New Drug Pipeline Monitor - July 2008
About the PMPRB
The Patented Medicine Prices Review Board (PMPRB) is an independent quasi-judicial body established by Parliament in 1987.
The PMPRB has a dual role: to ensure that prices at which patentees sell their patented medicines in Canada are not excessive; and to report on pharmaceutical trends of all medicines and R&D spending by patentees.
The PMPRB reports annually to Parliament, through the Minister of Health, on its activities, on pharmaceutical trends relating to all medicines, and on the R&D spending by patentees.
The NPDUIS Initiative
The National Prescription Drug Utilization Information System (NPDUIS) provides critical analyses of drug price, utilization, and cost trends in Canada to support drug plan policy decision-making for participating federal, provincial, and territorial governments.
The NPDUIS initiative is a partnership between the PMPRB and the Canadian Institute for Health Information. It was established in 2001 by the federal/provincial/territorial Ministers of Health.
Acknowledgements
This report was prepared by the Patented Medicine Prices Review Board (PMPRB) under the provisions of the National Prescription Drug Utilization Information System (NPDUIS).
The PMPRB recognizes the contributions of the members of the NPDUIS Steering Committee for their expert oversight and guidance in the preparation of this report.
1. Introduction
This is the second edition of the New Drug Pipeline Monitor (NDPM) report. The NDPM identifies drugs currently under development that may have an impact on federal, provincial and territorial (F/P/T) drug plan expenditures in the near future. It concentrates on new drugs that are expected to have a significant impact in terms of therapeutic value and uptake.
Each NDPM report provides a list of “pipeline” drugs currently under development. Drugs are selected based on a set of criteria that includes the phase of development, the indication and therapeutic area, the drug description and clinical impact. Each report also updates the list from the previous edition. Drugs may be removed from the list based on new information.
2. Updating the NDPM List
The NDPM is intended to provide a timely list of drugs that are moving through the development and approval process. For this reason, it is necessary to check the status of these drugs on a regular basis and make updates where necessary.
2.1 Adding New Drugs
The NDPM list is updated based on information contained in the BioPharm Insight® database. This comprehensive database summarizes information provided by the companies that are responsible for researching and marketing drugs. A pharmacist regularly monitors this data to identify possible additions to the list. These are examined in reference to the selection criteria outlined in the first edition of the NDPM: the phase of development, indication and therapeutic area, drug description and clinical or other impacts. Only drugs meeting all of the criteria are added to the list.
An initial search of the BioPharm Insight database conducted for this report found 554 potential additions to the NDPM list (Table 1). After applying the selection criteria outlined in the first edition, that number was reduced to 6 drugs. Table 2 provides details on the drugs selected.
As in the previous edition, the initial search showed a high proportion of new cancer drugs in Phase III clinical trials or with a NDA/BLA filed. Most of these were eliminated when the remaining selection criteria were applied. Ultimately, only one additional cancer drug was added to the list.
Table 1. Number of potential candidates for addition to the NDPM list by therapeutic area
| Therapeutic area |
In Phase III or NDA/BLA filed |
With key descriptors |
With positive preliminary results |
Selected for NDPM |
| Cancer |
130 |
67 |
10 |
1 |
| Cardiovascular* |
50 |
29 |
11 |
1 |
| Central nervous system |
71 |
37 |
12 |
0 |
| Dermatology |
12 |
2 |
1 |
0 |
| Diagnostic/imaging agents |
9 |
5 |
3 |
0 |
| Eye & ear |
10 |
3 |
1 |
0 |
| Gastrointestinal |
30 |
10 |
2 |
0 |
| Genitourinary |
18 |
2 |
1 |
1 |
| Hematological |
14 |
6 |
4 |
0 |
| HIV infections |
15 |
0 |
0 |
0 |
| Hormonal system* |
49 |
17 |
9 |
2 |
| Immune system |
27 |
15 |
5 |
2 |
| Infectious diseases |
42 |
13 |
2 |
0 |
| Musculoskeletal |
26 |
7 |
3 |
0 |
| Pain |
39 |
8 |
5 |
0 |
| Respiratory |
13 |
7 |
2 |
0 |
| TOTAL |
554 |
227 |
70 |
6 |
* One drug (tolvaptan) is indicated for both the cardiovascular and hormonal systems. It is included in both therapeutic counts, but only counted once in the totals.
Table 2. Drugs added to the NDPM list
Chemical/
Trade name/ Codes/
Company |
Therapeutic class*/
Indication/ Route |
Regulatory status |
Therapeutic considerations |
|
Ecallantide
DX-88
Dyax Corp. Genzyme Corp.
|
ATC B02 Antihemorrhagics
Hereditary angioedema (HAE)
Intravenous
|
Phase III trials
Orphan drug status (US and Europe)
Under FDA review (fast track)
|
DX-88 is a small protein that inhibits an enzyme in the blood called kallikrein. Kallikrein is a key component responsible for the regulation of inflammatory and blood clotting processes. Activated kallikrein is thought to play a role in a number of inflammatory and autoimmune diseases or conditions including hereditary angioedema, blood loss following major surgical procedures (such as cardiac bypass grafting and hip replacement), and rheumatoid arthritis. Due to its high specificity to kallikrein, DX-88 may have fewer side effects and/or greater effectiveness in the treatment of inflammation than naturally occurring inhibitors.
Source: BioPharm Insight
|
|
Eculizumab
Soliris
Alexion Pharmaceuticals, Inc.
|
ATC L04 Immunosuppressive agents
Paroxysmal nocturnal hemoglobinuria (PNH)
Intravenous
|
Phase III trials
Orphan designation (US)
Approval (US and Europe)
|
Eculizumab, a long-acting C5 complement inhibitor, is a humanized monoclonal antibody drug designed to selectively block terminal complement activation, thereby preventing destruction of red blood cells by complement in patients with paroxysmal nocturnal hemoglobinuria (PNH). There currently is no therapy specifically available for the treatment of PNH. Eculizumab appeared to be safe and well tolerated and provided clinically and statistically significant improvements in intravascular hemolysis, anemia, fatigue and quality of life in patients with PNH.
Source: Eculizumab prescribing information for physicians, Alexion conference call March 26, 2007
|
|
Plerixafor
Mozobil
Genzyme Corp.
|
ATC L03
Immunostimulants
Multiple myeloma Non-Hodgkin lymphoma
Intravenous
|
Phase III trials
Orphan designation
(US)
|
Mozobil, a novel small molecule CXCR4 chemokine antagonist, has been shown in multiple earlier studies to rapidly and effectively increase the number of stem cells in circulation in the blood. Once circulating in the blood, stem cells can be collected for use in a stem cell transplant. Mozobil is currently under clinical investigation in North America and Europe as a potential new agent for stem cell mobilization in cancer patients undergoing a stem cell transplant.
Source: BioPharm Insight, Genzyme Press Release, August 2, 2007
|
|
Sapropterin
Kuvan, Phenoptin
Biomarin Pharmaceutical, Inc.
Daiichi Asubio Pharmaceuticals, Inc.
Merck Serono S.A.
|
ATC A16
Other alimentary tract and metabolism products
Phenylketonuria (PKU)
Oral
|
Phase III trials
Orphan designation (US)
Approval (US)
In development in Canada
|
Kuvan (Phenoptin) is an investigational oral small molecule therapeutic for the treatment of phenylketonuria (PKU). Sapropterin dihydrochloride,is the synthetic form of 6R-BH4 (tetrahydrobiopterin), a naturally occurring enzyme cofactor that works in conjunction with phenylalanine hydroxylase (PAH) to metabolize phenylalanine. Preliminary clinical data have suggested that Phenoptin has a potential to produce significant reductions in blood Phe levels in the subset of patients who are BH4-responsive. BioMarin and Serono estimate that Phenoptin could be a potential treatment option for approximately 30% to 50% of the estimated 50,000 individuals in the developed world who have been diagnosed with PKU.
Source: Biomarin Pharmaceutical, Inc. Press release Dec 3, 2007
|
|
Tolvaptan
Otsuka Maryland Research Institute (OMRI)
Otsuka Pharmaceutical Co., Ltd.
|
ATC C03
Diuretics
Congestive heart failure
Hyponatremia
Oral
|
Phase III
Fast track designation
NDA filed
|
Tolvaptan is a novel, investigational small molecule designed to be an antagonist of the vasopressin V2 receptor, which plays a role in the kidney's regulation of fluid excretion. The majority of patients hospitalized for acute decompensated heart failure (ADHF) have edema or excess body fluid, which is treated with diuretics to excrete the fluid. In contrast to diuretics, tolvaptan is designed to promote aquaresis, the excretion of electrolyte-free water. It has the potential to be the first available oral vasopressin receptor antagonist.
Source: BioPharm Insight
|
|
Rivaroxaban
Xarelto, Factor Xa inhibitor
Bayer Ag
Bayer Schering Pharma
Johnson & Johnson
|
ATC B01
Antithrombotic agents
Prevention of venous thromboembolism (VTE)
Acute coronary syndrome
Stroke
Oral
|
Phase III trials
MAA filed
In development in Canada
|
Rivaroxaban is a novel direct Factor Xa (FXa) inhibitor currently in clinical development for the prevention and treatment of arterial and venous thromboembolic disorders. Rivaroxaban inhibits thrombogenesis via selective and potent inhibition of FXa activity and does not require cofactors such as antithrombin. It is currently under Phase III trials for four indications: stroke prevention in atrial fibrillation, venous thromboembolism, deep vein thrombosis and venous thromboembolism (VTE) prophylaxis. EMEA approval has been filed for the use of rivaroboxan to prevent VTE after major orthopedic surgery of the lower limbs.
Source: BioPharm Insight
|
2.2 Status Updates
A pharmacist reviews the NDPM list from previous editions and checks for changes in drug status. The pharmacist has three options for each drug currently on the NDPM list:
- leave the drug and drug description unchanged
- update the drug description with information deemed appropriate and useful to drug plan managers
- remove the drug from the list
If there have been no significant changes in the drug's status since the previous review, the description will remain the same. Conversely, if there have been noteworthy changes to the drug's status, the drug's description will be updated. These changes could include (but are not limited to): detailed results regarding efficacy, initiation of trials for further expansion of indication or international approval. New information will be added to the therapeutic considerations section of the NDPM along with the date of the last revision. Changes such as company mergers or marketing contracts (that do not impact the intention to market in Canada) and minor changes in clinical trials (e.g., changes to the location) are not considered to be significant enough to change the status.
There are only a few situations in which removing a drug from the NDPM list would be necessary (Figure 1). In particular, a drug will be removed from the NDPM list if:
- development of the drug studies has halted (e.g., because of negative outcomes in clinical trials or insufficient evidence that the new drug provides therapeutic benefit over comparators)
- pharmaceutical companies indicate they do not have an interest in obtaining approval for distribution in Canada
- the drug gains market access thereby moving beyond its pipeline status
It should be noted that a change in circumstances could potentially bring a previously removed drug back onto the NDPM list. For example, a drug might have been removed because the clinical trials were suspended to investigate potential adverse reactions. After completing its investigation, the company might conclude there is not sufficient evidence of adverse reactions and resume clinical trials. The drug would again be captured in the BioPharm Insight database and might reappear on the NDPM list.
Table 3 provides the status updates made since the last edition of this report.
Figure 1. Updates and removals from the NDPM list
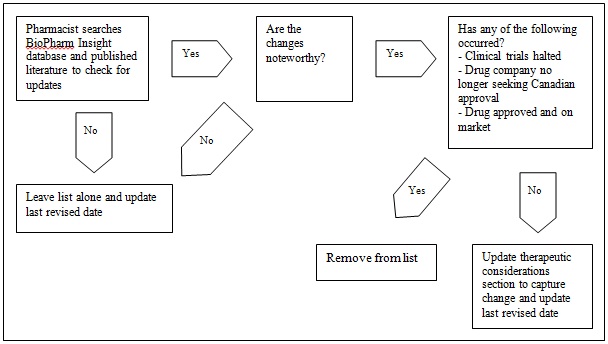
Table 3. Current NDPM list including status updates
Chemical/
Trade name/ Codes/
Company |
Therapeutic class/
Indication/
Route |
Regulatory status |
Therapeutic considerations |
|
Agomelatine
Valdoxan
Servier
|
ATC N06
Psychoanaleptics
Major depressive disorder
Oral
|
Phase III trials
In development in Canada
Under EMEA review
|
Agomelatine is the first melatonergic agonist and serotonergic antagonist. It combines antidepressant efficacy, even in severely depressed patients, with a favourable side effect profile. It has the additional benefit of sleep regulation in depressive patients.
|
|
Efaproxiral
Efaproxyn, Revaproxyn
RSR13
Allos Therapeutics
|
ATC L01
Antineoplastic agents
Brain metastases
Intravenous
|
Phase III trials
Under EMEA review
|
Efaproxiral is an allosteric hemoglobin modifier/radiation sensitizer. It has the potential to enhance the efficacy of standard radiation therapy. Efaproxiral is under investigation as an adjunct to whole-brain radiation therapy in patients with brain metastases from solid tumours (i.e., breast cancer, lung cancer). Early results show a statistically significant improvement in median survival and QoL in a subset of metastatic breast cancer patients.
|
|
Istradefylline
KW-6002
Kyowa Hakko
Kogyo Co Ltd.
|
ATC N04
Anti-Parkinson drugs
Parkinson's disease
Oral
|
Phase III trials
NDA Filed*
|
Istradefylline is the first selective adenosine A2A antagonist. A2A receptor antagonists improve motor deficits without inducing dyskinesia and counteract parkinsonian tremor. It has been shown to reduce complications, such as wearing-off, with existing treatments.
|
|
Lapatinib
Tykerb
GSK
|
ATC L01
Antineoplastic agents
Breast cancer
Oral
|
In development in Canada
Approved in US, Europe as part of combination therapy for treatment of advanced or metastatic breast cancer in women who have progressed on prior therapy*
|
Lapatinib is a small molecule that inhibits the tyrosine kinase components of EGFR (ErbB1) and HER2 receptors. In combination with capecitabine, it nearly doubled time to progression in women with refractory advanced or metastatic ErbB2 positive breast cancer whose disease had progressed following treatment with trastuzumab and other cancer therapies. It has a mild adverse effect profile.
|
|
Raltegravir
Isentress
MK-0518
Merck & Co Inc.
|
ATC J05
Antivirals for systemic use
HIV infections
Oral
|
Approval in US for use in combination with other antiretroviral agents for the treatment of HIV-1 infection in treatment-experienced adult patients who have evidence of viral replication and HIV-1 strains resistant to multiple antiretroviral agents*
Health Canada approved with condition use in combination with other antiretroviral medications for the treatment of HIV-1 infection in "treatment-experienced" adult patients who show evidence of viral replication and drug resistance*
|
Raltegravir is the first integrase inhibitor, a new class of antiretroviral agents.
|
|
Phenoxodiol
Marshall Edwards Inc.
|
ATC L01
Antineoplastic agents
Ovarian cancer
Oral
|
Phase III trials
|
Phenoxodiol is a synthetic analog of the plant isoflavone genistein, and represents a new generation of oncology drugs acting as multiple signal transduction regulators. When combined with either paclitaxel or cisplatin, overall survival has been substantially extended. It would be used as add-on therapy to increase survival.
|
|
Pirfenidone
Intermune Inc.
|
ATC R07
Respiratory system products
Pulmonary fibrosis
Oral
|
Phase III trials
Orphan
designation in US and Europe
|
Pirfenidone is a novel agent with anti-inflammatory, antioxidant, and antifibrotic properties. Early results suggest that pirfenidone may impact lung function and disease progression in patients with idiopathic pulmonary fibrosis. It is also in development for multiple sclerosis.
|
|
Satraplatin
LA 12
GPC Biotech AG
Spectrum Pharmaceuticals Inc.
Pharmion Corp.
|
ATC L01
Antineoplastic agents
Prostate cancer
Oral
|
Under FDA review (fast track)
MAA filed*
|
Satraplatin is an orally bioavailable platinum compound.
|
|
Sipuleucel-T
Provenge
Dendrion Corporation
|
ATC L01
Antineoplastic agents
Prostate cancer
Intravenous
|
Under FDA review (fast track)
NDA filed*
|
Sipuleucel-T is the first in a new class of active cellular immunotherapies (ACIs) designed to stimulate a patient's own immune system.
|
|
Tramiprosate
Alzhemed
Neurochem Inc.
|
ATC N06
Psychoanaleptics
Alzheimer's disease
Oral
|
Phase III trials
Fast track designation*
|
Tramiprosate is an amyloid â antagonist, designed to cross the blood-brain-barrier, bind to soluble Aâ‚ peptide and interfere with the amyloid cascade, thereby leading to the prevention or inhibition of amyloid deposition and the toxic effects of Aâ‚ peptide in the brain. It can potentially slow the progression of Alzheimer's disease. Early results show clinically significant benefits on cognitive and global performance measures, with stabilization of the disease in a proportion of mild patients (44%) after 3 years of treatment.
|
Note: Table Updated: February 14, 2008. New developments are bolded in the list.
* New development.