April 2013 NEWSletter
Volume 17, Issue No. 2
Table of Contents
- Since our last issue...
- News from the Chairperson
- Comings and Goings
- Diamond Jubilee Medal Awarded to Dr. Jean Gray
- Regulatory Burden Reduction – Upcoming Notice and Comment
- CPI-Based Price-Adjustment Factors for 2014
- NPDUIS – Release of Two New Analytical Reports
- Summary of March 27, 2013, Board Meeting
- Voluntary Compliance Undertakings
- Hearings – Update
- Upcoming events
Since our last issue...
Our recent key events
February 4: The Human Drug Advisory Panel (HDAP) held its quarterly meeting. The Queen's Diamond Jubilee Medal was awarded to Dr. Jean Gray.
February 6: Michelle Boudreau, Tanya Potashnik and Elena Lungu met with representatives of the Canadian Generic Pharmaceutical Association in Toronto.
February 7: The Evaluation of the Patented Medicine Prices Review Board and the corresponding Management Response and Action Plan to the 2011-12 PMPRB Program Evaluation were released on the PMPRB website.
February 13: Michelle Boudreau, Ginette Tognet, Béatrice Mullington, George Botulynsky, Tanya Potashnik and Robert Squires met with representatives of GlaxoSmithKline in Toronto and toured the facilities.
February 26–27: Michelle Boudreau spoke at the Pharmacare 2020 Symposium in Vancouver; Elena Lungu, Gary Warwick and Kirk Stanley attended the conference.
March 18: Michelle Boudreau and Ginette Tognet met with representatives of the German Federal Joint Committee — Gemeinsamer Bundesauschuss (GBA) in Berlin.
March 19: Michelle Boudreau and Ginette Tognet met with representatives of the Canadian Embassy in Berlin. They also met with representatives of the German Association of Research-Based Pharmaceutical Manufacturers (vfa) in Berlin.
March 19–22: Michelle Boudreau spoke at the Pharma Pricing & Market Access Outlook Europe 2013 Conference in London, UK; Tanya Potashnik and Ginette Tognet attended the conference.
March 22: Tanya Potashnik attended the Pharmaceutical Pricing and Reimbursement Information (PPRI) Networking Meeting in London, UK.
March 22: Michelle Boudreau and Ginette Tognet met with representatives of the UK Department of Health.
March 27: The Board held its quarterly meeting.
April 3: The Chairperson of the Board approved a Voluntary Compliance Undertaking submitted by Novo Nordisk Canada Inc. regarding the price of the patented medicine Novolin.
April 5: NPDUIS released two new analytical reports: The Use of Blood Glucose Test Strips in Select Public Drug Plans, 2008 and the New Drug Pipeline Monitor – April 2013.
April 10: Michelle Boudreau and Tanya Potashnik met with representatives of AstraZeneca in Toronto.
April 11: Michelle Boudreau met with representatives of Janssen Pharmaceuticals, Inc., in Toronto.
April 24: The Hearing Panel in the matter of Galderma Canada Inc. and the patented medicine Tactuo released its decision, concluding the hearing.
April 29: The Chairperson of the Board approved a Voluntary Compliance Undertaking submitted by Abbott Laboratories Inc. regarding the price of the patented medicine Mavik.
PMPRB speeches and presentations are available at News and Events/Speech Series.
News from the Chairperson
It is with profound sadness that we learned that Dr. Robert Goldwyn Elgie, former Chairperson of the PMPRB, passed away on April 3, 2013.
Dr. Elgie was a remarkable man. A lawyer and neurosurgeon, Robert Elgie had a long and distinguished career in public service. He was Chief of Medical Staff at the Scarborough General Hospital, sat in the Ontario Legislature as MPP for York East and held several cabinet posts, such as Minister of Labour and Minister of Consumer and Commercial Relations, to name a few. He went on to chair the Ontario Workmen's Compensation Board, the Nova Scotia Workmen's Compensation Board, participated in the establishment of the Health Law Institute at Dalhousie University and served as its first Director.
Dr. Elgie was appointed Chair of the Patented Medicine Prices Review Board in 1995 and made an invaluable contribution to the organization. During his 10-year tenure, he showed leadership and dedication in every action he took. He shared his passion for learning as well as his curiosity and desire to get to the root of a question. He was knowledgeable, quick-witted and generous. He genuinely cared for people and was interested in their points of view.
Robert Elgie believed that we all have a duty to leave our world a better place. He did just that. In May 2001, Dalhousie University awarded him a Doctor of Laws, honoris causa, in recognition of his outstanding personal achievements. His lifelong contribution to public service was recognized in 2003 when he was awarded the Order of Canada. He continued to serve as founding Chair of Ontario's Greenbelt Council until 2011 and as Chair of the Ontario Press Council.
I am joined by Board Staff, Members and former Members in offering Dr. Elgie's family and friends our most heartfelt condolences. He will be greatly missed.
Mary Catherine Lindberg
Comings and Goings
We would like to extend our congratulations and appreciation to Marian Eagen, Guy Roberge and Marielle Racicot, all of whom recently retired from the Public Service after productive and dedicated careers. We wish them the very best in their future endeavours — they will all be missed.
We welcome both Ramona Kenney and Liane Lavallée to the Corporate Services Branch of the PMPRB. Ramona, who recently worked for the Office of the Commissioner of Review Tribunals, CCP/OAS, is the new Director of the Corporate Services Branch. Liane is the new Chief of Human Resources Services. She comes to us from Human Resources and Skills Development Canada where she was Director, Diversity and Official Languages. We would also like to welcome John Buffone, who recently joined the PMPRB as a Regulatory Officer in the Regulatory Affairs and Outreach Branch. John came to the PMPRB from Industry Canada where he was a Research Analyst for the Information and Communications Technologies Branch.
In addition, we welcome back Theresa Morrison and Candice Popkie, who both recently returned to the PMPRB from maternity leave. Lynn Harrison, who was replacing Candice during her absence, has resumed her substantive position at the Office of the Privacy Commissioner. We wish her the best of luck.
Diamond Jubilee Medal Awarded to Dr. Jean Gray
Dr. Jean Gray, recipient of the Queen Elizabeth II Diamond Jubilee Medal
Dr. Jean Gray, member of the PMPRB's Human Drug Advisory Panel, received the Queen Elizabeth II Diamond Jubilee Medal on February 4, 2013, during a small ceremony in the PMPRB offices.
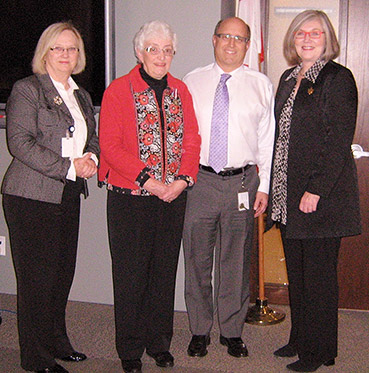
In the picture, Dr. Gray (centre) is joined by (left to right) Mary Catherine Lindberg, Chairperson of the PMPRB, Dr. Mitchell Levine, Vice-Chair of the PMPRB, and Margaret Kennedy of the Royal College of Physicians and Surgeons of Canada.
“Dr. Gray has made a tremendous contribution in the area of health care in Canada. We at the PMPRB are grateful that she has shared her wealth of knowledge and provided strong leadership on the PMPRB's Human Drug Advisory Panel.” Mary Catherine Lindberg

Regulatory Burden Reduction – Upcoming Notice and Comment
In alignment with the Government's Red Tape Reduction Plan and the Economic Action Plan, the PMPRB has committed to examining its price review process to identify possible ways to reduce the regulatory burden on patentees and increase efficiency without adversely affecting its mandate to protect consumers.
To date, the Board has approved moving forward on consulting on two priority initiatives to amend:
- the Consumer Price Index (CPI) Adjustment Methodology; and
- the Patented Medicines Regulations by moving to one annual filing of price and sales information for existing patented medicines by patentees, and to modify the requirement for patentees to submit information for the first day of sales of new patented products.
The PMPRB will consult on these initiatives in the customary way. On May 16, 2013, a Notice and Comment will be posted on the PMPRB website seeking stakeholder comment and feedback on proposals related to the two initiatives. The proposed amendments to the Regulations will then follow a formal consultation, the Federal Regulatory Development Process, through Cabinet and publication in the Canada Gazette.
Additional information on the PMRPB's commitment to examining its price review process is available in the PMPRB Report on Plans and Priorities 2013-2014 and in the Management Response and Action Plan to the 2012 Program Evaluation.
CPI-Based Price-Adjustment Factors for 2014
The Patent Act specifies the factors to be used by the PMPRB in determining whether the price of a patented drug product sold in Canada is excessive. One of these factors is the Consumer Price Index (CPI). The Board's Compendium of Policies, Guidelines and Procedures requires the cumulative increase in a product's price over any three-year period be no more than the increase in the CPI over the same period. The Compendium also sets a cap on year-over-year price increases equal to one and one-half times the CPI-inflation rate for the year in question.
To allow patentees to set prices in advance, the Board's CPI-Adjustment Methodology provides for the calculation of the CPI-adjustment factors based on forecast changes in the CPI. The Board informs patentees of these CPI-adjustment factors each year in its NEWSletter.
The following table provides CPI-adjustment factors for 2014. These factors were based on forecasts of annual CPI-inflation rates (provided by Finance Canada) of 1.3% and 2.0% for 2013 and 2014, respectively, as well as the actual 2012 CPI-inflation rate of 1.5%. (See Government of Canada, Budget 2013: Jobs, Growth and Long-Term Prosperity, March 21, 2013, Table 2.1.)
Forecast 2014 Price-Adjustment Factors for Patented Drug Products
| Benchmark Year |
(1) 2011 |
(2) 2012 |
(3) 2013 |
| Price-Adjustment Factor |
1.049 |
1.033 |
1.020 |
These figures imply (1) a maximum allowable cumulative price increase between 2011 and 2014 of 4.9% for patented drug products with Canadian sales in 2011 (that is, products whose “benchmark year” is 2011); (2) a maximum allowable cumulative price increase between 2012 and 2014 of 3.3% for products whose first Canadian sales occurred in 2012; and (3) a maximum allowable cumulative price increase between 2013 and 2014 of 2.0% for products whose first Canadian sales occurred in 2013.
In addition, the forecast inflation rate of 2.0% for 2014 implies a year-over-year price increase cap (applicable to all drug products, regardless of benchmark year) of 3.0% (= 1.5 x 2.0%) for 2014.
Although we are currently proposing changes to the CPI Adjustment Methodology, the existing Methodology will be applied until any potential changes are implemented. All proposed changes are subject to consultation prior to implementation.
NPDUIS – Release of Two New Analytical Reports
Two new analytical reports were published by the PMPRB on April 5, 2013: The Use of Blood Glucose Test Strips in Select Public Drug Plans, 2008 and the latest edition of the New Drug Pipeline Monitor.
The Use of Blood Glucose Test Strips in Select Public Drug Plans, 2008
This study focuses on the use of blood glucose test strips using data from public drug plans in Saskatchewan, Manitoba and Nova Scotia. It provides
(i) an overview of the cost and utilization of blood glucose test strips,
(ii) an international price comparison, and
(iii) a treatment group analysis that assesses the extent to which the actual utilization in 2008 compares with the recognized Canadian recommendations: the Canadian Diabetes Association (CDA) 2008 guidelines; the Canadian Agency for Drugs and Technologies in Health (CADTH) 2009 recommendations; and the CDA 2011 comments in the briefing document for healthcare providers.
The results of this study may be used as a reference point for any existing and future recommendations, including the CDA 2013 guidelines.
The New Drug Pipeline Monitor – April 2013
The New Drug Pipeline Monitor (NDPM) provides information on drugs currently under development that may have an impact on Canadian drug plan expenditure. Drugs are selected based on their phase of development, their indication and mechanism of action, and their potential impact on clinical practice. Twelve new drugs were added to the fourth edition of the NDPM. Five of these drugs were identified as biologics, an emerging class of drugs.
Summary of March 27, 2013, Board Meeting
The Board held a meeting by teleconference at which it officially welcomed its newest Board Member, Richard Bogoroch.
The Board approved the consultation plan and proposals aimed at increasing the efficiency of its price review process and reducing the regulatory burden for patentees. The Board will be consulting stakeholders on amending its Consumer Price Index (CPI) Adjustment Methodology, and the feasibility of reducing patentees' regulatory filings on price and sales to one per year. The Notice and Comment will be posted on the PMPRB website on May 16, 2013.
The Board's next meeting is scheduled for May 9, 2013.
Voluntary Compliance Undertakings
A Voluntary Compliance Undertaking (VCU) is a written undertaking by a patentee to adjust its price to conform to the Board's Guidelines. Under the Guidelines, patentees are given an opportunity to submit a VCU when Board Staff concludes, following an investigation, that the price set forth by the patentee for a patented drug product sold in Canada appears to have exceeded the Guidelines. A VCU can also be submitted by a patentee after a Notice of Hearing is issued.
Since the January issue of the NEWSletter, the Chairperson accepted two VCUs for the patented medicines Novolin® and Mavik.
Novolin®, Novo Nordisk Canada Inc.
On April 3, 2013, the Chairperson of the Board approved a VCU submitted by Novo Nordisk Canada Inc. regarding the price of Novolin®.
Under the terms of the VCU, Novo Nordisk Canada Inc. reduced the prices of Novolin® to the 2013 National Non-Excessive Average Prices (N-NEAPs). Novo Nordisk has made a payment to the Government of Canada in the amount of $6,503,426.81 to offset the cumulative excess revenues it received from January 1, 2009, to December 31, 2012. Novo Nordisk also provided notification to customers of the price reduction of Novolin®.
Finally, Novo Nordisk will ensure that the prices of Novolin® remain within the Guidelines in all future periods during which the drug product is under the PMPRB's jurisdiction.
Novolin® is indicated for the treatment of Diabetes Mellitus, where treatment with insulin is indicated.
Mavik, Abbott Laboratories Limited
On April 29, 2013, the Chairperson of the Board approved a VCU submitted by Abbott Laboratories Limited regarding the price of Mavik.
The terms of the VCU require that, on or before May 27, 2013, Abbott reduce the price of the Mavik 0.5 mg capsule to the 2013 N-NEAP and adjust the National Average Transaction Price (N-ATP) in 2014 if at the end of 2013, the Highest International Price is below the 2013 N-NEAP. Abbott is to notify its customers of the price reduction on or before May 10. Abbott will offset the cumulative excess revenues received from January 1, 2011, to December 31, 2012, by making a payment of $118,168.48 to the Government of Canada on or before May 27. Abbott is to make a further payment to offset any excess revenues received during the period of January 1, 2013, to the date of reduction of the price of Mavik, as calculated by Board Staff, on or before August 29, 2013.
Abbott will ensure that the the price of Mavik remains within the Guidelines in all future periods during which it is under the PMPRB's jurisdiction.
Mavik is indicated for the treatment of hypertension. It may be used alone or in combination with other antihypertensive medication such as hydrochlorothiazide.
Hearings – Update
The PMPRB's regulatory mandate is to ensure that prices charged by patentees for their patented medicines sold in Canada are not excessive.
In the event that the price of a patented medicine appears to be excessive, the Board can hold a public hearing and, if it finds that the price is excessive, it may issue an order to reduce the price and to offset revenues received as a result of excessive prices. Board decisions are subject to judicial review in the Federal Court of Canada.
The Board did not issue a Notice of Hearing during this past quarter.
In the matter of Galderma Canada Inc. and the medicine Tactuo
On April 24, 2013, the Hearing Panel approved a Voluntary Compliance Undertaking regarding Tactuo, which concludes this matter.
Galderma is to ensure that the National Average Transaction Price (N-ATP) for 2013 does not exceed the 2013 National Non-Excessive Average Price (N-NEAP). Galderma will also offset the cumulative excess revenues it received from May 11, 2011, to December 3, 2012, by making a payment of $419,468.12 to the Government of Canada on or before May 24, 2013. Galderma will make an additional payment, on or before March 3, 2014, to offset any excess revenues that may have been received during the period January 1 to December 31, 2013, as calculated by Board Staff.
Galderma will ensure that the N-ATP of Tactuo remains within the Guidelines in all future periods during which Tactuo is under the PMPRB's jurisdiction.
Tactuo is indicated for the treatment of acne vulgaris, characterized by comedones, inflammatory papules/pustules with or without occasional nodules in patients 12 years of age and older.
This VCU is available at Hearings and Decisions / Decisions and Orders and at Voluntary Compliance Undertakings.
Status of Board Proceedings
| Patented Drug Product |
Indication / Use |
Patentee |
Date of Notice of Hearing |
Status |
|
Apo-Salvent CFC-Free
|
Asthma
|
Apotex Inc.
|
July 8, 2008
|
Ongoing
|
|
Copaxone – Redetermination
|
Multiple sclerosis
|
Teva Canada
|
New panel struck February 2010
|
Order:
February 23, 2012
Application for judicial review: March 20, 2012
Federal Court decision: April 30, 2013
|
|
ratio-Salbutamol HFA
|
Asthma
|
ratiopharm Inc.(now Teva Canada)
|
July 18, 2008
|
Order: May 27, 2011
Application for judicial review: June 27, 2011
Federal Court hearing: November 4–5, 2013
|
|
Tactuo
|
Acne
|
Galderma Canada Inc. |
September 26, 2012 |
Board decision: April 24, 2013
|
| Patentee |
Issue |
Date of Notice of Application |
Status |
|
Apotex Inc.
|
Failure to file (jurisdiction)
|
March 3, 2008
|
Ongoing
|
|
ratiopharm Inc.(now Teva Canada)
|
Failure to file (jurisdiction)
|
August 28, 2008
|
Board Order: June 30, 2011
Amended: October 17, 2011
Application for judicial review: July 29, 2011
Federal Court hearing: November 4–5, 2013
|
|
Sandoz Canada Inc.
|
Failure to file (jurisdiction)
|
March 8, 2010
|
Board Decision: August 1, 2012
Re-issued: October 1, 2012
Application for judicial review: August 31, 2012
Federal Court hearing date to be announced
|
Upcoming events
May
May 5–7: Elena Lungu to present a poster on the NPDUIS report : The Use of Blood Glucose Test Strips in Select Public Drug Plans, 2008 at the Canadian Agency for Drugs and Technologies in Health (CADTH) Symposium, St. John's, Nfld.
May 6: Human Drug Advisory Panel (HDAP) quarterly meeting
May 9: Quarterly Board meeting
May 16: Notice and Comment related to the two initiatives for regulatory burden reduction to be posted on the PMPRB website under Consultations
May 16: RA&O webinar on the DIP Methodology
May 22–24: Michelle Boudreau to attend the 6th Annual Life Sciences Invitational Forum, Cambridge, Ont.
May 26–28: Michelle Boudreau, Sylvie Dupont and Martine Richard to attend the Council of Canadian Administrative Tribunals' (CCAT) 6th International Conference, Toronto
May 28–30: Elena Lungu to present a poster on the NPDUIS Drug Expenditure Decomposition report and present the Blood Glucose Test Strips report at the Canadian Association for Health Services and Policy Research (CAHSPR) Conference, Vancouver
May 31: PMPRB Annual Report for 2012 to be submitted to the Minister of Health for tabling in Parliament
June
June 4–5: Michelle Boudreau to speak at the Drug Pricing & Reimbursement in Canada Forum, Toronto
July
July 30: Deadline for patentees to file Form 2
July 31: Release of the July 2013 NEWSletter
September
September 12–13: Quarterly Board meeting
September 16: Human Drug Advisory Panel (HDAP) quarterly meeting
December
December 12–13: Quarterly Board meeting