adjusted for the latest annual inflation projections by the federal Department of Finance. The forecast CPI is also published annually in the PMPRB´s April NEWSletter.
2.7 CPI-Adjustment Factor: The forecast CPI divided by the base CPI, rounded to three decimal places.
2.8 CPI-Adjusted Price: This is the benchmark price multiplied by the CPI-adjustment factor for the benchmark year.
2.9 Cap: In any year, the price increase of a patented drug product may not exceed 1.5 times the forecast change in the annual CPI. In times of high inflation (greater than 10%), the limit will be 5 percentage points more than the forecast change in the CPI.
2.10 Example of the application of the CPI-Adjustment Methodology at the national level:
Forecast Period: Jan – Dec 2009 First sale: 1998 Benchmark Year: 2006
National Average Transaction Price in Benchmark Year: $10.00
National Average Transaction Price in 2008: $10.39
CPI-adjusted price: 1.065 (CPI-adjustment factor for 2006) × $10.00 = $10.65
Cap: 1.030 (1.5 x Forecast CPI for 2009 of 2.0%) × $ 10.39 = $10.70
The 2009 National Non-Excessive Average Price for the patented drug product is $10.65.
Schedule 10 – DIP Methodology
1. DIP Methodology
1.1 Defining the DIP Methodology If a price increase in excess of that allowable under the CPI-Adjustment Methodology is claimed by the patentee as due to the reduction or termination of benefit(s) – and the patentee provides the necessary evidence of the benefit(s) – the drug product´s National Non-Excessive Average Price and/or Market-Specific Non-Excessive Average Price may increase beyond the level allowable under the CPI-Adjustment Methodology.
1.2 Eligible Benefits Benefits are defined as “any reduction given as a promotion or in the form of rebates, discounts, refunds, free goods, free services, gifts or any other benefits of a like nature.”
1.3 Evidence of Benefits Required
Patentees wishing to invoke the DIP Methodology are expected to:
- Demonstrate that the recipient of the benefit was aware in advance that it was receiving a benefit not offered to all customers;
- Identify the type and value of benefit(s) and when/how it was offered;
- Provide evidence of the termination or reduction of a benefit(s);
- Whether the same customer is still receiving other benefits.
The exact form of this required evidence (e.g., a contract), and type of data (e.g., quantity of free goods, price discount, rebate value) will depend on the specifics of each case.
The DIP Methodology will not apply to cases where the apparent excessive increase in the National Average Transaction Price was due solely to a sales mix shift or when the price was reduced to offset excess revenues (see Schedule 13).
1.4 Application of the DIP Methodology to the Calculation of Non-Excessive Average Prices When the National Average Transaction Price and the Market-Specific Average Transaction Prices of a drug product increase by more than that allowable under the CPI-Adjustment Methodology and the patentee provides the required evidence, the maximum price to which the drug may increase without being presumed to be excessive is as follows:
- If the benefit(s) was offered after introduction, the Market-Specific Average Transaction Prices where benefits had been offered may increase up to the highest previous Average Transaction Price in that market without being presumed to be excessive.
- If the benefit(s) was offered at introduction, and for every subsequent reporting period, the Market-Specific Average Transaction Prices where benefits had been offered may increase up to the level of the highest current Market-Specific Average Transaction Price in any comparable market where no benefits are offered without being presumed to be excessive. Comparable markets are defined as hospital, wholesaler and pharmacy markets within the class of customer markets and each province and territory within the provincial/territorial markets.
1.5 Example of the application of the DIP Methodology
| |
Hospital MS-ATP* |
Wholesaler MS-ATP* |
Pharmacy MS-ATP* |
National ATP** |
| Year 1 (Introduction) |
$8.00 |
$9.00 |
$10.00 |
$9.00 |
| Year 2 |
$6.00 |
$8.00 |
$10.00 |
$8.00 |
| Year 3 |
$10.00 |
$9.00 |
$10.00 |
$9.67 |
* MS-ATP refers to Market-Specific Average Transaction Price
** National Average Transaction Price
In year 3, the National Average Transaction Price increases beyond what would generally be permitted by the CPI-Adjustment Methodology. The patentee claims and provides evidence as follows:
i) Hospital:
- Contracts offering a 20% discount off list price were negotiated through a Group Purchasing Organizations from date of first sale.
- Certain hospitals were offered even deeper discounts in year 2 due to the high volume of sales.
- In year 3, a new competitor entered the hospital market and the contracts were not renewed. Those hospitals that did purchase the patentee´s drug product paid full list price of $10.00, which is not presumed to be excessive due to the evidence of benefits provided.
ii) Wholesaler:
- Wholesalers were charged $9.00 in the first year and then the price was temporarily discounted in year 2 to preferred clients. By year 3, the one-year discount ended.
- The patentee provided evidence of the preferred pricing in year 2. No evidence of an introductory benefit was provided. The price of $9.00 is not presumed to be excessive due to the evidence of a benefit.
Schedule 11 – Criteria for Commencing an Investigation
The Guidelines provide that the Board may establish criteria for the commencement of an investigation into an apparent excessive price.
The criteria balance the need for pricing flexibility on the part of patentees with the PMPRB´s mandate of ensuring that the prices of patented drug products are not excessive. The Board publishes its criteria for commencing an investigation to improve transparency and to provide patentees with greater certainty as to their responsibilities.
A price is generally considered to be non-excessive if the National and Market-Specific Average Transaction Prices are equal to or below the Maximum Average Potential Price for the introductory period and below their respective Non-Excessive Average Prices for all subsequent periods.
In order to allocate its resources to investigations as efficiently as possible the Board has developed criteria for when a price that exceed the Guidelines will become the subject of an investigation.
| Criteria for Commencing an Investigation |
Board Staff will commence an investigation into the price of a patented drug product when any of the following criteria are met:
- The National Average Transaction Price or any Market-Specific Average Transaction Price of a new drug product exceeds the Maximum Average Potential Price during the introductory period by more than 5%.
- The National Average Transaction Price of an existing drug product exceeds the National Non-Excessive Average Price by more than 5%.
- Excess revenues for a new or existing drug product are $50,000 or more.
- PMPRB receives a complaint that a price is excessive.
|
Where the National Average Transaction Price exceeds the National Non-Excessive Average Price by an amount too small to trigger an investigation in one year, the patentee is expected to reduce the price of the patented drug product and to offset any excess revenues, as per the PMPRB´s Policy on the Offset of Excess Revenues (see Part II – Policies, under section 7). Evidence of persistent pricing above the Maximum Average Potential Price during the introductory period or above the National Non-Excessive Average Price during subsequent periods, even by amounts which do not trigger the investigation criteria, may result in an investigation.
Should the National Average Transaction Price of a patented drug product or its cumulative excess revenues meet the criteria, an investigation will be initiated in conformity with the Guidelines. Patentees will be advised of the compliance status and cumulative excess revenues for each of their patented drug products as part of the compliance reports they receive from the PMPRB.
Schedule 12 – “Any Market” Price Reviews
For New Patented Drug Products
- Board Staff undertakes the appropriate introductory price tests based on the drug product's level of therapeutic and determines the Maximum Average Potential Price.
- The Maximum Average Potential Price will apply to every market at introduction - i.e., national, each class of customer (i.e., hospital, pharmacy and wholesaler) and each province/territory.
Example 1
| Maximum Average Potential Price = |
$10 |
|
No price would be presumed excessive, since neither the National Average Transaction Price nor the Market-Specific Average Transaction Prices exceed the Maximum Average Potential Price. |
| National ATP* = |
$9 |
 |
| Market-Specific ATP* (Hospital) = |
$8 |
 |
| Market-Specific ATP* (Wholesaler) = |
$9 |
 |
| Market-Specific ATP* (Pharmacy) = |
$10 |
 |
* ATP means Average Transaction Price
Example 2
| Maximum Average Potential Price = |
$10 |
|
Although the National Average Transaction Price is not presumed to be excessive, one Market-Specific Average Transaction Price (pharmacy) exceeds the Maximum Average Potential Price and would therefore be presumed to be excessive. |
| National ATP* = |
$9 |
 |
| Market-Specific ATP* (Hospital) = |
$6 |
 |
| Market-Specific ATP* (Wholesaler) = |
$9 |
 |
| Market-Specific ATP* (Pharmacy) = |
$12 |
X |
* ATP means Average Transaction Price
For Existing Patented Drug Products
- Following the introductory period, Board Staff will only actively monitor the National Average Transaction Price of a patented drug product and compare it to the National Non-Excessive Average Price based on the application of the CPI-Adjustment Methodology and the Highest International Price Comparison test.
- Board Staff will further investigate the changes in prices at the level of Specific Markets (class of customer; and/or province/territory) when:
- the National Average Transaction Price exceeds the National Non-Excessive Average Price to such an extent as to trigger the criteria for commencing an investigation (see Schedule 11);
- a complaint is received; or,
- required as part of the monitoring of compliance with a VCU or Board Order.
- When the National Average Transaction Price triggers the investigation criteria, Board Staff will review the pricing trends in each market (i.e., hospital, pharmacy, wholesaler and each province/territory) to identify which market(s) caused the National Average Transaction Price to appear excessive.
- To do this, each specific market¡¯s Average Transaction Price will be compared against the Non-Excessive Average Price for that market on application of the CPI-Adjustment Methodology and the Highest International Price Comparison test.
- Three possibilities could arise:
- No Market-Specific Average Transaction Price is presumed to be excessive. This could occur if the apparent increase in the National Average Transaction Price was solely due to a shift in the sales mix ¡§C i.e., the quantity sold in each market changed such that proportionately more was sold in a market with a higher Market-Specific Average Transaction Price than in the previous reporting period.
- One or more markets are found to have taken price increases that appear to be excessive. The patentee will be expected to reduce the Market-Specific Average Transaction Price(s) to the level of the Non-Excessive Average Price for the respective market(s). The National Average Transaction Price following the price reduction would then generally be considered to be non-excessive. Rather than calculate excess revenue based solely on the market(s) where the price was excessive, the excess revenues will be calculated based on the amount generated at the level of the National Average Transaction Price.
- The patentee may provide evidence that the increase in the particular market¡¯s price was due solely to the reduction or termination of a ¡¡ãbenefit¡¡À in that market. In this case, it may be appropriate to deviate from the CPI-Adjustment Methodology, as per the DIP Methodology outlined in Schedule 10.
Schedule 13 – Offset of Excess Revenues
Approaches to offset excess revenues
1.1 Subject to subsection 1.3.1, if the investigation criteria have not been triggered, patentees will be given the opportunity to take a voluntary price reduction to offset excess revenues.
1.2 Once the investigation criteria have been triggered, patentees will only be permitted to offset cumulative excess revenues pursuant to the specific terms of an approved VCU or an issued Board decision.
Timeframes to offset excess revenues
1.3 Patentees are expected to offset excess revenues in a timely manner. The following parameters will generally be applied in the determination of repayment terms.
1.3.1 Excess revenue balances below the amount sufficient to trigger the investigation criteria that are carried for six consecutive six month reporting periods (3 years) will be expected to be offset through a VCU. Failing this, Board Staff will refer the matter to the Chairperson.
1.3.2 In the context of a VCU, and subject to the specific terms of the VCU, patentees will generally be allowed:
- 30 days following the Board´s acceptance of the VCU to make payment; or
- Until the end of the following reporting period to offset excess revenues through a price reduction. Any excess revenues remaining at the end of the specified period would be due in payment.
Resumption of price level following excess revenue offset through a price reduction
1.4 The following exception to the CPI-Adjustment Methodology applies when a price reduction below the Non-Excessive Average Price is taken in one or more markets specifically to offset excess revenues. Following the repayment of excess revenues, the Average Transaction Prices in those markets may return in the next reporting period up to the Market-Specific Non-Excessive Average Prices prior to the price reduction.
1 ICN Pharmaceuticals, Inc. v. Canada (Staff of the Patented Medicine Prices Review Board) (C.A.) [1997] 1 F.C. 32, aff´g [1996] F.C.J. No. 206 (T.D.)
2 Supra 1
3 The table above is based on the Oxford Centre for Evidence-based Medicine Levels of Evidence (May 2001) – produced by Bob Phillips, Chris Ball, Dave Sackett, Doug Badenoch, Sharon Straus, Brian Haynes, Martin Dawes since November 1998.