ISSN : 1495-057X
N° de catalogue : H78-2013F-PDF
La version PDF
Le Conseil d'examen du prix des médicaments brevetés a pour mandat de veiller à ce que les brevetés ne vendent pas leurs médicaments brevetés au Canada à des prix excessifs ainsi que de faire rapport des tendances pharmaceutiques de tous les médicaments et des dépenses de R-D des brevetés.
Aperçu statistique de 2013
Mandat de réglementation
Conformité
- Cent-quinze (115) nouveaux produits médicamenteux brevetés pour usage humain ont fait l’objet d’un rapport au CEPMB.
- Les prix de 93 nouveaux produits médicamenteux brevetés ont été jugés conformes aux Lignes directrices.
- Au total, 1 343 nouveaux produits médicamenteux brevetés pour usage humain relevaient de la compétence du CEPMB.
Application
Jusqu’au 30 mai 2014 :
- Le Conseil a accepté 6 Engagements de conformité volontaire en vertu desquels des prix ont été réduits et des recettes excessives totalisant 10,5 millions de dollars ont été remboursées au moyen de paiements au gouvernement du Canada.
- Le Conseil a mené à terme deux audiences : Copaxone (réexamen) et Tactuo, les deux sur le prix.
- Aucune décision du Conseil n’est en attente.
- Le Conseil compte deux affaires dans son calendrier d’audiences : Apotex Inc. et Apo-Salvent exempt de CFC.
- La Cour fédérale a émis des décisions dans trois affaires : ratio-Salbutamol HFA, ratiopharm Inc. et Sandoz Canada Inc.
Mandat de rapport
Tendances observées au niveau des ventes
- Les ventes de produits médicamenteux brevetés ont augmenté de 6,5 %, totalisant 13,6 milliards de dollars.
- La part des ventes de produits médicamenteux brevetés exprimée en pourcentage de la valeur de l’ensemble des ventes a augmenté, passant de 59,3 % en 2012 à 61,8 % en 2013.
- Les agents antinéoplasiques et les agents immunomodulateurs ont le plus contribué à l’accroissement des ventes.
Tendances observées au niveau des prix des produits médicamenteux brevetés
- Les prix départ-usine des produits médicamenteux brevetés, mesurés à l’aide de l’indice des prix des médicaments brevetés, ont augmenté de 0,5 % en moyenne, alors que l’indice des prix à la consommation augmenté de 0,9 %.
- Les prix au Canada des produits médicamenteux brevetés se situaient au troisième rang des prix les plus élevés par rapport aux prix pratiqués dans les sept pays de comparaison, moins élevés que les prix pratiqués en Allemagne et aux États-Unis.
Recherche-développement
- Les brevetés ont fait rapport de dépenses de R-D totalisant 752,8 millions de dollars, ce qui représente un recul de 15,9 % par rapport à 2012.
- Les brevetés membres de Rx&D ont fait rapport de dépenses de R-D totalisant 652,0 millions de dollars, ce qui représente un recul de 16,7 % par rapport à 2012.
- Les ratios des dépenses de R-D par rapport aux recettes tirées des ventes ont enregistré un recul en 2013 :
- Chez tous les brevetés, le ratio est passé de 5,3 % en 2012 à 4,5 %.
- Chez les brevetés membres de Rx&D, le ratio est passé de 6,6 % en 2012 à 5,4 %.
Lettre à la Ministre
Le 30 mai 2014
L'honorable Rona Ambrose, C.P., députée
Ministre de la Santé
Chambre des communes
Ottawa (Ontario)
K1A 0A6
Madame la ministre,
J'ai le plaisir de vous présenter, conformément aux articles 89 et 100 de la Loi sur les brevets, le Rapport annuel du Conseil d'examen du prix des médicaments brevetés pour l'exercice terminé le 31 décembre 2013.
Croyant le tout conforme, je vous prie d'agréer, Madame la ministre, l'assurance de mes sentiments distingués.
Mary Catherine Lindberg
Présidente
Message de la présidente
À la lumière de notre 25e anniversaire, la dernière année a marqué l'aube d'une nouvelle ère en ce qui concerne l'historique des rapports du CEPMB. Au moment où nous nous tournons vers l'avenir, il paraît évident que notre organisme devra composer avec de multiples évolutions concurrentes. Les responsables de la réglementation et les intervenants de l'industrie pharmaceutique ont rarement eu la chance de témoigner d'une période si intéressante. Bien sûr, il peut s'agir d'une bénédiction ou d'une malédiction, selon la perspective adoptée. Au CEPMB, toutefois, nous sommes du premier avis, et c'est dans cette optique que nous cherchons à rapprocher les liens parfois très écartés au sein de notre environnement afin d'établir nos priorités stratégiques en vue des prochaines années.
Au cours des dernières années, nous avons observé une très faible croissance des dépenses relatives aux médicaments d'ordonnance au Canada, une tendance tout à fait opposée à celle observée à la fin des années 1990 et au début des années 2000. En effet, les dépenses relatives aux médicaments ont affiché une croissance de seulement 2,3 % en 2012, et ce taux était de 1,3 % l'année précédente, soit le plus faible taux observé depuis des décennies. Les experts s'entendent pour dire que cette tendance découle principalement des initiatives récentes d'achats groupés par les provinces, de l'arrivée à échéance des brevets d'un nombre important de « médicaments vedettes » et de la réduction du taux de lancement de nouveaux médicaments de ce type. Les avis sont toutefois plus partagés sur la question de savoir s'il s'agit d'une tendance à court ou à long terme, et sur l'incidence éventuelle de la population vieillissante au Canada et de la récente émergence de médicaments onéreux dans certaines catégories thérapeutiques quant à la viabilité des régimes privés et publics d'assurance-médicaments pour l'avenir.
Comme l'on peut s'y attendre, les dépenses relatives aux médicaments brevetés ont suivi cette tendance générale au cours des dernières années, bien que l'augmentation de 6,5 % observée pour l'année de rapport à l'étude contraste vivement avec la diminution de 0,3 % observée pour l'année précédente. Malgré les dernières tendances en faveur de la stabilisation, la croissance des ventes de médicaments brevetés au Canada continue de dépasser la croissance au sein des pays de comparaison nommés dans le Règlement, sauf les États-Unis. De même, les prix des médicaments brevetés au Canada se situent actuellement au troisième rang parmi les pays de comparaison, à presque égalité avec l'Allemagne. Alors que les prix augmentent, le ratio de R-D est à la baisse, représentant actuellement 5,4 % pour tous les brevetés, soit le plus faible taux depuis avant l'adoption du projet de loi C-22. Même si le Canada prend plusieurs mesures pour aborder les coûts des médicaments d'ordonnance, il en va de même pour la plupart des pays auxquels nous comparons les prix et la R-D. Il est essentiel de surveiller de près les évolutions au pays et à l'étranger afin de s'assurer que nos règlements, nos lignes directrices et nos procédures opérationnelles demeurent pertinents et efficaces.
Les éléments susmentionnés nous aideront à définir nos priorités stratégiques en vue de l'avenir. Or, au cours de la dernière année, nous nous sommes concentrés sur l'amélioration de nos programmes en surveillant l'incidence des modifications apportées aux Lignes directrices et sur la publication d'études qui font état des dernières tendances du marché et qui tiennent compte des intérêts immédiats des payeurs publics et privés. Nous avons également poursuivi nos activités de liaison, élargi et diversifié nos échanges avec les intervenants et participé de façon plus active aux consultations avec nos partenaires fédéraux, provinciaux et internationaux.
En cadrant avec le Plan d'action pour la réduction du fardeau administratif du gouvernement, nous avons achevé un processus de consultation sur des initiatives proposées pour réduire le fardeau réglementaire. Par conséquent, nous avons simplifié la méthodologie de rajustement du prix selon l'indice des prix à la consommation (IPC). La méthodologie simplifiée entrera en vigueur en 2015. De plus, nous avons proposé des modifications au Règlement sur les médicaments brevetés afin de faire la transition vers un seul dépôt annuel au lieu de deux, et nous envisageons la prépublication des modifications proposées dans la Partie I de la Gazette du Canada cette année, aux fins de consultation officielle.
À la veille de la publication du présent rapport, nous avons appris que la Cour fédérale avait rendu ses décisions dans les affaires de ratiopharm et de Sandoz. Comme c'est le cas pour toute nouvelle jurisprudence, nous examinerons attentivement l'incidence éventuelle de ces décisions quant à la compétence et au mandat du Conseil, et nous continuerons à collaborer étroitement avec le Bureau du procureur général en cas de recours.
Malgré tous ces nouveaux défis et enjeux, nous continuons à prioriser la protection des intérêts des consommateurs, tout en reconnaissant la valeur des médicaments novateurs pour les patients. Cela ne sera rendu possible que grâce aux efforts de collaboration et au dévouement des membres du Conseil et de son personnel.
Enfin, il serait négligent de ma part de ne pas prendre le temps de reconnaître les cadres clés du CEPMB qui ont récemment quitté l'organisme ou qui ont pris leur retraite cette année, notamment Martine Richard, avocate générale, ainsi que Catherine Lombardo, Béatrice Mullington et Anna Chodos de la Direction de la réglementation et de la liaison auprès des brevetés. On peut qualifier ces dernières de fonctionnaires au sens le plus noble du terme, et cette affirmation s'applique tout particulièrement à Sylvie Dupont, notre Directrice du Secrétariat du Conseil et communications de longue date, qui a été l'une des premières employées du CEPMB. Éternelle professionnelle jusqu'au bout des ongles, Sylvie a diligemment tenté, sans toutefois réussir, de me déconseiller de la mentionner dans le présent rapport annuel puisque ce serait « sans précédent ». J'exerce alors ma prérogative de présidente, à cette occasion exceptionnelle, afin d'exprimer nos sentiments de profonde gratitude à Sylvie pour son dévouement et tous les excellents conseils qu'elle a su fournir, à moi et à mes prédécesseurs, au cours de ses nombreuses années de service. Sylvie, tu nous manqueras. À toutes celles qui nous ont quittés, nous tenons à vous remercier de votre long service dévoué et nous vous souhaitons une longue et heureuse retraite bien méritée.
Au nom de mes collègues, je réaffirme notre engagement à assurer l'exécution efficace du mandat du CEPMB au service de la population canadienne.
Mary Catherine Lindberg
Le Conseil d’examen du prix des médicaments brevetés
Le Conseil d'examen du prix des médicaments brevetés (CEPMB) est un organisme indépendant qui détient des pouvoirs quasi judiciaires. Il a été créé par le Parlement en 1987 en vertu de la Loi sur les brevets.
Le CEPMB protège les intérêts des Canadiens en s'assurant que les produits médicamenteux brevetés ne sont pas vendus au Canada à des prix excessifs. Il le fait en examinant les prix auxquels les brevetés vendent les produits médicamenteux brevetés sur les marchés canadiens. Si un prix semble être excessif, le Conseil peut tenir des audiences publiques et ordonner la réduction des prix et (ou) le remboursement des recettes excessives. Le CEPMB doit également déclarer les tendances observées au niveau des ventes de produits pharmaceutiques et de l'établissement des prix pour tous les médicaments, ainsi que les dépenses de recherche-développement (R-D) des brevetés.
Le ministre de la Santé est responsable de l'application des dispositions de la Loi sur les brevets (la Loi) formulées aux articles 79 à 103. Le CEPMB fait partie du portefeuille de la Santé, qui est également constitué de Santé Canada, de l'Agence de la santé publique du Canada, des Instituts de recherche en santé du Canada et de l'Agence canadienne d'inspection des aliments. Le portefeuille de la Santé aide le ministre de la Santé à maintenir et à améliorer la santé des Canadiens.
Même s'il fait partie du portefeuille de la Santé, le CEPMB exerce son mandat en toute indépendance vis-à-vis du ministre de la Santé. Le CEPMB fonctionne également d'une façon indépendante des autres organismes, à savoir Santé Canada, qui autorise la vente des médicaments au Canada après avoir vérifié leur innocuité, leur efficacité et leur qualité; les régimes publics fédéral, provinciaux et territoriaux d'assurance-médicaments qui autorisent l'inscription des médicaments sur leurs formulaires de médicaments admissibles à un remboursement; et le Programme commun d'examen des médicaments, géré par l'Association canadienne des médicaments et des technologies de la santé, qui évalue l'efficience des médicaments avant leur inscription sur les formulaires des régimes publics d'assurance-médicaments participants.
Compétence
Réglementation
Le CEPMB vérifie les prix auxquels les brevetés vendent leurs produits médicamenteux brevetés pour usage humain ou pour usage vétérinaire distribués sous ordonnance ou en vente libre au Canada, afin de s'assurer qu’ils ne sont pas excessifs. Cela comprend les ventes aux grossistes, aux hôpitaux, aux pharmacies et autres clients pour usage humain ou pour usage vétérinaire. Le CEPMB réglemente le prix de chaque médicament breveté. Cela comprend chaque concentration et chaque forme posologique finale de tout médicament.
La compétence du Conseil ne s'applique pas exclusivement aux produits médicamenteux dont le brevet porte sur l'ingrédient actif, mais aussi aux médicaments auxquels un brevet est lié, que ce soit au niveau de son procédé de fabrication, de son mode d'administration, de sa forme posologique, de l'indication/utilisation, de la préparation ou autre.
Les produits médicamenteux brevetés ne sont pas par définition exclusivement des produits de marque. En effet, certains fabricants de produits génériques sont assujettis à la compétence du Conseil du fait qu'ils vendent en vertu d'une licence d'exploitation le même produit que le produit de marque ou, encore, qu'ils sont titulaires d'un brevet visant le procédé de conditionnement ou de traitement de produits génériques.
Le CEPMB n'est pas habilité à réglementer les prix des médicaments non brevetés et n'a aucun droit de regard sur les prix auxquels les grossistes et les pharmacies vendent les médicaments ni sur les honoraires des pharmaciens. La distribution, l'ordonnance et le remboursement des médicaments échappent aussi à sa compétence.
En vertu de la Loi, les brevetés doivent informer le CEPMB de leur intention de vendre un nouveau produit médicamenteux breveté. Après leur première vente, les brevetés doivent faire rapport au CEPMB du prix de vente de leur produit médicamenteux et de la quantité vendue. Par la suite, à la fin de chaque semestre, ils doivent faire rapport des prix et des ventes au Canada des différentes concentrations de leurs produits médicamenteux.
Même s’ils ne sont pas tenus de faire approuver au préalable les prix de vente de leurs produits médicamenteux, les brevetés doivent respecter à la lettre les dispositions de la Loi pour s’assurer que les prix auxquels ils vendent leurs produits médicamenteux au Canada ne sont excessifs. Lorsque, à l’issue d’une audience publique, il apparaît que le prix d’un produit médicamenteux vendu sur un marché canadien est excessif, le Conseil peut rendre une ordonnance qui oblige le breveté à réduire le prix de son produit médicamenteux et à appliquer les mesures qui lui sont dictées pour rembourser les recettes excessives qu’il a encaissées.
Rapport
Chaque année, le CEPMB rend compte de ses activités au Parlement par le truchement du ministre de la Santé. Le rapport annuel présente une analyse des tendances relatives à la vente et aux prix de tous les produits médicamenteux et fait rapport des dépenses de R-D des brevetés.
Au moyen de l’initiative du Système national d’information sur l’utilisation des médicaments prescrits (SNIUMP), le CEPMB effectue des analyses critiques des tendances des prix des produits médicamenteux d’ordonnance, de l’utilisation faite de ces produits et des coûts en produits médicamenteux au Canada. Les résultats de ces analyses éclairent le processus de décision des régimes d’assurance-médicaments fédéraux, provinciaux et territoriaux participants.
Gouvernance
Le Conseil est composé d’au plus cinq membres siégeant à temps partiel, dont un président et un vice-président. Tous les membres du Conseil sont nommés par le gouverneur en conseil. En vertu de la Loi, le président du Conseil assume également les fonctions de chef de la direction et, en cette qualité, est chargé de la gouverne et de la supervision des activités du CEPMB.
Les membres du Conseil, y compris le président, sont collectivement responsables de la mise en oeuvre des dispositions applicables de la Loi. Ensemble, ils établissent les lignes directrices, les règles, les règlements administratifs et les autres politiques du Conseil, comme le prévoit la Loi et consultent au besoin des intervenants, y compris les ministres provinciaux et territoriaux de la Santé et les représentants de groupes de consommateurs, l’industrie pharmaceutique et d’autres personnes.
En date du 30 mai 2014, il y avait un seul poste vacant au Conseil.
Membres du Conseil

Présidente
Mary Catherine Lindberg,
B. Sc. Pharm.
Mary Catherine Lindberg a d’abord été nommée membre et vice-présidente du Conseil d’examen du prix des médicaments brevetés en juin 2006. Le 19 mai 2010, Mme Lindberg assumait les fonctions de la présidence pendant la vacance du poste. Elle a été officiellement nommée présidente du Conseil le 3 mars 2011.
Mme Lindberg a occupé de 2002 à 2009 le poste de directrice exécutive du Ontario Council of Academic Hospitals, un regroupement de 25 hôpitaux universitaires affiliés à une université et à sa faculté de médecine. Avant d’occuper ce poste, Mme Lindberg était sous-ministre adjointe des Services de santé, au ministère de la Santé et des Soins de longue durée de l’Ontario. Elle s’occupait entre autres du Régime d’assurance-maladie de l’Ontario et des Programmes de médicaments.
Mme Lindberg a fait ses études en pharmacie à l’Université de la Saskatchewan et a son permis de pratique en Saskatchewan et en Ontario.

Vice-président
Mitchell Levine,
B. Sc., M. Sc., M.D., FRCPC, FISPE
Le Dr Mitchell Levine a été nommé membre et vice-président du Conseil le 3 mars 2011.
Le Dr Levine est professeur au sein des départements de médecine et d’épidémiologie clinique et biostatistiques à la faculté des sciences de la santé de l’Université McMaster à Hamilton, en Ontario. Il est également directeur du Centre for Evaluation of Medicines à St. Joseph’s Healthcare, à Hamilton.
Le Dr Levine a obtenu son diplôme en médecine à l’Université de Calgary en 1979, qui a été suivi d’études supérieures en médecine interne (FRCPC) et en pharmacologie clinique à l’Université de Toronto (de 1981 à 1987). Il a obtenu un diplôme de maîtrise ès sciences en épidémiologie clinique de l’Université McMaster en 1988.
Avant sa nomination au Conseil, le Dr Levine était membre du Groupe consultatif sur les médicaments pour usage humain du CEPMB. Il agit comme consultant spécial en pharmacologie clinique au ministère de la Santé et des Soins de longue durée de l’Ontario. De plus, il est rédacteur en chef du Journal de la thérapeutique des populations et de la pharmacologie clinique et est corédacteur de l’ACP Journal Club: Evidence-Based Medicine.
Membres

Normand Tremblay,
ASC, M. Sc., Adm. A., C.M.C.
Normand Tremblay a été nommé membre du Conseil le 31 mai 2012.
M. Tremblay enseigne la gestion stratégique à l’Université du Québec à Trois-Rivières. Il fait bénéficier le Conseil d’une vaste expérience en planification stratégique et opérationnelle.
M. Tremblay a siégé comme membre au Conseil national de recherches du Canada de 2007 à 2010. Il est membre de l’Ordre des administrateurs agréés du Québec.

Richard Bogoroch, LL. B.
Richard Bogoroch a été nommé membre du Conseil le 13 décembre 2012.
M. Bogoroch est le fondateur et directeur associé de Bogoroch & Associates LLP, le successeur de Bogoroch and Associates, un cabinet d’avocats spécialisé en litige civile établi à Toronto en novembre 1999. Bogoroch & Associates LLP exerce principalement en litige lié aux blessures personnelles graves, à la mort dommageable, à la faute médicale, à la responsabilité des produits et aux réclamations d’invalidité.
M. Bogoroch a obtenu un B. C.L. en 1978 et un LL. B en 1979 de l’Université McGill. Il a été reçu au Barreau de l’Alberta en 1980 et appelé au Barreau de l’Ontario en 1983. Richard a complété son stage à Thomson Rogers et, suite à son appel au Barreau en 1983, s’est joint au cabinet. En 1993, il a été certifié en tant que spécialiste en contentieux civil par le Barreau du Haut-Canada. Il a agi à titre d’associé au cabinet Thomson Rogers de 1987 à 1999. M. Bogoroch est ancien directeur de l’Ontario Centre for Advocacy Training et de l’Advocates’ Society. Il est également ancien président de de l’Association du Barreau canadien – comité provincial de l’Ontario sur le pouvoir judiciaire. M. Bogoroch a donné de nombreux exposés et écrit bon nombre de documents sur les diverses facettes du contentieux du dommage corporel dans le cadre des programmes de formation juridique permanente offerts par l’Advocates’ Society, le Barreau du Haut-Canada, l’Association du Barreau de l’Ontario, l’Ontario Trial Lawyers Association, l’Institut canadien, l’Osgoode Hall Law School, Insight, et autres. Depuis 1999, il est professeur invité dans le cadre de l’atelier intensif en techniques de plaidoirie à la Osgoode Hall Law School. De 2011 à 2014, il a présidé ou co-présidé le programme annuel du programme de formation permanente d’Osgoode sur le droit de préjudice personnel. Depuis 2011, il co-préside le programme « Tricks of the Trade » de l’Advocates’ Society, son programme annuel de formation juridique permanente sur le droit de préjudice personnel.
M. Bogoroch a été reconnu par LEXPERT en tant qu’avocat de premier rang en droit du préjudice personnel et a été nommé parmi les « meilleurs avocats » en litige en matière de préjudice personnel.
Structure organisationnelle et personnel
Directeur exécutif
Le directeur exécutif avise le Conseil, supervise le travail du personnel et en assume le leadership.
Réglementation et liaison auprès des brevetés
La Direction de la réglementation et de la liaison auprès des brevetés fait l’examen des prix des produits médicamenteux brevetés vendus au Canada pour s’assurer qu’ils ne sont pas excessifs. De plus, elle encourage les brevetés à se conformer volontairement aux Lignes directrices du Conseil, veille à la bonne application des politiques de conformité et fait enquête sur les plaintes reçues concernant les prix de certains produits médicamenteux brevetés. De plus, la Direction sensibilise les brevetés sur les Lignes directrices du Conseil et les informe de leurs obligations en matière de présentation de rapports.
Politiques et analyse économique
La Direction des politiques et de l’analyse économique formule lorsqu’il y a lieu des conseils stratégiques concernant les changements qui pourraient être apportés aux Lignes directrices et à d’autres politiques du Conseil. Elle effectue des recherches, ainsi que des analyses des tendances des prix des produits médicamenteux, et en présente les résultats dans des rapports. Enfin, elle effectue les études à l’appui des activités de conformité et d’application et les études que lui commande le ministre de la Santé.
Services généraux
La Direction des services généraux offre conseils et services en matière de gestion des ressources humaines, des installations, de la santé et sécurité au travail, de la technologie et de la gestion de l’information. Elle s’occupe également de la planification stratégique et financière de même que des rapports, des vérifications, de l’évaluation et de la liaison auprès des agences centrales fédérales compétentes.
Secrétariat du Conseil et communications
La Direction du Secrétariat du Conseil et des communications planifie et orchestre le programme des communications du CEPMB, les relations avec les médias, le suivi aux demandes de renseignements du grand public et le processus relatif aux plaintes officielles. Elle gère les réunions et les audiences du Conseil, dont les dossiers de procédure. Ils coordonnent les activités du Conseil relatives à l’application de la Loi sur l’accès à l’information et de la Loi sur la protection des renseignements personnels.
Avocate générale
L’avocate générale fournit des opinions juridiques au CEPMB et dirige l’équipe de la poursuite dans les audiences du Conseil.
Budget
En 2013-2014, le Conseil disposait d’un budget de 10,944 millions de dollars et d’un effectif approuvé de 74 équivalents temps plein (ETP).
Tableau 1 Budget et effectif 2012
| |
2012-2013 |
2013-2014 |
2014-2015 |
| * L’affectation à but spécial est réservée aux coûts externes liés à la tenue d’audiences publiques (conseillers juridiques, témoins experts, etc.). Les fonds non dépensés sont retournés au Trésor. |
| Budget |
11,058 M$ |
10,944 M$ |
10,927 M$ |
| Salaires |
7,034 M$ |
6,920 M$ |
6,903 M$ |
| Opérations |
1,554 M$ |
1,554 M$ |
1,554 M$ |
| Affectation à but spécial* |
2,470 M$ |
2,470 M$ |
2,470 M$ |
| ETP |
76 |
74 |
73 |
Communications et liaison auprès des brevetés
Le programme des communications est responsable de la planification et de la gestion des communications externes du CEPMB. Il assure également la visibilité de l’organisme et la participation des intervenants. Des renseignements sont échangés sous diverses formes et au moyen de différents supports, avec les consommateurs, les partenaires provinciaux et territoriaux, l’industrie et d’autres intervenants. Les principales activités du programme comprennent, entre autres, les relations avec les médias, répondre aux demandes de renseignements du public, et renseigner le public par la publication de mises à jour sur les audiences devant le Conseil et les décisions du Conseil et de résultats de recherche.
Le groupe des Communications cherche tout particulièrement à adapter le CEPMB aux nouvelles exigences de son environnement opérationnel, en évaluant son efficacité et en envisageant constamment des produits de communication de rechange.
À titre de source fiable et impartiale de renseignements exhaustifs et exacts sur les prix des médicaments, le CEPMB s’engage à développer et à entretenir de façon continue une collaboration avec ses intervenants.
Les intervenants de l’industrie sont consultés et informés de tout changement de l’environnement opérationnel et sont rapidement informés de toute mise à jour au processus de réglementation. Afin de faciliter l’accès à l’information, la Direction de la réglementation et de la liaison auprès des brevetés tient régulièrement des séances d’information à l’intention des brevetés.
Publications
Outre ses publications régulières, dont le Rapport annuel et le bulletin d’information trimestriel La Nouvelle, le CEPMB publie des rapports de recherche du SNIUMP en réponse aux exigences de programme ou de l’organisme.
Le CEPMB publie ses documents uniquement en format électronique dans le but de réduire les coûts et l’incidence environnementale de l’imprimerie. Il compte davantage sur son site Web et les médias sociaux à des fins de collaboration avec ses intervenants. Le CEPMB est toujours résolu à réaliser son mandat de façon ouverte et transparente.
Réglementation des prix des médicaments brevetés
Le CEPMB protège les intérêts des consommateurs canadiens en s’assurant que les produits médicamenteux brevetés ne sont pas vendus au Canada à des prix excessifs. Il le fait en examinant les prix auxquels les brevetés vendent chaque produit médicamenteux breveté sur les marchés canadiens aux grossistes, aux hôpitaux et aux pharmacies.
Exigences en matière de rapport
Les brevetés sont tenus par la loi de produire des renseignements relatifs à la vente de leurs produits médicamenteux au Canada. La Loi sur les brevets (la Loi) et le Règlement sur les médicaments brevetés (le Règlement) dictent les rapports que doivent soumettre les brevetés, et le personnel du Conseil examine de façon continue les renseignements sur les prix afin de s’assurer que les prix ne sont pas excessifs, et ce, jusqu’à échéance de tous les brevets applicables.
Il existe divers facteurs servant à déterminer si le prix d’un produit médicamenteux est excessif, comme l’énonce l’article 85 de la Loi. Le Compendium des politiques, des Lignes directrices et des procédures (les Lignes directrices) fournit des renseignements sur les tests appliqués aux prix pour décider si le prix auquel un breveté vend son produit est inférieur au prix maximal permis. Les Lignes directrices ont été élaborées en collaboration avec les intervenants, dont les ministres de la Santé provinciaux et territoriaux, les associations de consommateurs et l’industrie pharmaceutique. Lorsqu’à l’issue d’une enquête, on juge qu’il y a un problème relativement au prix d’un produit médicamenteux breveté, on offre au breveté la possibilité de réduire volontairement son prix et (ou) de rembourser ses recettes excessives aux termes des modalités d’un Engagement de conformité volontaire. Si le breveté ne souscrit pas aux résultats de l’enquête et choisit de ne pas présenter un Engagement, le président du Conseil peut émettre un Avis d’audience. Après l’audition de la preuve, si le Conseil conclut que le prix est excessif, il peut rendre une ordonnance obligeant le breveté à réduire le prix de son produit et (ou) à rembourser les recettes excessives. Le breveté peut également soumettre un Engagement de conformité volontaire après l’émission d’un Avis d’audience afin de résoudre l’affaire. Des copies de la Loi, du Règlement, des Lignes directrices et du Guide du breveté sont affichées sur le site Web du CEPMB.
Défaut de présenter ses rapports
Le CEPMB compte sur la ponctualité des brevetés en ce qui a trait à la présentation de leurs rapports sur tous les produits médicamenteux brevetés qu’ils vendent au Canada auxquels un brevet s’applique. En 2013, cinq produits médicamenteux ont été déclarés au CEPMB pour la première fois, même s’ils avaient été brevetés et vendus avant 2013. De plus, trois produits médicamenteux déclarés antérieurement au CEPMB et dont le brevet était arrivé à échéance, ont de nouveau été déclarés comme ayant un brevet applicable.
Le tableau 2 présente les produits médicamenteux qui étaient brevetés et vendus au Canada avant de faire l’objet d’un rapport au CEPMB.
Tableau 2 Défaut de présenter ses rapports sur les ventes de produits médicamenteux brevetés
| Breveté |
Nom de marque |
Nom générique |
Année où le médicament est devenu assujetti à la compétence du Conseil |
Année où le médicament est devenu assujetti à la compétence du Conseil et dont le brevet était nouveau |
| Alcon Canada Inc. |
Triesence |
Acétonide de triamcinolone |
2012 |
|
| CSL Behring |
Fibrogammin |
Facteur XIII (humain) |
1997 |
|
| GlaxoSmithKline Inc. |
Arixtra |
Fondaparinux |
|
2007 |
| Novartis Pharmaceuticals Canada Inc. |
Lamisil |
Hydrochloride de terbinafine |
|
2005 |
| Paladin Laboratories Inc. |
GlucaGen |
Glucagon |
2010 |
|
| Paladin Laboratories Inc. |
GlucaGen HypoKit |
Glucagon |
2010 |
|
| Sanofi-aventis Canada Inc. |
Deflazacort |
Deflazacort |
2006 |
|
| Sanofi pasteur Limited |
Adacel-Polio |
Vaccin DaPT-IPV |
|
2011 |
Défaut de présenter les données sur les prix et sur les ventes (Formulaire 2)
Le défaut de présenter ses rapports fait référence au défaut partiel ou complet d’un breveté de présenter les rapports qu’il est tenu de présenter en vertu de la Loi et du Règlement. Le Conseil n’a pas été appelé à rendre des ordonnances pour défaut de présenter ses rapports en 2013.
Examen scientifique
Groupe consultatif sur les médicaments pour usage humain
Tous les nouveaux produits médicamenteux brevetés ayant fait l’objet d’un rapport au CEPMB sont soumis à une évaluation scientifique dans le cadre du processus d’examen du prix. Le Groupe consultatif sur les médicaments pour usage humain (GCMUH) a été créé par le Conseil dans le but d’offrir une expertise et des conseils indépendants au personnel du Conseil. Le GCMUH entreprend un examen lorsqu’un breveté présente une demande relative à l’amélioration thérapeutique. De plus, les membres du groupe examinent et évaluent les renseignements scientifiques pertinents et disponibles, notamment toute preuve présentée par le breveté quant au niveau d’amélioration thérapeutique proposé, le choix de produits médicamenteux aux fins de comparaison et les posologies comparables.
Examen du prix
Le CEPMB examine le prix moyen de chaque concentration et chaque forme posologique d’un médicament breveté. Dans la plupart des cas, cette unité est conforme au numéro d’identification du médicament (DIN) attribué par Santé Canada au moment où la vente au Canada du médicament est approuvée.
Nouveaux produits médicamenteux brevetés ayant fait l’objet d’un rapport au CEPMB en 2013
Aux fins du présent rapport, tout produit médicamenteux breveté lancé ou vendu sur le marché canadien avant l’attribution de son premier brevet entre le 1er décembre 2012 et le 30 novembre 2013 est réputé avoir été breveté en 2013.
Il y avait 115 nouveaux produits médicamenteux brevetés pour usage humain ayant été rapportés comme vendus en 2013. Certains constituent une ou plusieurs concentrations d’une nouvelle substance active et d’autres, de nouvelles présentations de médicaments existants. Dix (8,7 %) des 115 nouveaux produits médicamenteux brevetés ont été commercialisés au Canada avant d’avoir obtenu un premier brevet canadien qui les aurait automatiquement assujettis à la compétence du CEPMB. Le tableau 3 indique l’année de la première commercialisation de ces produits médicamenteux.
Tableau 3 Nombre de nouveaux produits médicamenteux brevetés pour usage humain en 2013 selon l’année de leur première vente
| Année de la première vente |
Nbre de produits médicamenteux |
| 2013 |
105 |
| 2012 |
4 |
| 2011 |
2 |
| 2006 |
1 |
| 2000 |
3 |
| Total |
115 |
La liste des nouveaux produits médicamenteux brevetés ayant fait l’objet d’un rapport au CEPMB est mise à jour chaque trimestre et affichée sur le site Web sous la rubrique « Réglementation des prix ». Cette liste présente de l’information sur l’état d’avancement de l’examen (p. ex. la question de savoir si le prix du médicament est sous examen, conforme aux Lignes directrices, sous enquête ou assujetti à un Engagement de conformité volontaire ou à un Avis d’audience).
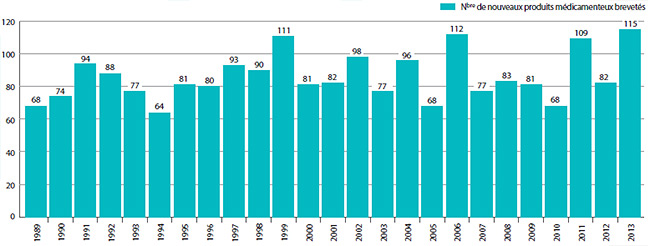
Le graphique 1 illustre le nombre de nouveaux produits médicamenteux brevetés pour usage humain ayant fait l’objet d’un rapport au CEPMB de 1989 à 2013.
Des 115 nouveaux produits médicamenteux brevetés :
- les prix de tous les produits médicamenteux brevetés ont été soumis à un examen :
- les prix de 93 produits médicamenteux brevetés ont été jugés conformes aux Lignes directrices;
- les prix de 7 produits médicamenteux brevetés semblaient excessifs aux termes des Lignes directrices d’un montant ne justifiant pas d’enquête;
- les prix de 15 produits médicamenteux brevetés semblaient excessifs aux termes des Lignes directrices; ainsi, des enquêtes ont été lancées.
Pour la liste complète des 115 nouveaux produits médicamenteux brevetés, y compris l’état d’avancement de l’examen de leur prix, consultez l’Annexe 2.
GRAPHIQUE 1 Nouveaux produits médicamenteux brevetés pour usage humain
Source : CEPMB
Examen des prix des produits médicamenteux brevetés existants pour usage humain en 2013
Aux fins du présent rapport, l’expression « produits médicamenteux brevetés existants » désigne tous les produits médicamenteux brevetés vendus sur le marché canadien et ayant fait l’objet d’un rapport au CEPMB avant le 1er décembre 2013.
Au moment de la rédaction du présent rapport, il y avait 1 228 produits médicamenteux brevetés existants :
- les prix de 1 005 produits médicamenteux brevetés étaient conformes aux Lignes directrices;
- les prix de 157 produits médicamenteux brevetés étaient excessifs aux termes des Lignes directrices d’un montant ne justifiant pas d’enquête;
- les prix de 51 produits médicamenteux brevetés existants faisaient l’objet d’une enquête :
- 9 enquêtes provoquées par le prix de lancement en 2012;
- 42 enquêtes lancées en fonction des prix annuels.
- les prix de 2 produits médicamenteux étaient sous enquête;
- les prix de 13 produits médicamenteux étaient visés par un Engagement de conformité volontaire;
- 1 autre produit médicamenteux fait toujours l’objet d’une audience, même s’il n’était plus breveté en 2013.
Un aperçu de l’état d’avancement de l’examen en 2013 du prix des produits médicamenteux brevetés pour usage humain nouveaux et existants est présenté au tableau 4.
Tableau 4 Produits médicamenteux brevetés pour usage humain vendus au Canada en 2013 – État d’avancement de l’examen du prix en date du 31 mars 2014
|
Nouveaux produits médicamenteux lancés sur le marché en 2013 |
Produits médicamenteux brevetés existants |
Total |
| Total |
115 |
1 228 |
1 343 |
| Conformes aux Lignes directrices |
93 |
1 005 |
1 098 |
| Sous examen |
0 |
2 |
2 |
| Ne justifient pas d’enquête |
7 |
157 |
164 |
| Sous enquête |
15 |
51 |
66 |
| Engagements de conformité volontaire |
0 |
13 |
13 |
| Audiences sur le prix |
0 |
0 |
0 |
Mise à jour : Rapport annuel 2012
- L’examen de l’ensemble des produits médicamenteux brevetés pour usage humain ayant fait l’objet d’un rapport « sous examen » dans le rapport annuel de 2012 est terminé.
- Des 59 enquêtes mentionnées dans le rapport annuel de 2012, 39 d’entre elles se sont soldées par les résultats suivants :
- la fermeture de l’enquête lorsqu’il apparaît que le prix est conforme aux Lignes directrices;
- un Engagement de conformité volontaire par lequel le breveté s’engage à réduire le prix de son produit et à rembourser les recettes excessives au moyen d’un paiement et (ou) d’une réduction du prix d’un autre produit médicamenteux breveté (voir la section « Engagements de conformité volontaire »);
- une audience publique dont l’objet est de déterminer si le prix du produit médicamenteux est ou non excessif, y compris une ordonnance corrective rendue par le Conseil (voir la section «Audiences »).
Produits médicamenteux brevetés en vente libre et produits médicamenteux brevetés pour usage vétérinaire
Le personnel du Conseil fait l’examen des prix des produits médicamenteux brevetés en vente libre et des produits médicamenteux brevetés pour usage vétérinaire à la suite de la réception d’une plainte. Une plainte a été reçue concernant le prix du Vetoryl, vendu au Canada par Vetoquinol Canada Inc.
Conformément au Règlement sur les médicaments brevetés (le Règlement), le breveté a présenté ses données sur les prix et les ventes rétrospectivement jusqu’à la date de première vente en 2009. Les prix des trois concentrations du Vetoryl (10 mg, 30 mg et 60 mg) étaient conformes au Lignes directrices à la date de première vente en 2009. Les prix sont demeurés conformes aux Lignes directrices jusqu’à la fin de 2013. Conformément au Règlement, le breveté fera rapport de ses données sur les prix et les ventes sur une période de deux ans, jusqu’à la fin de 2015. Un tableau sommaire de l’examen du prix peut être consulté sur le site Web du CEPMB.
Demandes de renseignements et plaintes officielles
En 2013, Le CEPMB a reçu de nombreuses demandes de renseignements concernant le statut de conformité de différents produits médicamenteux. Le personnel du Conseil était en mesure de confirmer que les prix des médicaments brevetés en question étaient conformes aux Lignes directrices. Si le prix d’un produit médicamenteux breveté avait été excessif aux termes des Lignes directrices, le personnel du Conseil aurait entamé une enquête.
De plus, le CEPMB a reçu trois plaintes officielles quant aux prix du Differin, du Nexium et du Spiriva. Des enquêtes quant aux prix de ces trois produits médicamenteux brevetés ont été entamées. On a conclu que les prix du Nexium et du Spiriva étaient conformes aux Lignes directrices. L’enquête quant au prix du Differin est en cours.
Si l’on conclut que le prix d’un produit médicamenteux est excessif en vertu des Lignes directrices, le breveté peut soumettre un Engagement de conformité volontaire aux fins d’approbation de la présidente du Conseil. La présidente peut aussi juger que l’intérêt public justifie la tenue d’une audience publique. Dans les deux cas, la décision et les résultats sont affichés sur le site Web du CEPMB.
Engagements de conformité volontaire et audiences
Engagements de conformité volontaire
L’Engagement de conformité volontaire est un engagement écrit par lequel le breveté s’engage à rendre le prix de son produit médicamenteux conforme aux Lignes directrices du Conseil. En vertu des Lignes directrices, les brevetés peuvent soumettre un Engagement de conformité volontaire lorsque le personnel du Conseil détermine, à la suite d’une enquête, que le prix auquel le produit médicamenteux breveté est vendu au Canada est excessif aux termes des Lignes directrices. Il peut également soumettre un Engagement de conformité volontaire après l’émission d’un Avis d’audience.
En 2013, la présidente a approuvé cinq Engagements de conformité volontaire visant 14 produits médicamenteux. En plus de la réduction du prix de certains produits médicamenteux, des recettes excessives totalisant 10 281 679,63 $ ont été remboursées au moyen de paiements versés au gouvernement du Canada.
En 2014, à ce jour, la présidente a accepté un Engagement de conformité volontaire dans l’affaire Copaxone, mettant ainsi fin à cette instance.
Les brevetés doivent s’assurer que les prix de leurs produits médicamenteux brevetés sont conformes aux Lignes directrices du Conseil et ce, au cours de toutes les périodes où les produits médicamenteux relèvent de la compétence du CEPMB.
Tableau 5 Engagements de conformité volontaire en 2013 jusqu’au 30 mai 2014
| Produit médicamenteux breveté |
Usage thérapeutique |
Breveté |
Date d’approbation |
Remboursement des recettes excessives |
| Réduction de prix |
Paiement à la Couronne |
|
1 Le brevet lié à ce médicament est expiré.
2 Au cours de l’exercice 2013-2014, le CEPMB a remboursé à Teva Canada Innovation la somme de 2 801 285,00 (plus les intérêts) suite à l’ordonnance de la Cour fédérale dans sa décision du 14 mai 2013.
|
| Engagements de conformité volontaire en 2013 |
| Novolin® (8 produits médicamenteux) |
Diabète sucré |
Novo Nordisk Canada Inc. |
Avril |
✓ |
6 503 426,81 $ |
| Mavik (1 produit médicamenteux) |
Hypertension |
Abbott Laboratories Limited |
Avril |
✓ |
118 168,48 $ |
| Airomir1 (1 produit médicamenteux) |
Asthma |
Graceway Canada Inc. |
Avril |
|
206 583,48 $ |
| Tactuo (1 produit médicamenteux) |
Acné |
Galderma Canada Inc. |
Avril |
|
Ordonnance du Conseil : 419 468,12 $ |
| Elocom (3 produits médicamenteux) |
Psoriasis et atopic dermatite |
Merck Canada Inc. |
Juillet |
✓ |
3 034 032,74 $ |
| Total |
|
|
|
|
10 281 679,63 $ |
| Engagements de conformité volontaire en 2014 jusqu’au 30 mai |
| Copaxone2 (1 produit médicamenteux) |
Sclérose en plaques |
Teva Canada Innovation G.P.-S.E.N.C. |
Février |
|
Ordonnance du Conseil :248 222,32 $ |
| Total |
|
|
|
|
10 529 901,95 $ |
Audiences
Lorsque le prix d’un médicament breveté semble excessif, le Conseil peut tenir une audience publique et, s’il est démontré que le prix du produit médicamenteux est excessif, rendre une ordonnance obligeant le breveté à réduire le prix de son produit et à rembourser les recettes excessives qu’il a tirées de la vente de son produit à un prix excessif. Les décisions du Conseil peuvent être assujetties à une révision judiciaire devant la Cour fédérale du Canada.
En 2013, le Conseil a rendu des décisions et (ou) des ordonnances pour mener à terme une affaire : Tactuo, sur le prix. En février 2014, le Conseil a aussi mené à terme l’affaire Copaxone.
Le Conseil compte deux affaires dans son calendrier d’audiences : Apotex Inc. et Apo-Salvent exempt de CFC. Les résultats de ces affaires seront examinés à la lumière des décisions de la Cour fédérale dans trois autres affaires : ratio-Salbutamol HFA, ratiopharm et Sandoz, sur des questions relatives à la compétence du Conseil.
Sommaire
En 2013 et jusqu’au 30 mai 2014, des recettes excessives totalisant 10 529 901,95 $ ont été remboursées au moyen de paiements versés au gouvernement du Canada en vertu d’Engagements de conformité volontaire et d’ordonnances du Conseil.
Depuis 1993, 95 Engagements de conformité volontaire ont été approuvés et 26 audiences publiques ont été entamées. Ces mesures ont donné lieu à des réductions de prix et au remboursement des recettes excessives au moyen de réductions supplémentaires de prix ou de paiements versés au gouvernement du Canada. Plus de 147 millions de dollars ont été recueillis en vertu d’Engagements de conformité volontaire et d’ordonnances du Conseil par l’entremise de paiements versés au gouvernement du Canada et (ou) aux clients, dont les hôpitaux et les cliniques médicales.
Affaires interjetées auprès de la Cour fédérale
L’audition de l’affaire de Copaxone (réexamen) (T-586-12) devant la Cour fédérale a eu lieu le 5 février 2013. La Cour a rendu sa décision le 30 avril 2013; elle a accueilli la demande de Teva, a annulé la décision du Conseil rendue le 23 février 2012 et a renvoyé l’affaire à un différent panel d’audience du Conseil pour réexamen. Le nouveau panel a reçu une demande conjointe des parties et a accepté un Engagement de conformité volontaire visant le remboursement des recettes excessives. Une ordonnance mettant fin à cette affaire a été rendue le 14 février 2014.
Trois autres décisions du Conseil ont fait l’objet de révisions judiciaires devant la Cour fédérale : ratio-Salbutamol HFA (T-1058-11; T-1825-11); ratiopharm Inc. (actuellement Teva Canada) (T-1252-11); et Sandoz Canada Inc. (T-1616-12). Les audiences devant la Cour ont été tenues en novembre 2013, et la Cour a rendu ses décisions le 27 mai 2014. La Cour fédérale a fait droit aux demandes de révision judiciaire et a renvoyé les affaires au Conseil avec une directive de statuer que ratiopharm Inc. et Sandoz Canada Inc. ne sont pas des brevetés.
Tableau 6 État d’avancement des audiences devant le Conseil en 2012 jusqu’au 30 mai 2014
| Produit médicamenteux breveté |
Usage thérapeutique |
Breveté |
Date de l’Avis d’audience |
État d’avancement |
| Apo-Salvent exempt de CFC |
Asthme |
Apotex Inc. |
Le 8 juillet 2008 |
En cours |
| Copaxone – Réexamen |
Sclérose en plaques |
Teva Canada |
Nouveau panel nommé en février 2010 |
Décision de la Cour fédérale rendue le 30 avril 2013
Affaire renvoyée au nouveau panel pour réexamen
Ordonnance du Conseil rendue le 14 février 2014
Audience conclue suite à l’acceptation d’un Engagement de conformité volontaire – Recettes excessives remboursées : 248 222,32 $
|
| ratio-Salbutamol HFA |
Asthme |
ratiopharm Inc. (actuellement Teva Canada) |
Le 18 juillet 2008 |
Décision du Conseil rendue le 27 mai 2011
Ordonnance du Conseil rendue le 17 octobre 2011
Révision judiciaire entendue par la Cour fédérale du 4 au 6 novembre 2013; décision rendue le 27 mai 2014
|
| Tactuo |
Acné |
Galderma Canada Inc. |
Le 26 septembre 2012 |
Ordonnance du Conseil rendue le 24 avril 2013 Audience conclue suite à l’acceptation d’un Engagement de conformité volontaire – Recettes excessives remboursées : 419 468,12 $ |
| Breveté |
Objet de l’audience |
Date de l’Avis de demande |
État d’avancement |
| Apotex Inc. |
Défaut de soumettre ses rapports (questions relatives à la compétence du Conseil) |
Le 3 mars 2008 |
En cours |
| ratiopharm Inc. (actuellement Teva Canada) |
Défaut de soumettre ses rapports (questions relatives à la compétence du Conseil) |
Le 28 août 2008 |
Ordonnance du Conseil rendue le 30 juin 2011; modifiée le 17 octobre 2011
Révision judiciaire entendue par la Cour fédérale du 4 au 6 novembre 2013; décision rendue le 27 mai 2014
|
| Sandoz Canada Inc. |
Défaut de soumettre ses rapports (questions relatives à la compétence du Conseil) |
Le 8 mars 2010 |
Ordonnance du Conseil rendue le 1er août 2012; rendue de nouveau le 1er octobre 2012
Révision judiciaire entendue par la Cour fédérale les 19 et 20 novembre 2013; décision rendue le 27 mai 2014
|
Réduction du fardeau réglementaire
En cadrant avec le Plan d’action pour la réduction du fardeau administratif, le CEPMB s’est engagé à revoir son processus d’examen des prix afin de cerner des moyens possibles d’accroître l’efficience et de réduire le fardeau réglementaire imposé aux brevetés sans porter atteinte à son mandat visant à protéger les consommateurs.
À ce jour, l’examen s’est focalisé sur deux initiatives, notamment :
- la méthodologie de rajustement du prix du médicament pour tenir compte des variations de l’indice des prix à la consommation (IPC);
- la faisabilité d’une transition vers un seul dépôt annuel par les brevetés des données relatives aux médicaments brevetés existants.
Suite à une consultation au sujet de ces initiatives, le Conseil a approuvé la mise en oeuvre d’une nouvelle méthodologie de rajustement du prix selon l’IPC retardé à compter de 2015. La nouvelle approche utilisera l’IPC réel de 2013 pour déterminer les facteurs de rajustement du prix selon l’IPC pour 2015. L’augmentation maximale du prix permise sera annoncée sur une base annuelle dans le numéro de janvier de La Nouvelle.
On tiendra compte de cette modification, lorsqu’elle entrera en vigueur, dans le cadre la mise à jour de juin 2014 du Compendium des politiques, des Lignes directrices et des procédures.
Suite aux commentaires reçus de la part des intervenants concernant les modifications proposées au Règlement sur les médicaments brevetés relativement à la fréquence de dépôt des renseignements sur les prix et les ventes, on a annoncé une consultation officielle conformément au Processus fédéral d’élaboration de règlements par l’entremise du Cabinet et d’une publication dans la Gazette du Canada.
De plus, le CEPMB a élaboré et publié de nouvelles normes de service afin de préciser les attentes et d’accroître la prévisibilité du système réglementaire fédéral. Les normes de service suivantes ont été élaborées et publiées sur le site Web du CEPMB :
- Norme de service pour l’examen scientifique des nouveaux produits médicamenteux brevetés
- Norme de service pour l’examen du prix des nouveaux produits médicamenteux brevetés
- Norme de service pour l’examen du prix des produits médicamenteux brevetés existants
Les mesures et modifications proposées visent à réduire le fardeau réglementaire imposé aux brevetés, et à accroître l’efficience du processus d’examen du prix tout en protégeant le mandat de base du CEPMB visant la protection des consommateurs.
Tendances relatives aux ventes des produits médicamenteux brevetés
Le CEPMB fait rapport des tendances observées au chapitre des ventes de produits pharmaceutiques et des prix de tous les médicaments, ainsi que des dépenses de recherche-développement des brevetés. De plus, il dirige des études et mène des analyses portant sur diverses questions relatives aux prix et aux coûts des produits pharmaceutiques.
En vertu du Règlement sur les médicaments brevetés (le Règlement), les brevetés doivent faire rapport au CEPMB de leurs ventes de produits médicamenteux brevetés au Canada, à savoir les quantités vendues et les recettes nettes tirées des ventes de chaque produit médicamenteux, par catégorie de clients et par province ou territoire. Le CEPMB utilise ces éléments d’information dans ses analyses des tendances aux niveaux des ventes, des prix et de l’utilisation faite des produits médicamenteux brevetés1. La présente section donne les résultats statistiques clés de cette analyse.
Ventes et prix
La population canadienne consacre aujourd’hui une partie beaucoup plus grande de son budget à l’achat de produits médicamenteux qu’elle ne le faisait il y a une dizaine d’années; toutefois, il est important de préciser qu’une augmentation des dépenses en produits médicamenteux n’est pas nécessairement attribuable à une augmentation des prix. Par exemple, selon les rapports annuels des années 1995 à 2003, la valeur des ventes de produits médicamenteux brevetés a augmenté de plus de 10 % par année, alors que les taux moyens de variation des prix n’atteignaient même pas 1 %. Dans ces cas, ce sont le volume et la composition de l’utilisation faite des produits médicamenteux qui sont à l’origine de la croissance de la valeur des ventes.
Différents facteurs peuvent être à l’origine de tels changements, dont les suivants :
- augmentation de la population totale;
- variations de la composition démographique de la population (p. ex. vieillissement de la population et, partant, une plus grande incidence de problèmes de santé);
- augmentation de l’incidence des problèmes de santé nécessitant une pharmacothérapie;
- nouvelles pratiques d’ordonnance des médecins (p. ex. tendance à prescrire des nouveaux produits médicamenteux plus onéreux pour traiter une condition qui était jusque-là traitée avec des produits existants souvent vendus à moindre prix, ou ordonnance de concentrations plus fortes ou plus fréquentes);
- recours plus régulier à des pharmacothérapies en remplacement d’autres formes de traitement;
- recours à de nouveaux produits médicamenteux pour traiter des conditions pour lesquelles il n’existait pas encore un traitement efficace.
Tendances observées au niveau des ventes
Le tableau 7 présente la valeur des ventes par les brevetés au Canada des produits médicamenteux brevetés pour les années 1990 à 2013. Les ventes de produits médicamenteux brevetés ont totalisé 13,6 milliards de dollars en 2013, soit 6,5 % de plus qu’en 2012, où ce montant totalisait 12,8 milliards de dollars. En guise de comparaison, la croissance annuelle des ventes de produits médicamenteux brevetés était de 27,0 % en 1999, et s’est maintenue à deux chiffres jusqu’en 2003.
La dernière colonne du tableau 7 présente la valeur des ventes des produits médicamenteux brevetés exprimée en pourcentage de la valeur des ventes de tous les produits médicamenteux brevetés et non brevetés. Entre 1990 et 2003, le pourcentage de la valeur des ventes est passé respectivement de 43,2 % à un sommet de 72,7 %. En général, ce pourcentage a reculé depuis 2003, avec un léger redressement de la tendance au cours des trois dernières années, ce qui signifie que les ventes des produits médicamenteux de marque non brevetés et des produits médicamenteux génériques ont augmenté davantage au cours de cette période que celles de produits médicamenteux brevetés.
Tableau 7 Ventes de produits médicamenteux brevetés, 1990-2013
| Année |
Produits médicamenteux brevetés |
Valeur des ventes de produits médicamenteux brevetés exprimée en pourcentage de la valeur des ventes de tous les médicaments (%)* |
| Ventes (milliards $) |
Variation (%) |
|
* Le dénominateur dans ce ratio comprend la valeur des ventes des produits médicamenteux de marque brevetés, des produits médicamenteux de marque non brevetés, et des produits médicamenteux génériques. L’estimation de la valeur totale des ventes utilisée pour calculer le ratio à compter de 2005 se fonde sur les données tirées de la base de données MIDAS d’IMS Health. Pour les années antérieures, les données d’IMS Health n’ont été utilisées que pour calculer la valeur des ventes des produits médicamenteux génériques. Quant à la valeur des ventes des produits médicamenteux de marque non brevetés, elle était alors estimée à l’aide des données fournies par les brevetés. Pour mettre un terme aux anomalies attribuables aux variations annuelles de la liste des brevetés, le CEPMB n’utilise plus cette approche. Les ratios rapportés pour les années avant 2005 gonflaient légèrement la part des produits médicamenteux brevetés. Ce léger écart n’invalide toutefois pas la forte tendance à la hausse observée pour les années 1990 à 2003.
Sources : CEPMB et MIDAS©, 2005-2013, IMS Health Incorporated ou sociétés affiliées. Tous droits réservés2
|
| 2013 |
13,6
|
6,5
|
61,8
|
| 2012 |
12,8
|
-0,3
|
59,3
|
| 2011 |
12,9
|
4,0
|
58,6
|
| 2010 |
12,4
|
-3,8
|
56,0
|
| 2009 |
12,9
|
2,4
|
59,2
|
| 2008 |
12,6
|
2,4
|
61,7
|
| 2007 |
12,3
|
3,4
|
63,2
|
| 2006 |
11,9
|
3,5
|
67,8
|
| 2005 |
11,5
|
4,5
|
70,6
|
| 2004 |
11,0
|
7,8
|
72,2
|
| 2003 |
10,2
|
14,3
|
72,7
|
| 2002 |
8,9
|
17,5
|
67,4
|
| 2001 |
7,6
|
18,9
|
65,0
|
| 2000 |
6,3
|
16,7
|
63,0
|
| 1999 |
5,4
|
27,0
|
61,0
|
| 1998 |
4,3
|
18,9
|
55,1
|
| 1997 |
3,7
|
22,6
|
52,3
|
| 1996 |
3,0
|
12,8
|
45,0
|
| 1995 |
2,6
|
10,8
|
43,9
|
| 1994 |
2,4
|
-2,1
|
40,7
|
| 1993 |
2,4
|
9,4
|
44,4
|
| 1992 |
2,2
|
14,0
|
43,8
|
| 1991 |
2,0
|
13,1
|
43,2
|
| 1990 |
1,7
|
–
|
43,2
|
Facteurs à la source de la croissance des ventes
Le tableau 8 décompose en différents éléments la croissance des ventes enregistrée entre 2012 et 2013. Ces éléments sont les suivants :
- produits médicamenteux brevetés dont le brevet est arrivé à échéance ou dont le brevet a été cédé au domaine public (« effet du retrait du médicament »);
- produits médicamenteux brevetés lancés sur le marché canadien en 2013 (« effet du nouveau médicament »);
- variations des prix des produits médicamenteux brevetés vendus au Canada en 2012 et en 2013 (« effet du prix »);
- écarts de quantités vendues de ces produits médicamenteux en 2012 et en 2013 (« effet du volume »);
- interactions des variations de prix et de quantité (« effets croisés »).
La première rangée du tableau 8 présente les incidences d’après leur valeur monétaire et la deuxième rangée les présente au moyen de la variation des ventes en 2013 par rapport à 2012. Pour des fins de comparaison, la troisième rangée présente les incidences avec les taux moyens annuels de variation des ventes pour la période de 2008 à 20123.
Les résultats de ce tableau révèlent que l’augmentation du volume des ventes des nouveaux produits médicamenteux et des produits médicamenteux existants était à l’origine de la croissance observée au niveau de ventes en 2013 par rapport à 2012. Pris seul, l’effet du volume était assez important pour atténuer la forte incidence négative de l’effet du retrait du médicament.
Tableau 8 Décomposition des variations au chapitre des ventes de produits médicamenteux brevetés
| |
Variation totale |
Effet du retrait du médicament |
Effet du nouveau médicament |
Effet du prix |
Effet du volume |
Effets croisés |
| Source : CEPMB |
| Incidence sur les ventes, 2013/2012 (millions $) |
859,81 |
-268,20 |
285,86 |
149,87 |
790,12 |
-97,84 |
| Proportion de la variation totale, 2013/2012 (%) |
100,00 |
-31,19 |
33,25 |
17,43 |
91,90 |
-11,38 |
| Proportion moyenne de la variation totale, 2008-2012 (%) |
100,00 |
180,69 |
-136,98 |
-20,49 |
33,18 |
43,61 |
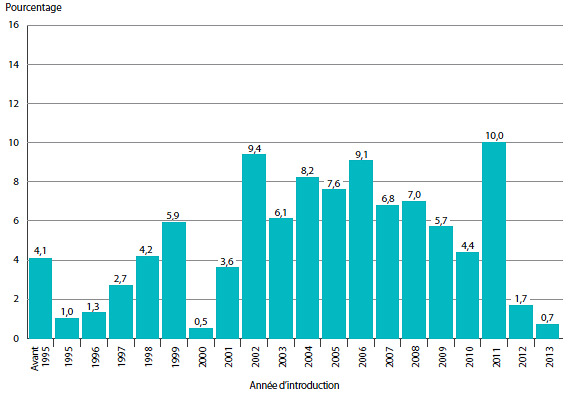
Le graphique 2 présente pour 2013 une ventilation des ventes de produits médicamenteux brevetés selon l’année de leur première vente au Canada. Au cours de la dernière partie des années 1990 et du début des années 2000, la croissance des ventes a été associée à une succession de nouveaux médicaments « vedettes » qui ont donné lieu à des volumes de ventes très élevés. Malgré l’expiration récente des brevets (chute des brevets), ces produits représentent toujours un pourcentage important des ventes en 2013. Depuis le début des années 2000, les changements observés dans l’environnement pharmaceutique canadien ainsi que la diminution du taux de mise en marché de nouveaux produits médicamenteux de grande vente ont ralenti la croissance.
GRAPHIQUE 2 Pourcentage des ventes de produits médicamenteux brevetés selon l'année de lancement, 2013
Source : CEPMB
Ventes selon la catégorie thérapeutique
Pour ses analyses de prix au niveau du groupe thérapeutique, le CEPMB classe généralement les produits médicamenteux à l’aide du Système de classification anatomique, thérapeutique, chimique (ATC) de l’Organisation mondiale de la Santé (OMS). Ce système hiérarchique classe les produits médicamenteux selon leur utilisation thérapeutique principale et leur composition chimique. Au premier niveau de ce système, à savoir au niveau 1, le système ATC classe les médicaments selon la partie de l’anatomie à laquelle ils sont principalement associés.
Le tableau 9 ventile les ventes des produits médicamenteux brevetés effectuées au Canada en 2013 selon le groupe thérapeutique principal, à savoir le premier niveau de la classification ATC. Il présente les ventes effectuées en 2013 dans les différents groupes de produits médicamenteux, leur part de l’ensemble des ventes, ainsi que le taux d’augmentation de la valeur de leurs ventes par rapport à 2012. Les valeurs présentées dans la dernière colonne correspondent à la composante de la croissance de l’ensemble des ventes attribuables aux produits médicamenteux du groupe4. La mesure ainsi obtenue permet de dégager que ce sont les agents antinéoplasiques et immunomodulateurs qui ont le plus contribué à la croissance de la valeur des ventes. Il convient de noter le recul important des ventes de médicaments agissant sur le système cardiovasculaire et, accessoirement, sur le système génito-urinaire et hormones sexuelles.
Tableau 9 Ventes des produits médicamenteux brevetés selon leur groupe thérapeutique principal, 2013
| Groupe thérapeutique |
Ventes en 2013
(millions $) |
Part de ventes
en 2013 (%) |
Croissance :
2013/2012
(millions $) |
Croissance :
2013/2012 (%) |
Incidence sur la variation
des dépenses (%) |
|
* Pour des raisons de confidentialité, les données sur ces deux groupes ont été combinées.
Source : CEPMB
|
| A : Tube digestif et métabolisme |
1 425,0 |
10,4 |
160,8 |
12,7 |
19,3 |
| B : Sang et organes sanguinoformateurs |
784,3 |
5,7 |
-7,3 |
-0,9 |
-0,9 |
| C : Système cardiovasculaire |
1 023,8 |
7,5 |
-319,0 |
-23,8 |
-38,4 |
| D : Produits dermatologiques |
130,0 |
1,0 |
15,2 |
13,2 |
1,8 |
| G : Système génito-urinaire et hormones sexuelles |
509,7 |
3,7 |
-63,3 |
-11,0 |
-7,6 |
| H : Préparations hormonales systémiques |
62,4 |
0,5 |
7,1 |
12,7 |
0,8 |
J : Antiinfectieux généraux pour usage systémique;
P : Produits antiparasitaires* |
1 515,6 |
11,1 |
57,1 |
3,9 |
6,9 |
L : Agents antinéoplastiques et
agents immunomodulateurs |
3 947,6 |
28,9 |
646,0 |
19,6 |
77,7 |
| M : Système musculo-squelettaire |
431,4 |
3,2 |
11,0 |
2,6 |
1,3 |
| N : Système nerveux |
1 853,8 |
13,6 |
-46,5 |
-2,4 |
-5,6 |
| R : Système respiratoire |
1 274,9 |
9,3 |
212,1 |
20,0 |
25,5 |
| S : Organes sensoriels |
643,1 |
4,7 |
137,8 |
27,3 |
16,6 |
| V : Divers |
75,1 |
0,5 |
20,3 |
36,9 |
2,4 |
Tendances observées au niveau des prix
Le CEPMB utilise l’indice des prix des médicaments brevetés (IPMB) pour faire le suivi des tendances des prix des produits médicamenteux brevetés. L’IPMB mesure la variation moyenne des prix auxquels les brevetés vendent leurs produits médicamenteux brevetés sur le marché canadien (prix départ-usine) par rapport à l’année précédente. L’indice est calculé à l’aide de la formule qui correspond à la moyenne de la variation des prix au niveau du produit médicamenteux pondérée en fonction des ventes5. La méthodologie utilisée rappelle celle qu’utilise Statistique Canada pour compiler l’indice des prix à la consommation (IPC). L’IPMB est fondée sur l’information sur les prix moyens par transaction et sur les ventes pour une période de six mois, dont les brevetés font rapport au Conseil.
Il est important de bien comprendre la relation théorique qui existe entre l’IPMB et les coûts des produits médicamenteux. L’IPMB ne mesure pas les effets des changements de l’utilisation faite des produits médicamenteux. Cette mesure est prise par un autre indice appelé l’indice du volume des ventes de médicaments brevetés – l’IVVMB (voir la section « Utilisation faite des produits médicamenteux brevetés »). L’IPMB ne mesure pas non plus l’incidence sur les coûts des nouvelles habitudes d’ordonnance des médecins ou de l’arrivée sur le marché de nouveaux produits médicamenteux. L’IPMB est conçu pour isoler la composante de variation des ventes attribuable aux variations des prix des produits médicamenteux brevetés.
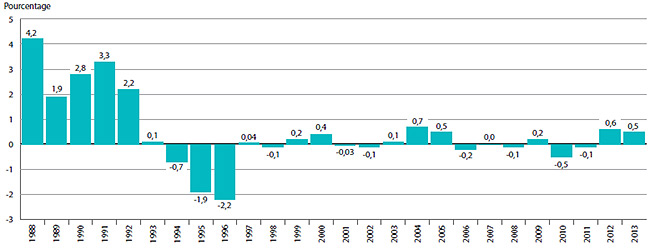
Le graphique 3 présente le taux annuel de variation de l’IPMB pour les années 1988 à 2013. Selon la mesure prise par l’IPMB, les prix départ-usine des produits médicamenteux brevetés ont augmenté (0,5 %), en moyenne, en 2013 par rapport à 2012.
GRAPHIQUE 3 Taux annuel de variation de l’indice des prix des médicaments brevetés (IPMB), 1988-2013
Source : CEPMB
La Loi sur les brevets prévoit que le CEPMB doit tenir compte des variations de l’IPC, entre autres facteurs, lorsqu’il est appelé à déterminer si le prix d’un médicament breveté est ou non excessif. Le graphique 4 présente les variations annuelles de l’IPMB par rapport aux variations de l’IPC pour les mêmes années. L’inflation générale des prix, mesurée au moyen de l’IPC, a été supérieure à l’augmentation moyenne des prix des produits médicamenteux brevetés presque chaque année depuis 1988. En 2013, l’IPC a augmenté de 0,9 %, alors que l’IPMB a légèrement augmenté, en moyenne, de 0,5 %.
GRAPHIQUE 4 Taux annuel de variation de l’indice des prix des médicaments brevetés (IPMB) et de l’indice des prix à la consommation (IPC), 1988-2013
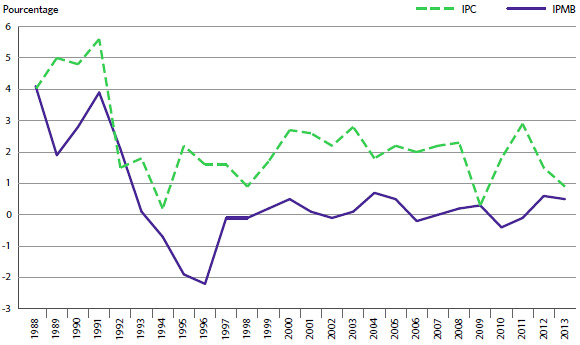
Source : CEPMB, Statistique Canada
Il n’est pas surprenant que l’IPMB ait rarement augmenté au même rythme que l’IPC. Les Lignes directrices du CEPMB prévoient en effet que les prix des produits médicamenteux brevetés ne peuvent augmenter davantage que le taux moyen d’augmentation de l’indice des prix à la consommation calculé sur une période de trois ans. (Les Lignes directrices limitent également les augmentations annuelles de prix à une fois et demie le taux d’inflation calculé à l’aide de l’IPC.) Cette exigence a pour effet de limiter les augmentations des prix des produits à celles de l’IPC sur une période de trois années6. En pratique, les variations de l’IPC n’atteignent jamais cette limite, étant donné qu’un grand nombre de brevetés n’augmentent pas les prix de leurs médicaments dans toute la mesure autorisée par les Lignes directrices, ou lorsqu’ils ne les réduisent pas.
Variation du prix selon le groupe thérapeutique
Le tableau 10 présente les taux moyens de variation des prix des produits médicamenteux brevetés selon leur groupe thérapeutique principal. Ce tableau a été établi en appliquant la méthode de calcul de l’IPMB aux données sur les prix des différents produits médicamenteux brevetés ventilées selon le groupe thérapeutique principal (niveau 1 de la classification ATC). La dernière colonne du tableau présente le résultat de la décomposition de la variation globale de l’IPMB où chaque entrée représente la composante attribuable aux produits médicamenteux du groupe thérapeutique correspondant. Selon cette mesure, la légère augmentation de l’IPMB (0,5 %) indique que les prix des produits médicamenteux des différentes catégories thérapeutiques sont relativement stables. Veuillez noter que toutes les catégories thérapeutiques ont affiché, en moyenne, un taux de variation des prix inférieur au taux d’inflation de l’IPC.7.
Tableau 10 Variation de l’indice des prix des médicaments brevetés (IPMB) selon le groupe thérapeutique principal, 2013
| Groupe thérapeutique |
Pourcentage des
ventes en 2013 (%) |
Variation des prix de
2012 à 2013 (%) |
Contribution :
variation de l'IPMB (%) |
|
* Pour des raisons de confidentialité, les données sur ces deux groupes ont été combinées.
** Il se peut que les valeurs n’équivaillent pas à 100,0 en raison de l’arrondissement.
Source : CEPMB
|
| A : Tube digestif et métabolisme |
10,4 |
5,5 |
0,6 |
| B : Sang et organes sanguinoformateurs |
5,7 |
-5,6 |
-0,3 |
| C : Système cardiovasculaire |
7,5 |
0,6 |
0,0 |
| D : Produits dermatologiques |
1,0 |
0,0 |
0,0 |
| G : Système génito-urinaire et hormones sexuelles |
3,7 |
-2,2 |
-0,1 |
| H : Préparations hormonales systémiques |
0,5 |
2,0 |
0,0 |
J : Antiinfectieux généraux pour usage systémique;
P : Produits antiparasitaires* |
11,1 |
-0,9 |
-0,1 |
| L : Agents antinéoplastiques et agents immunomodulate |
urs 28,9 |
0,3 |
0,1 |
| M : Système musculo-squelettaire |
3,2 |
2,4 |
0,1 |
| N : Système nerveux |
13,6 |
1,0 |
0,1 |
| R : Système respiratoire |
9,3 |
0,8 |
0,1 |
| S : Organes sensoriels |
4,7 |
1,1 |
0,1 |
| V : Divers |
0,5 |
-0,6 |
0,0 |
| Tous les groupes thérapeutiques |
100,0** |
0,5 |
0,5 |
Variation des prix selon la catégorie de clients
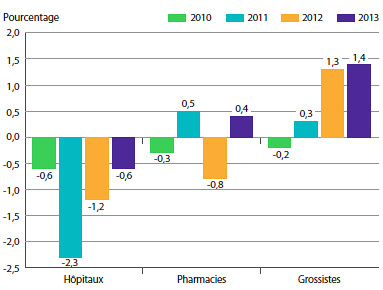
Le graphique 5 présente les taux moyens de variation des prix selon la catégorie de clients8. Ces taux ont été obtenus en appliquant la méthodologie de l’IPMB aux données sur les ventes faites aux hôpitaux, aux pharmacies et aux grossistes9. Pour 2013, les taux de variation des prix étaient de 0,6 %, 0,4 % et 1,4 %, respectivement.
GRAPHIQUE 5 Taux annuel de variation de l’indice des prix des médicaments brevetés (IPMB) selon la catégorie de clients, 2010-2013
Source : CEPMB
Variation des prix selon la province ou le territoire
Le graphique 6 présente les taux moyens de variation des prix des produits médicamenteux brevetés selon la province ou le territoire. Ces taux ont été obtenus en appliquant la méthodologie du calcul de l’IPMB aux données sur les prix ventilées selon la province ou le territoire dans lequel les ventes ont été effectuées. Ces résultats révèlent que, entre 2012 et 2013, les prix des produits médicamenteux brevetés aux T.N.-O. ont reculé, en moyenne. Le Nouveau-Brunswick a connu la plus importante augmentation moyenne des prix (3,6 %).
GRAPHIQUE 6 Taux annuel de variation des prix par province ou territoire*, par catégorie de clients**, 2013
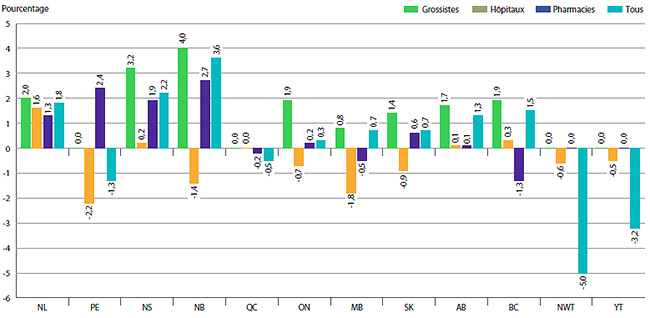
* Les valeurs pour le Nunavut sont comprises dans celles des Territoires du Nord-Ouest (T.N.-O.).
** Les résultats pour la catégorie « Tous » dans le graphique 6 comprennent ceux de la catégorie « Autre ».
Source : CEPMB
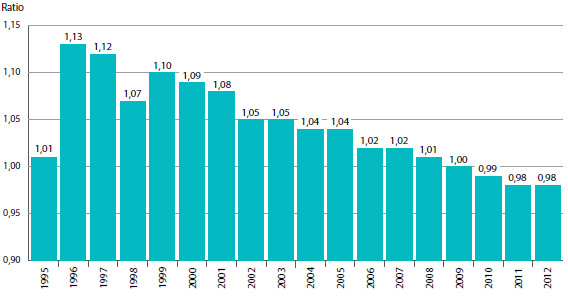
Variation du prix d'un produit médicamenteux breveté dans les années qui suivent son lancement sur le marché canadien
Le prix d’un produit médicamenteux breveté varie-t-il beaucoup au cours des années qui suivent son lancement sur le marché canadien? Le graphique 7 répond à cette question en présentant le ratio moyen des prix de vente des produits médicamenteux en 2013 par rapport aux prix auxquels ils ont été offerts au moment de leur lancement sur le marché canadien.
Les résultats présentés dans le graphique 7 ne révèlent pas une tendance à la hausse ou à la baisse des prix après la période de lancement des produits médicamenteux sur le marché canadien. En 2013, le prix moyen d’un produit médicamenteux breveté se situait dans une marge de quelques points de son prix de lancement, et ce, pour toute année de lancement du produit médicamenteux sur le marché canadien10.
GRAPHIQUE 7 Ratio moyen du prix de 2013 par rapport au prix de lancement, par année de lancement
Source : CEPMB
Variation des prix selon le pays
La Loi et son Règlement obligent les brevetés à faire rapport au CEPMB des prix de leurs produits médicamenteux brevetés accessibles au public qu’ils pratiquent dans les sept pays de comparaison nommés dans le Règlement. Ces pays sont la France, l’Allemagne, l’Italie, la Suède, la Suisse, le Royaume-Uni et les États-Unis.
Le CEPMB utilise ces données aux fins suivantes :
- pour effectuer ses comparaisons des prix internationaux prévues dans les Lignes directrices;
- pour comparer les prix des produits médicamenteux pratiqués au Canada avec les prix pratiqués dans d’autres pays.
Le graphique 8 présente les taux moyens annuels de variation des prix pour le Canada et pour les sept pays de comparaison. Ces valeurs ont été obtenues en appliquant la méthodologie de l’IPMB (avec pondération pour tenir compte des tendances des ventes au Canada) aux données sur les prix pratiqués dans les différents pays de comparaison soumises par les brevetés. À titre d’information, les résultats pour les États Unis sont fondés sur des prix moyens qui tiennent compte des prix de la Classification fédérale des approvisionnements (US Federal Supply Schedule ou FSS)11.
Selon le graphique 8, les prix des produits médicamenteux brevetés aux États-Unis ont augmenté en 2013 d’un taux moyen de 11,2 %. Les augmentations de prix ont été beaucoup plus modestes au Royaume-Uni, alors que les prix en France, en Italie, en Suisse et en Suède ont enregistré un recul.
GRAPHIQUE 8 Taux moyens annuels de variation des prix pratiqués au Canada et dans les pays de comparaison, 2013
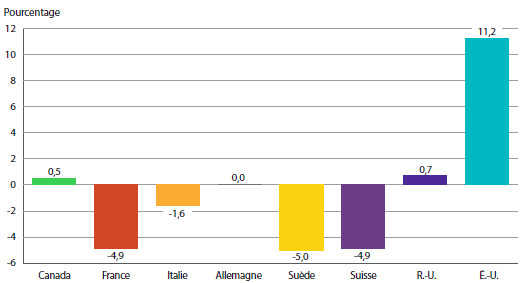
Source : CEPMB
Comparaison des prix pratiqués au Canada avec ceux pratiqués dans les pays de comparaison
Les tableaux 11 et 12 présentent des statistiques détaillées qui permettent de comparer les prix des produits médicamenteux pratiqués dans les sept pays de comparaison avec ceux pratiqués au Canada. Chaque tableau présente deux séries de ratios de prix moyens. Ils sont différents l’un de l’autre selon la méthode de conversion en équivalents dollars canadiens des prix exprimés dans la devise des différents pays. Les deux tableaux présentent également le nombre de produits médicamenteux (DIN) et le volume des ventes couverts par chaque ratio de prix12.
Les ratios de prix moyens donnés dans les tableaux 11 et 12 sont des moyennes d’arithmétique pondérées de ratios de prix obtenues pour des produits médicamenteux individuels, dont la pondération est fondée sur les tendances des ventes au Canada. Les ratios moyens de prix interprétés de cette façon donnent des réponses exactes aux questions comme celle-ci :
« Combien les Canadiens auraient-ils payé, en plus ou en moins, leurs produits médicamenteux brevetés en 2013 s’ils les avaient achetés aux prix pratiqués dans le pays X? »
Par exemple, vous pouvez voir dans le tableau 11 que le ratio du prix moyen en France par rapport au prix moyen au Canada calculé avec la moyenne arithmétique est de 0,72 pour 2013. Ce ratio signifie que les Canadiens auraient payé leurs médicaments brevetés 28 % de moins en 2012 s’ils avaient pu les acheter aux prix pratiqués en France.
Pendant bon nombre d’années, le CEPMB a fait rapport des ratios des prix moyens pratiqués dans les pays de comparaison par rapport aux prix moyens pratiqués au Canada après avoir converti les prix pratiqués dans les pays de comparaison en équivalents dollars canadiens à l’aide des moyennes des taux de change (ou, plus précisément, les moyennes mobiles des taux de change sur une période de 36 mois que le CEPMB utilise généralement lorsqu’il applique ses Lignes directrices). Le tableau 11 compare également les ratios des prix des produits médicamenteux dans les pays de comparaison par rapport à leurs prix au Canada, après avoir converti la devise à l’aide de la parité des pouvoirs d’achat. Le taux de parité des pouvoirs d’achat représente le coût de la vie relatif dans ces deux pays exprimé dans leurs devises respectives. En pratique, le coût de la vie est calculé à l’aide d’un panier de produits et de services aux prix courants.
Étant donné que les taux de parité des pouvoirs d’achat représentent le coût de la vie dans chacun des pays, ils constituent un moyen simple de rajuster les prix pour tenir compte des différences de revenus et d’autres valeurs monétaires dans la comparaison des prix pratiqués dans les différents pays. Appliqués au calcul des ratios moyens des prix pratiqués dans les pays de comparaison par rapport aux prix pratiqués au Canada, les taux de parité des pouvoirs d’achat fournissent des statistiques qui nous permettent de répondre à des questions comme celle-ci :
« Dans quelle mesure les Canadiens auraient-ils dû sabrer dans leur consommation de biens et de services pour acheter des produits médicamenteux brevetés, ou encore, auraient-ils pu augmenter leur consommation de biens et de services si, en 2013, ils avaient vécu et acheté leurs produits médicamenteux brevetés dans le pays X? »
On ne peut répondre à telle question en limitant la comparaison aux prix des produits médicamenteux. Il faut en effet calculer ce que chaque prix représente en termes de biens et de services sacrifiés. C’est précisément ce que permettent de faire les parités des pouvoirs d’achat.
Comparaisons bilatérales des prix
Le tableau 11 compare les prix pratiqués dans les sept pays de comparaison avec ceux pratiqués au Canada. D’après les résultats de la conversion des différentes devises aux taux de change du marché, les prix des produits médicamenteux brevetés pratiqués au Canada se situent habituellement dans la gamme des prix observés parmi les pays de comparaison. Les prix en France, en Italie, au Royaume-Uni, en Suède et en Suisse étaient beaucoup moins élevés qu’au Canada, alors que ceux en Allemagne étaient un peu plus élevés. Comme dans les années antérieures, les prix déclarés étaient beaucoup plus élevés aux États Unis qu’au Canada, et que dans les autres pays de comparaison.
Tableau 11 Ratios moyens des prix pratiqués dans les pays de comparaison par rapport aux prix pratiqués au Canada, comparaisons bilatérales, 2013
| |
Canada |
France |
Italie |
Allemagne |
Suède |
Suisse |
Royaume- Uni |
États-Unis |
| Source : CEPMB |
| Taux de change du marché |
| Ratio moyen des prix en 2013 |
1,00 |
0,72 |
0,79 |
1,04 |
0,90 |
0,95 |
0,77 |
2,07 |
| Average price ratio 2012 |
1,00 |
0,76 |
0,80 |
1,11 |
0,90 |
1,01 |
0,78 |
2,02 |
| Parités des pouvoirs d’achat |
| Ratio moyen des prix en 2013 |
1,00 |
0,78 |
0,97 |
1,23 |
0,85 |
0,78 |
0,86 |
2,53 |
| Ratio moyen des prix en2012 |
1,00 |
0,79 |
0,91 |
1,24 |
0,84 |
0,82 |
0,89 |
2,42 |
| Nombre de produits médicamenteux brevetés |
1,306 |
760 |
772 |
919 |
903 |
847 |
913 |
1088 |
| Ventes (millions $) |
13 676,49 |
11 062,06 |
10 704,40 |
11 832,60 |
11 575,50 |
11 446,00 |
11 596,30 |
12 811,50 |
Il importe de prendre note qu’il n’est pas toujours possible de trouver un prix correspondant dans chaque pays de comparaison pour chacun des produits médicamenteux brevetés vendus au Canada. Le tableau 11 illustre la fréquence de disponibilité d’un prix de comparaison pratiqué dans chacun des pays de comparaison. Par exemple, parmi les 1 306 produits médicamenteux brevetés assujettis à la
compétence du CEPMB en 2013, un prix départ-usine publiquement disponible n’était disponible pour la France que dans 58 % des cas, alors que le même chiffre pour les États-Unis s’élevait à 83 %, de loin le taux le plus élevé. Vu le caractère intégré de la chaîne d’approvisionnement du Canada et des États Unis, il arrive souvent que les États-Unis constituent le seul pays pour lequel un prix de comparaison est disponible relativement à un produit vendu au Canada. Dans tel cas, ce prix est considéré comm étant le prix international médian pour les fins de la méthodologie du CEPMB.
Les ratios moyens de prix obtenus suite à la conversion des devises aux taux de parité des pouvoirs d’achat présentent des différences entre le Canada et les pays de comparaison. Si l’on tient compte des différences du coût de la vie dans les sept pays de comparaison, le Canada se présente comme le pays où les coûts de consommation pour les produits médicamenteux brevetés sont les plus élevés. En effet, ils donnent à penser que les Canadiens ont dû sacrifier en 2013 un taux plus élevé de leur pouvoir d’achat pour se procurer des médicaments brevetés que n’ont dû le faire les consommateurs des pays de comparaison, exclusion faite des consommateurs de l’Allemagne et des États-Unis.
Le graphique 9 présente ces résultats dans une perspective historique. En 2005, les prix au Canada étaient généralement égaux ou inférieurs aux prix correspondants pratiqués dans tous les pays de comparaison, sauf en Italie. En 2013, les prix au Canada étaient généralement plus élevés que ceux pratiqués au Royaume-Uni, en France et en Italie, et un peu plus élevés que ceux pratiqués en Suède et en Suisse.
GRAPHIQUE 9 Ratios moyens des prix pratiqués dans les pays de comparaison par rapport aux prix pratiqués au Canada : 2005, 2013
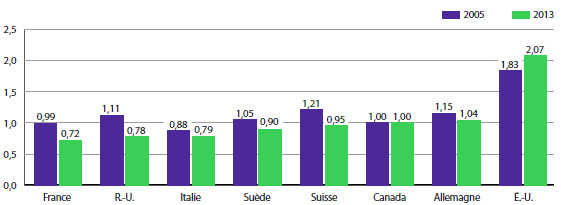
Source : CEPMB
Comparaisons multilatérales des prix
Le tableau 12 présente les ratios moyens des prix pratiqués dans les pays de comparaison par rapport aux prix pratiqués au Canada, et ce, pour plusieurs mesures multilatérales des prix pratiqués dans les pays de comparaison. Le « prix international médian » correspond à la médiane des prix de vente des produits médicamenteux dans les sept pays de comparaison. D’autres ratios de prix multilatéraux comparent la moyenne minimale, maximale et simple des prix pratiqués dans les pays de comparaison par rapport aux prix au Canada.
Tableau 12 Ratios moyens des prix pratiqués dans les pays de comparaison par rapport aux prix pratiqués au Canada, comparaisons multilatérales, 2013
| |
Médiane |
Minimum |
Maximum |
Moyenne |
| Source : CEPMB |
| Ratio moyen des prix aux taux de change du marché |
1,06 |
0,82 |
2,10 |
1,19 |
| Ratio moyen des prix aux parités de pouvoir d’achat |
1,11 |
0,88 |
2,54 |
1,34 |
| Nombre de produits médicamenteux brevetés |
1 227 |
1 227 |
1 227 |
1 227 |
| Ventes (millions $) |
13 418,5 |
13 418,5 |
13 418,5 |
13 418,5 |
Sous l’angle des résultats obtenus avec les taux de change du marché, le ratio de la médiane des prix pratiqués dans les pays de comparaison par rapport aux prix pratiqués au Canada s’est maintenu à 1,06 en 2013 (la valeur correspondante pour 2012 était de 1,07). Il convient de noter que la moyenne des prix pratiqués dans les pays de comparaison semble produire des ratios des prix pratiqués dans les pays de comparaison par rapport aux prix pratiqués au Canada plus élevés que les médianes des prix pratiqués dans les pays de comparaison. Cette situation s’explique par l’influence des prix pratiqués aux États-Unis où les prix sont beaucoup plus élevés que dans tous les autres pays. Même si les prix pratiqués aux États-Unis sont presque toujours pris en compte dans la détermination du prix moyen pratiqué dans les pays de comparaison, ce n’est pas toujours le cas en ce qui concerne la médiane des prix pratiqués dans les pays de comparaison. Néanmoins, les États-Unis exercent une influence notable sur le ratio de la médiane des prix pratiqués dans les pays de comparaison par rapport aux prix pratiqués au Canada en raison du phénomène récurrent mentionné à la section précédente, où les États-Unis constituent le seul pays pour lequel un prix départ-usine est disponible relativement à un produit médicamenteux breveté vendu au Canada.
Le graphique 10 replace ces résultats dans leur contexte historique, présentant l’historique des ratios moyens des prix pratiqués dans les pays de comparaison par rapport aux prix pratiqués au Canada de 2001 à 2013. Même si le ratio a beaucoup changé au cours de cette période, il est demeuré au-dessus de la parité.
GRAPHIQUE 10 Ratio moyen du prix international médian pratiqué dans les pays de comparaison par rapport aux prix pratiqués au Canada, aux taux de change du marché, 2001-2013
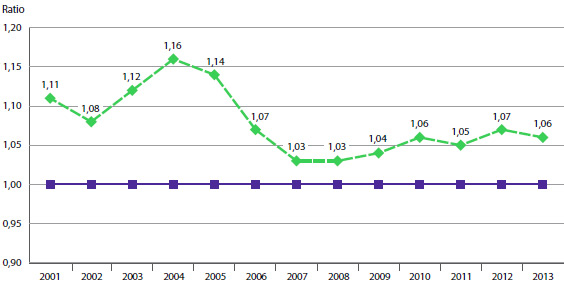
Source : CEPMB
Le graphique 11 présente avec encore plus de détails les ratios des prix des produits médicamenteux brevetés pratiqués dans les pays de comparaison par rapport aux prix pratiqués au Canada, ventilés selon la moyenne de la valeur des ventes présentée dans le tableau 12. Ce graphique ventile les ventes de médicaments brevetés effectuées en 2013 selon le ratio de la médiane des prix des produits médicamenteux brevetés pratiqués dans les pays de comparaison par rapport aux prix pratiqués au Canada (ou, pour être plus précis, selon la fourchette dans laquelle s’inscrit le ratio)13. Ces résultats révèlent une dispersion importante des ratios de prix au niveau du produit. Alors que les produits dont les ratios du prix international médian par rapport au prix au Canada se situent entre 0,90 et 1,10 accaparaient 21,8 % des ventes, ceux dont les ratios se situent sous 0,90 en accaparaient 51,4 % et ceux dont les ratios dépassaient 1,10 en accaparaient 26,9 %.
GRAPHIQUE 11 Distribution d’intervalle des ventes selon le ratio du prix international médian pratiqué dans les pays de comparaison par rapport aux prix pratiqués au Canada, 2013
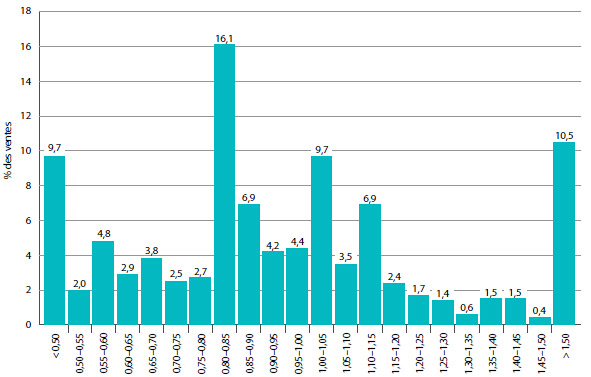
Source : CEPMB
Utilisation faite des produits médicamenteux brevetés
Les données sur les prix et sur la valeur des ventes utilisées pour calculer l’IPMB servent également à déterminer les tendances des quantités de médicaments brevetés vendus au Canada. Le CEPMB calcule à cette fin l’indice de volume des ventes de médicaments brevetés (IVVMB). Le graphique 12 présente pour les années 1988 à 2013 les taux moyens de croissance de l’utilisation des produits médicamenteux brevetés, mesurée à l’aide de l’IVVMB. Les résultats obtenus confirment que la croissance de l’utilisation faite des produits médicamenteux brevetés est le principal facteur d’augmentation de la valeur des ventes. Les taux de croissance de l’utilisation des dernières années talonnent en effet de près les taux de croissance de la valeur des ventes. Cette tendance de suivi s’est maintenue en 2013, le taux d’utilisation des produits médicamenteux brevetés ayant augmenté, en moyenne, de 6,5 % entre 2012 et 2013 et les ventes ayant augmenté de 6,5 %.
GRAPHIQUE 12 Taux annuel de variation de l’indice du volume des ventes de médicaments brevetés (IVVMB), 1988-2013
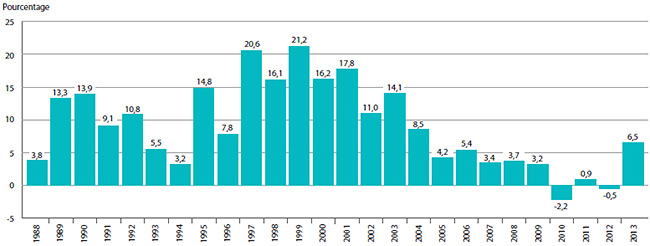
Source : CEPMB
Croissance de l'utilisation faite des produits médicamenteux brevetés selon le groupe thérapeutique principal
Le tableau 13 présente les taux moyens de croissance de l’utilisation faite des produits médicamenteux brevetés. Ces taux sont ventilés selon les groupes thérapeutiques principaux. Les résultats présentés dans ce tableau ont été obtenus en appliquant la méthodologie de l’IVVMB aux données du niveau 1 de la classification ATC. Comme dans le tableau 10, la dernière colonne donne un aperçu de la contribution des différents groupes thérapeutiques à la variation de l’IVVMB.
En 2013, les niveaux d’utilisation ont augmenté dans huit groupes thérapeutiques. La croissance modérée de l’utilisation des produits agissant sur le tube digestif et le métabolisme, sur les agents antinéoplastiques et immunomodulateurs, sur le système respiratoire ainsi que sur les organes sensoriels représente la plus grande partie de la croissance de l’utilisation faite de l’ensemble des produits. Quant aux produits médicamenteux agissant sur le système cardiovasculaire, sur le système génito-urinaire et hormones sexuelles et sur le système nerveux, leur taux d’utilisation a reculé.
Tableau 13 Variation de l’indice du volume des ventes des médicaments brevetés (IVVMB) selon le groupe thérapeutique principal, 2013
| Groupe thérapeutique |
Pourcentage des ventes en 2013 (%) |
Variation du volume de 2012 à 2013 (%) |
Contribution : Variation de l’IVVMB (%) |
|
* Pour des raisons de confidentialité, les données sur ces deux groupes ont été combinées.
** Il se peut que les valeurs n’équivaillent pas à 100,0 en raison de l’arrondissement.
Source : CEPMB
|
| A : Tube digestif et métabolisme |
10,4 |
11,9 |
1,2 |
| B : Sang et organes sanguinoformateurs |
5,7 |
3,4 |
0,2 |
| C : Système cardiovasculaire |
7,5 |
-20,7 |
-1,6 |
| D : Produits dermatologiques |
1,0 |
2,3 |
0,0 |
| G : Système génito-urinaire et hormones sexuelles |
3,7 |
-3,0 |
-0,1 |
| H : Préparations hormonales systémiques |
0,5 |
14,8 |
0,1 |
J : Antiinfectieux généraux pour usage systémique;
P : Produits antiparasitaires* |
11,1 |
4,7 |
0,5 |
| L : Agents antinéoplastiques et agents immunomodulateurs |
28,9 |
18,2 |
5,3 |
| M : Système musculo-squelettaire |
3,2 |
0,8 |
0,0 |
| N : Système nerveux |
13,6 |
-1,5 |
-0,2 |
| R : Système respiratoire |
9,3 |
12,7 |
1,2 |
| S : Organes sensoriels |
4,7 |
26,5 |
1,2 |
| V : Divers |
0,5 |
16,1 |
0,1 |
| Tous les groupes thérapeutiques |
100,0 |
6,5 |
6,5 |
Dépenses en produits médicamenteux au Canada par rapport aux marchés mondiaux
IMS Health14 fait régulièrement rapport des ventes médicaments dans différents pays. Selon les données sur les ventes provenant de cette source, le graphique 13 illustre la répartition de ces ventes entre le Canada et les sept pays que le CEPMB utilise dans ses examens de prix15. En ce qui concerne le Canada, les ventes de produits médicamenteux en 2013 ont représenté 2,5 % de l’ensemble des ventes sur les principaux marchés mondiaux.
GRAPHIQUE 13 Distribution des ventes de produits médicamenteux entre les grands marchés mondiaux, 2013
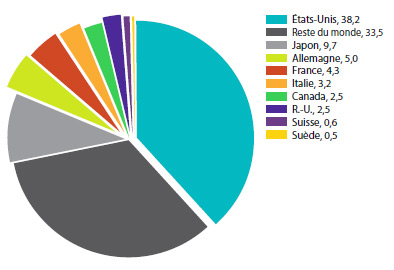
Source : MIDAS©, 2005-2013, IMS Health Incorporated ou sociétés affiliées. Tous droits réservés16.
Le graphique 14 présente la part des ventes du Canada sur les principaux marchés canadiens de 2005 à 2013. Pendant toutes ces années, la part des ventes du Canada s’est maintenue entre 2,4 % et 2,7 %.
GRAPHIQUE 14 Pourcentage des ventes de produits médicamenteux du Canada sur les principaux marchés mondiaux, 2005-2013
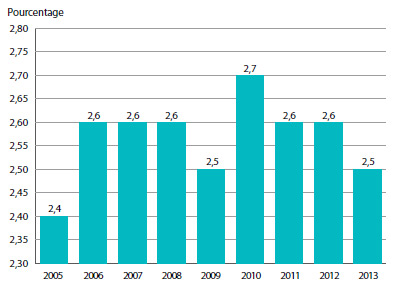
Source : MIDAS©, 2005-2013, IMS Health Incorporated ou sociétés affiliées. Tous droits réservés16.
Le graphique 15 compare la croissance des ventes de produits médicamenteux au Canada à celle des sept pays de comparaison, ensemble et séparément. Entre 2005 et 2013, les ventes au Canada de produits médicamenteux ont augmenté à un taux annuel moyen de près de 3,8 %. Cela équivaut au taux moyen de croissance des ventes dans les sept pays de comparaison pour la même période.
GRAPHIQUE 15 Taux moyen de croissance des ventes de produits médicamenteux aux taux de change constants du marché de 2013, par pays, 2005-2013
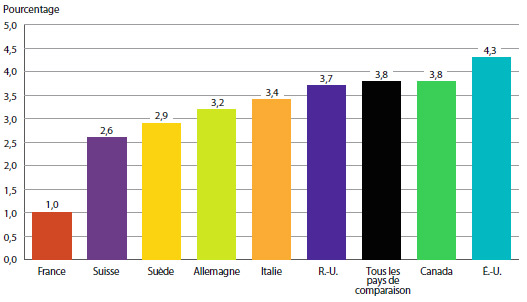
Source : MIDAS©, 2005-2013, IMS Health Incorporated ou sociétés affiliées. Tous droits réservés16.
Le graphique 16 compare les taux annuels de croissance des ventes de produits médicamenteux au Canada et dans l’ensemble des pays de comparaison. En 2013, pour la quatrième année consécutive, les ventes ont augmenté à un taux plus lent au Canada que dans les pays de comparaison.
GRAPHIQUE 16 Taux moyen annuel de variation des ventes de produits médicamenteux aux taux de change constants du marché de 2012, au Canada et dans les pays de comparaison, 2006-2013
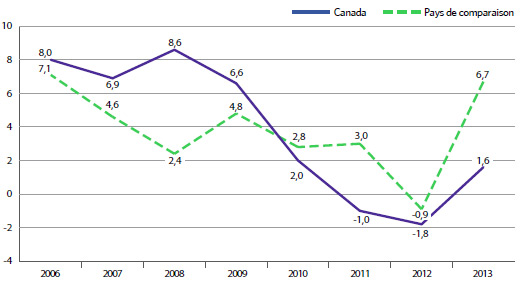
Source : MIDAS©, 2005-2013, IMS Health Incorporated ou sociétés affiliées. Tous droits réservés16.
La comparaison des dépenses en produits médicamenteux peut aussi être faite à l’aide de la proportion du produit intérieur brut consacrée à l’achat de médicaments17. Le graphique 17 présente les dépenses en produits médicamenteux exprimées en pourcentage du produit intérieur brut (PIB) du Canada et des sept pays de comparaison (données de 2011). Dans les sept pays de comparaison, les dépenses en produits médicamenteux ont accaparé entre 1,0 % et 2,1 % du PIB. Sur cette échelle, la valeur du Canada (1,9 %) s’inscrit près de la limite supérieure.
GRAPHIQUE 17 Dépenses en produits médicamenteux, exprimées en pourcentage du PIB, 2011
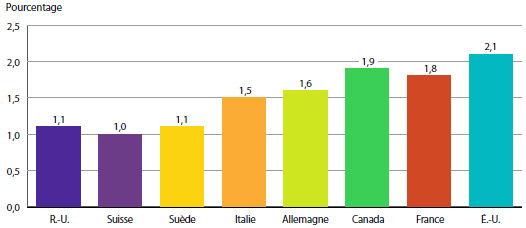
Source : OCDE
Le tableau 14 présente le ratio des dépenses par rapport au PIB dans son contexte historique. Entre 2000 et 2011, les dépenses en produits médicamenteux au Canada ont augmenté au deuxième taux le plus élevé après les États-Unis, soit presque le double du taux de croissance du PIB.
Tableau 14 Dépenses en produits médicamenteux, exprimées en pourcentage du PIB, 2011
| |
Part des ventes en produits médicamenteux, 2011 (% du PIB) |
Part des ventes en produits médicamenteux, 2000 (% du PIB) |
Croissance des dépenses en produits médicamenteux, 2000-2011 (%) |
Croissance du PIB,
2000-2011 (%) |
| Source: OECD |
| Canada |
1,86 |
1,42 |
160,38 % |
98,87 |
| France |
1,81 |
1,81 |
81,07 % |
81,11 |
| Allemagne |
1,59 |
1,43 |
99,70 % |
79,23 |
| Italie |
1,49 |
1,74 |
63,91 % |
91,36 |
| Suède |
1,15 |
1,18 |
60,30 % |
64,55 |
| Suisse |
1,03 |
1,11 |
57,48 % |
69,05 |
| Royaume-Uni |
1,07 |
1,14 |
43,90 % |
53,09 |
| États-Unis |
2,07 |
1,46 |
125,70 % |
59,12 |
Le tableau 15 présente la distribution des ventes de produits médicamenteux selon le groupe thérapeutique au Canada, dans chaque pays de comparaison ainsi que dans l’ensemble des pays de comparaison18. Les résultats font ressortir un degré d’uniformité remarquable entre les différents pays.
Tableau 15 Distribution des ventes de produits médicamenteux brevetés (exprimée en pourcentage) selon le groupe thérapeutique principal, au Canada et dans les pays de comparaison, 2013
| Groupe thérapeutique |
Canada |
Pays de comparaison |
France |
Italie |
Allemagne |
Suède |
Suisse |
Royaume-Uni |
États-Unis |
|
* Il se peut que les valeurs n’équivaillent pas à 100,0 en raison de l’arrondissement.
Source : MIDAS©, 2005–2013, IMS Health Incorporated ou sociétés affiliées. Tous droits réservés16.
|
| A : Tube digestif et métabolisme |
12,4 |
12,4 |
10,3 |
11,1 |
11,2 |
9,7 |
11,1 |
10,9 |
13,0 |
| B : Sang et organes sanguinoformateurs |
4,1 |
5,1 |
7,5 |
8,2 |
6,1 |
8,0 |
5,2 |
4,4 |
4,4 |
| C : Système cardiovasculaire |
11,8 |
9,5 |
11,8 |
12,8 |
8,8 |
5,3 |
10,6 |
7,6 |
9,2 |
| D : Produits dermatologiques |
3,2 |
3,0 |
2,5 |
2,1 |
2,7 |
2,6 |
3,6 |
3,1 |
3,1 |
| G : Système génito-urinaire et hormones sexuelles |
5,3 |
5,1 |
3,2 |
4,0 |
3,5 |
4,8 |
4,6 |
4,3 |
5,7 |
| H : Préparations hormonales systémiques |
1,3 |
2,2 |
2,0 |
2,1 |
2,1 |
2,6 |
1,6 |
2,4 |
2,2 |
| J : Antiinfectieux généraux pour usage systémique |
7,5 |
10,7 |
12,0 |
13,0 |
9,7 |
7,2 |
10,6 |
10,7 |
10,5 |
| L : Agents antinéoplastiques et agents immunomodulateurs |
17,2 |
17,7 |
18,0 |
17,2 |
22,1 |
23,2 |
21,1 |
17,9 |
17,0 |
| M : Système musculo-squelettaire |
3,8 |
3,2 |
3,3 |
3,8 |
3,7 |
3,3 |
4,9 |
2,6 |
3,1 |
| N : Système nerveux |
18,3 |
17,2 |
14,5 |
11,8 |
14,4 |
18,1 |
15,2 |
18,4 |
18,2 |
| P : Produits antiparasitaires |
0,2 |
0,2 |
0,2 |
0,0 |
0,1 |
0,2 |
0,2 |
0,2 |
0,2 |
| R : Système respiratoire |
7,4 |
7,4 |
6,2 |
5,7 |
7,0 |
9,0 |
6,4 |
9,8 |
7,5 |
| S : Organes sensoriels |
3,7 |
2,7 |
3,1 |
2,1 |
2,7 |
2,7 |
3,4 |
3,6 |
2,6 |
| V : Divers |
4,0 |
3,8 |
5,3 |
6,0 |
5,8 |
3,3 |
1,7 |
4,1 |
3,2 |
| Tous les groupes thérapeutiques* |
100,0 |
100,0 |
100,0 |
100,0 |
100,0 |
100,0 |
100,0 |
100,0 |
100,0 |
Analyse des dépenses de recherche-développement
La Loi sur les brevets (la Loi) confère au CEPMB le mandat de faire le suivi des dépenses des brevetés en recherchedéveloppement (R-D) et de faire rapport des tendances observées (la Loi ne confère toutefois pas au CEPMB un droit de regard sur le montant des dépenses des brevetés dans la recherche-développement ni sur le type de recherchedéveloppement). Le présent chapitre fournit les statistiques clés sur la situation actuelle des investissements dans la recherche-développement pharmaceutique au Canada.
Sources des données
Les résultats statistiques présentés dans le présent rapport ont été tirés des rapports semestriels que les brevetés ont soumis au CEPMB.
La Loi oblige les brevetés à faire rapport au CEPMB des recettes qu’ils tirent des ventes de tous leurs produits médicamenteux pour usage humain et pour usage vétérinaire (y compris les recettes tirées des ventes de produits médicamenteux non brevetés et les recettes découlant d’ententes de production sous licence) ainsi que de leurs dépenses de R-D au Canada pour leurs différents produits médicamenteux (brevetés et non brevetés, pour usage humain et pour usage vétérinaire). Les brevetés transmettent ces renseignements au CEPMB au moyen de son formulaire 3 (Recettes et dépenses en recherche et développement fournies en application du paragraphe 88(1) de la Loi sur les brevets).
Le Règlement sur les médicaments brevetés (le Règlement) exige que chaque formulaire 3 déposé soit accompagné d’un document certifiant l’exactitude et la conformité des données fournies. Le CEPMB ne vérifie pas systématiquement l’information qui lui est présentée sur le formulaire 3, mais cherche plutôt les anomalies et les contradictions et, lorsqu’il y a lieu, demande aux brevetés de corriger leurs données ou de les étoffer. Pour garantir la juste interprétation des données, chaque breveté est invité à confirmer, avant la publication du ratio, l’exactitude du ratio de ses dépenses de R-D par rapport aux recettes tirées des ventes calculé par le CEPMB.
Défaut de soumettre son rapport sur ses dépenses de R-D
Les brevetés sont tenus de soumettre un formulaire 3 complet et exact dans les délais mentionnés dans le Règlement. Si un breveté omet de respecter les exigences en matière de présentation de rapport, le Conseil peut rendre une ordonnance exigeant le respect de ces exigences. Le Conseil n’a pas été appelé à rendre des ordonnances pour la période de déclaration de 2013.
Couverture
Notons que les sociétés pharmaceutiques qui n’ont fait aucune vente au Canada ne sont pas tenues de présenter un rapport sur leurs dépenses de R-D au CEPMB. Cela a deux conséquences.
D’abord, les résultats statistiques indiqués ici ne devraient pas être utilisés afin de couvrir toute la recherche pharmaceutique réalisée au Canada. Par exemple, une société peut vendre uniquement des produits médicamenteux non brevetés, mais elle peut toujours effectuer beaucoup de recherche au Canada. De même, une société peut effectuer de la recherche et ne vendre aucun produit19. Les résultats présentés ci-dessous ne tiendront pas compte des dépenses de R-D des entreprises dans l’une ou l’autre situation.
Ensuite, comme de nouveaux produits médicamenteux non brevetés sont entrés dans le marché canadien et que les brevets existants expirent, le nombre et l’identité des sociétés pharmaceutiques ayant soumis un rapport sur leurs dépenses de R-D varie d’année en année. Au total, 81 sociétés ont déclaré leurs activités de R-D en 2013. Parmi elles, 35 étaient membres des Compagnies de recherche pharmaceutique du Canada (Rx&D).
Définition de recettes tirées des ventes
Aux fins du présent rapport, les recettes tirées des ventes s’entendent du produit brut des ventes de produits médicamenteux au Canada ainsi que des recettes découlant d’ententes de vente sous licence (p. ex. redevances et droits versés au breveté liés au ventes effectuées au Canada par des titulaires de licence).
Définition de dépenses de R-D
En vertu de l’article 6 du Règlement, les brevetés ne doivent inclure dans leurs rapports que leurs dépenses de R-D qui auraient été admissibles au crédit d’impôt à l’investissement pour la recherche scientifique et le développement expérimental (RS-DE) aux termes de la Loi de l’impôt sur le revenu, dans sa version qui est entrée en vigueur le 1er décembre 198720. Ainsi, les dépenses de R-D peuvent inclure les dépenses courantes, les coûts d’immobilisation et l’amortissement autorisé. Les frais engagés pour les études de marché, la promotion des ventes, le contrôle de la qualité ou les essais systématiques de matériel, de dispositifs ou de produits ainsi que pour la collecte de données n’étant pas admissibles au crédit d’impôt à l’investissement, ils ne doivent pas figurer dans les dépenses de R-D déclarées par les brevetés.
Recettes tirées des ventes et dépenses de R-D totales
Le tableau 16 donne un aperçu des recettes tirées des ventes et des dépenses de R-D déclarées au cours de la période de 1988 à 2013.
La valeur des recettes brutes tirées des ventes de produits médicamenteux au Canada déclarées par les brevetés a totalisé 16,8 milliards de dollars en 2013, ce qui représente une augmentation de 0,4 % par rapport à 2012. Les recettes tirées des ventes déclarées par les brevetés membres de Rx&D ont totalisé 12,2 milliards de dollars pour la même période, soit 72,3 % de l’ensemble des recettes tirées des ventes. (De ce montant, moins de 1 % des recettes découlent d’ententes de vente sous licence.)
Les dépenses de R-D déclarées par les brevetés ont totalisé 752,8 millions de dollars en 2013, ce qui représente un recul de 15,9 % par rapport à 2012. Les dépenses de R-D déclarées par les brevetés membres de Rx&D ont totalisé 652,0 millions de dollars en 2013, ce qui représente un recul de 16,7 % par rapport à l’année précédente. Les brevetés membres de Rx&D ont effectué 86,6 % de toutes les dépenses de R-D déclarées pour 2013.
Ratio des dépenses de R-D par rapport aux recettes tirées des ventes
Le tableau 16 et le graphique 18 présentent également les ratios des dépenses de R-D par rapport aux recettes tirées des ventes. Il convient de noter que dans ce contexte, en contrepartie de l’adoption des modifications apportées à la Loi en 1987, Rx&D s’était engagé à augmenter graduellement ses dépenses annuelles de R-D pour qu’elles totalisent en 1996 au moins 10 % des recettes tirées des ventes21. Ce niveau de dépenses de R-D a été atteint en 1993, le taux ayant même dépassé 10 % certaines années. Toutefois, depuis 2003, le ratio des dépenses de R-D de tous les brevetés et des membres de Rx&D a diminué.
Le ratio de 2013 des dépenses de R-D par rapport aux recettes tirées des ventes parmi les brevetés était de 4,5 %, un recul par rapport au ratio de 5,3 % en 2012. Ces valeurs sont inférieures aux données observées en 1988. Le ratio global des dépenses de R-D par rapport aux recettes tirées des ventes a été inférieur à 10 % pendant les 13 dernières années consécutives.
Le ratio correspondant des dépenses de R-D par rapport aux recettes tirées des ventes des brevetés membres de R&D était de 5,4 % en 2013, un recul par rapport au ratio de 6,6 % en 201222. Ces valeurs se situent également près des données observées en 1988. Le ratio des brevetés membres de R&D a été inférieur à 10 % pendant les 11 dernières années consécutives.
Le tableau 21 à l’annexe 3 présente les ratios des dépenses de R-D par rapport aux recettes tirées des ventes de 2013. Des 81 brevetés ayant présenté des rapports sur leurs dépenses de R-D au CEPMB en 2013, 94,8 % ont présenté un ratio de R-D par rapport aux recettes tirées des ventes de moins de 10 %.
Tableau 16 Dépenses de R-D déclarées par les brevetés et ratio des dépenses de R-D par rapport aux recettes tirées des ventes des brevetés, 1988-2013
| Année |
Tous les brevetés |
Rx&D |
Ratio des dépenses de R-D par rapport aux recettes tirées des ventes : Tous les brevetés (%) |
Ratio des dépenses de R-D par rapport aux recettes tirées des ventes : Brevetés membres de Rx&D (%) |
| Nbre de brevetés |
Dépenses de R-D de tous les brevetés (millions $) |
Variation par rapport à l’année précédente (%) |
Recettes tirées des ventes (millions $) |
Variation par rapport à l’année précédente (%) |
Dépenses de R-D de tous les brevetés membres de Rx&D (millions $) |
Variation par rapport à l’année précédente (%) |
Recettes tirées des ventes des brevetés membres de Rx&D (millions $) |
Variation par rapport à l’année précédente (%) |
| Source : CEPMB |
| 2013 |
81 |
752,8 |
-15,9 |
16 817,9 |
0,4 |
652,0 |
-16,7 |
12 167,6 |
2,3 |
4,5 |
5,4 |
| 2012 |
85 |
894,8 |
-9,8 |
16 754,4 |
-5,8 |
782,8 |
-13,1 |
11 896,1 |
-11,5 |
5,3 |
6,6 |
| 2011 |
79 |
991,7 |
-15,8 |
17 798,8 |
4,7 |
901,2 |
-9,9 |
13 446,1 |
10,7 |
5,6 |
6,7 |
| 2010 |
82 |
1 178,2 |
-7,4 |
17 000,0 |
-0,3 |
1 000,2 |
-11,7 |
12 149,0 |
-11,8 |
6,9 |
8,2 |
| 2009 |
81 |
1 272,0 |
-2,9 |
17 051,9 |
4,5 |
1 132,9 |
-3,4 |
13 780,0 |
4,6 |
7,5 |
8,2 |
| 2008 |
82 |
1 310,7 |
-1,1 |
16 316,7 |
2,0 |
1 172,2 |
-1,0 |
13 178,2 |
-1,4 |
8,1 |
8,9 |
| 2007 |
82 |
1 325,0 |
9,5 |
15 991,0 |
7,3 |
1 184,4 |
24,8 |
13 359,8 |
20,0 |
8,3 |
8,9 |
| 2006 |
72 |
1 210,0 |
-1,9 |
14 902,0 |
4,7 |
949,0 |
-8,8 |
11 131,2 |
-5,8 |
8,1 |
8,5 |
| 2005 |
80 |
1 234,3 |
5,5 |
14 231,3 |
0,5 |
1 040,1 |
3,9 |
11 821,4 |
0,0 |
8,7 |
8,8 |
| 2004 |
84 |
1 170,0 |
-2,0 |
14 168,3 |
4,0 |
1 000,8 |
0,8 |
11 819,0 |
8,8 |
8,3 |
8,5 |
| 2003 |
83 |
1 194,3 |
-0,4 |
13 631,1 |
12,8 |
992,9 |
-3,6 |
10 865,7 |
5,2 |
8,8 |
9,1 |
| 2002 |
79 |
1 198,7 |
13,0 |
12 081,2 |
12,5 |
1 029,6 |
10,1 |
10 323,8 |
16,8 |
9,9 |
10,0 |
| 2001 |
74 |
1 060,1 |
12,6 |
10 732,1 |
15,3 |
935,2 |
14,7 |
8 835,4 |
14,3 |
9,9 |
10,6 |
| 2000 |
79 |
941,8 |
5,3 |
9 309,6 |
12,0 |
815,5 |
4,0 |
7 728,8 |
11,6 |
10,1 |
10,6 |
| 1999 |
78 |
894,6 |
12,0 |
8 315,5 |
19,2 |
784,3 |
9,9 |
6 923,4 |
22,8 |
10,8 |
11,3 |
| 1998 |
74 |
798,9 |
10,2 |
6 975,2 |
10,9 |
713,7 |
8,6 |
5 640,2 |
10,6 |
11,5 |
12,7 |
| 1997 |
75 |
725,1 |
9,0 |
6 288,4 |
7,4 |
657,4 |
10,3 |
5 098,2 |
4,9 |
11,5 |
12,9 |
| 1996 |
72 |
665,3 |
6,4 |
5 857,4 |
9,9 |
595,8 |
6,5 |
4 859,5 |
8,7 |
11,4 |
12,3 |
| 1995 |
71 |
625,5 |
11,5 |
5 330,2 |
7,5 |
559,5 |
9,8 |
4 468,8 |
1,4 |
11,7 |
12,5 |
| 1994 |
73 |
561,1 |
11,4 |
4 957,4 |
4,4 |
509,5 |
10,4 |
4 407,2 |
2,0 |
11,3 |
11,6 |
| 1993 |
70 |
503,5 |
22,1 |
4 747,6 |
14,0 |
461,4 |
24,0 |
4 321,4 |
14,4 |
10,6 |
10,7 |
| 1992 |
71 |
412,4 |
9,6 |
4 164,4 |
6,9 |
372,1 |
9,0 |
3 778,4 |
6,5 |
9,9 |
9,8 |
| 1991 |
65 |
376,4 |
23,2 |
3 894,8 |
18,1 |
341,4 |
24,7 |
3 546,9 |
19,5 |
9,7 |
9,6 |
| 1990 |
65 |
305,5 |
24,8 |
3 298,8 |
11,0 |
273,8 |
25,8 |
2 967,9 |
10,5 |
9,3 |
9,2 |
| 1989 |
66 |
244,8 |
47,4 |
2 973,0 |
9,4 |
217,6 |
34,7 |
2 685,5 |
7,3 |
8,2 |
8,1 |
| 1988 |
66 |
165,7 |
— |
2 718,0 |
— |
161,5 |
— |
2 502,3 |
— |
6,1 |
6,5 |
GRAPHIQUE 18 Ratio des dépenses de R-D par rapport aux recettes tirées des ventes chez les brevetés, 1988-2013
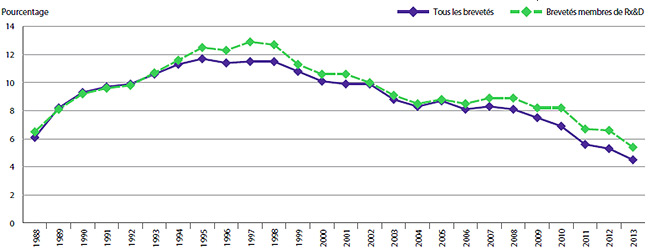
Source : CEPMB
Dépenses courantes selon le type de recherche
Le tableau 17 et le graphique 19 (ainsi que le graphique 21 de l’annexe 3) présentent des données sur la ventilation des dépenses courantes de R-D23 engagées en 2013 selon le type de recherche, à savoir la recherche fondamentale, la recherche appliquée et autres types de R-D admissible24.
Pour 2013, les brevetés ont fait état de dépenses de recherche fondamentale totalisant 67,6 millions de dollars ou 9,3 % du total des dépenses courantes de R-D, soit un recul de 41,0 % par rapport à l’année antérieure. Les brevetés ont déclaré des dépenses de recherche appliquée totalisant 487,8 millions de dollars, ou, encore, 66,9 % des dépenses courantes de R-D. Les essais cliniques ont accaparé 68,5 % des dépenses de recherche appliquée.
Tableau 17 Dépenses courantes de R-D selon le type de recherche, 2013 et 2012
| Type de recherche |
Dépenses en 2013 (millions $) |
Part en 2013 (%) |
Dépenses en 2012 (millions $) |
Part en 2012 (%) |
Variation annuelle des dépenses (%) |
| Source : CEPMB |
| Recherche fondamentale |
67,6 |
9,3 |
114,6 |
13,2 |
-41,0 |
| Chimique |
39,2 |
5,4 |
78,3 |
9,1 |
-49,9 |
| Biologique |
28,4 |
3,9 |
36,3 |
4,2 |
-21,8 |
| Recherche appliquée |
487,8 |
66,9 |
520,9 |
60,2 |
-6,4 |
| Processus de fabrication |
70,5 |
9,7 |
82,2 |
9,5 |
-14,2 |
| Essais précliniques I |
40,0 |
5,5 |
35,7 |
4,1 |
12,0 |
| Essais précliniques II |
42,9 |
5,9 |
41,5 |
4,8 |
3,4 |
| Essais cliniques de phase I |
35,8 |
4,9 |
34,3 |
4,0 |
4,4 |
| Essais cliniques de phase II |
70,1 |
9,6 |
82,1 |
9,5 |
-14,6 |
| Essais cliniques de phase III |
228,5 |
31,3 |
245,1 |
28,3 |
-6,8 |
| Autre R-D admissible |
173,9 |
23,8 |
230,1 |
26,6 |
-24,4 |
| Total |
729,3 |
100,0 |
865,6 |
100,0 |
-15,8 |
GRAPHIQUE 19 Dépenses courantes de R-D selon le type de recherche, 1988-2013
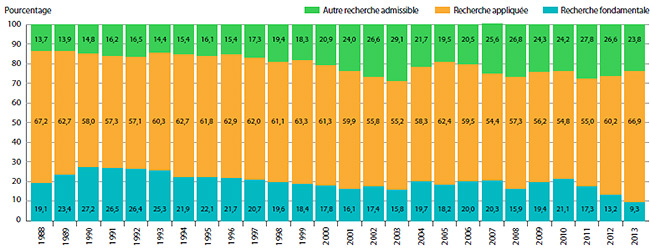
Source : CEPMB
Dépenses courantes de R-D selon le milieu de recherche
Les brevetés peuvent inclure dans leurs dépenses de R-D la recherche qu’ils effectuent eux-mêmes à l’interne ainsi que la recherche qu’ils font faire à l’externe, notamment par des universités, des hôpitaux et d’autres sociétés pharmaceutiques. Le tableau 18 révèle que, en 2013, 50,0 % des dépenses courantes de recherche ont été effectuées à l’interne. La proportion de la R-D effectuée à l’externe pour le compte des brevetés a représenté 11,3 % de l’ensemble des dépenses courantes de R-D. Quant à la recherche effectuée par les universités et par les hôpitaux, sa valeur a représenté 13,0 % des dépenses courantes de R-D.
Tableau 18 Dépenses courantes de R-D selon le milieu de recherche, 2013 et 2012
| Milieu de recherche |
Dépenses en 2013 (millions $) |
Part en
2013 (%) |
Dépenses en 2012 (millions $) |
Part en
2012 (%) |
Variation annuelle des dépenses (%) |
| Source : CEPMB |
| À l’interne |
| Brevetés |
364,9 |
50,0 |
425,3 |
49,1 |
-14,2 |
| À l’externe |
| Universités et hôpitaux |
94,7 |
13,0 |
131,0 |
15,1 |
-27,7 |
| Autres sociétés |
187,4 |
25,7 |
218,6 |
25,3 |
-9,3 |
| Autres |
82,3 |
11,3 |
90,7 |
10,5 |
-14,3 |
| Total |
729,3 |
100,0 |
865,6 |
100,0 |
-15,8 |
Dépenses courantes de R-D selon la provenance des fonds
Le tableau 19 présente des renseignements sur la provenance des fonds que les brevetés ont investis pour financer leurs activités de R-D. En 2013, les brevetés ont financé à même leurs propres fonds la majeure partie de leur R-D (87,7 % des dépenses courantes de R-D). Les fonds provenant du gouvernement n’ont servi à financer que 1,4 % de l’ensemble des dépenses courantes de R-D.
Tableau 19 Ensemble des dépenses de R-D selon la provenance des fonds, 2013 et 2012
| Provenance des fonds |
Dépenses
en 2013
(millions $) |
Part en
2013 (%) |
Dépenses
en 2012
(millions $) |
Part en
2012 (%) |
Variation annuelle des dépenses (%) |
| Source : CEPMB |
| Brevetés |
660,5 |
87,7 |
777,1 |
86,8 |
-15,0 |
| Gouvernements fédéral et provinciaux |
10,8 |
1,4 |
23,8 |
2,7 |
-55,0 |
| Autres |
81,5 |
10,8 |
93,9 |
10,5 |
-13,2 |
| Total |
752,8 |
100,0 |
894,8 |
100,0 |
-15,9 |
Dépenses courantes de R-D selon la région géographique
Le tableau 20 (ainsi que le tableau 23 et le tableau 24 de l’annexe 3) ventile les dépenses courantes de R-D selon la région géographique dans laquelle elles ont été engagées. Cette année encore, les dépenses ont été surtout engagées en Ontario et au Québec, qui ont accaparé 84,1 % de la valeur totale des dépenses courantes de R-D au Canada. La valeur des dépenses de R-D a reculé d’un taux annuel de 20,9 % dans l’Ouest du pays; elle a également reculé de 12,7 % en Ontario et de 17,7 % au Québec.
Tableau 20 Dépenses courantes de R-D selon la région géographique, 2013 et 2012
| Région géographique |
Dépenses
en 2013
(millions $) |
Part en
2013
(%) |
Dépenses
en 2012
(millions $) |
Part en
2012
(%) |
Augmentation
annuelle des
dépenses (%) |
| Source : CEPMB |
| Provinces de l’Atlantique |
20,1 |
2,8 |
21,9 |
2,5 |
-8,2 |
| Québec |
292,0 |
40,0 |
354,8 |
41,0 |
-17,7 |
| Ontario |
322,0 |
44,1 |
368,6 |
42,6 |
-12,7 |
| Provinces de l’Ouest |
95,2 |
13,1 |
120,3 |
13,9 |
-20,9 |
| Territoires |
0,0 |
0,0 |
0,0 |
0,0 |
0,0 |
| Total |
729,3 |
100,0 |
865,6 |
100,0 |
-15,8 |
Le contexte mondial
Le graphique 20 compare pour les années 2000 et 2011 les ratios des dépenses de R-D pharmaceutiques par rapport aux recettes tirées des ventes du Canada aux mêmes ratios des sept pays de comparaison du CEPMB25. Le ratio du Canada était de 10,1 % en 2000. Cette année-là, seule l’Italie présentait un ratio plus bas (6,2 %), tandis que la Suisse présentait le ratio le plus élevé (102,5 %).
En 2011, le Canada présentait le ratio le moins élevé (5,6 %) suivi de l’Italie (6,2 %). Le ratio de tous les autres pays de comparaison est demeuré bien supérieur à celui du Canada. Le ratio obtenu avec l’ensemble des dépenses de R-D et des ventes de tous les pays de comparaison était de 21,7 %, soit trois fois et demie celui du Canada.
GRAPHIQUE 20 Ratios des dépenses de R-D par rapport aux recettes tirées des ventes, au Canada et dans les pays de comparaison
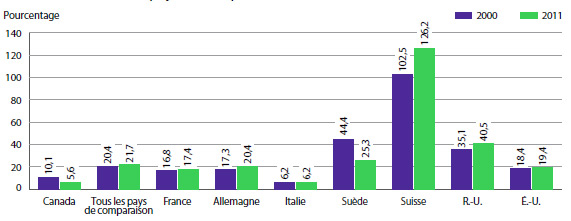
Source : CEPMB, Fédération européenne des industries pharmaceutiques (EFPIA) : The Pharmaceutical Industry in Figures 2013, PhRMA 2013 profile
On peut comparer les ratios des dépenses de R-D par rapport aux recettes tirées des ventes présentés dans le graphique 20 avec les ratios des prix moyens bilatéraux mentionnés au tableau 11 (voir la section « Comparaison des prix pratiqués au Canada avec ceux pratiqués dans les pays de comparaison »). Plusieurs pays de comparaison, dont les prix des produits médicamenteux brevetés sont, en moyenne, beaucoup moins élevés qu’au Canada, ont obtenu des ratios des dépenses de R-D par rapport aux recettes tirées des ventes bien supérieurs à ceux au Canada.
Tel que mentionné dans le rapport de l’an dernier, la région géographique où se produit la R-D pharmaceutique dépend de plusieurs facteurs. Parmi les facteurs dont tiennent compte les entreprises, mentionnons notamment la présence, la qualité et la rentabilité des meilleures institutions scientifiques et l’accès aisé à une infrastructure de haute qualité pour les essais cliniques. Bien qu’on cite souvent les niveaux de prix comme importants leviers politiques pour l’attraction de la R-D, les données n’appuient pas de telle corrélation au niveau national ou international.
Système national d’information sur l’utilisation des médicaments prescrits
Contexte
Le Système national d’information sur l’utilisation des médicaments prescrits (SNIUMP) est une initiative de recherche établie en septembre 2001 par les ministres fédéral, provinciaux et territoriaux de la Santé.
Il vise à fournir aux décideurs et aux gestionnaires des régimes publics d’assurance-médicaments des analyses critiques des tendances relatives aux prix, à l’utilisation et aux coûts des médicaments, afin de s’assurer que le système canadien des soins de santé a accès à des renseignements exhaustifs et précis sur l’utilisation des médicaments d’ordonnance et sur les facteurs à l’origine des augmentations des coûts.
Le CEPMB est autorisé à réaliser des activités en vertu de l’initiative du SNIUMP suite à une demande officielle du ministre fédéral de la Santé en vertu de l’article 90 de la Loi sur les brevets, et conformément au mandat du CEPMB qui consiste à faire rapport sur les tendances relatives aux produits pharmaceutiques.
Le Comité consultatif du SNIUMP, constitué de représentantsdes régimes publics d’assurance-médicaments de la Colombie-Britannique, de l’Alberta, de la Saskatchewan, du Manitoba, de l’Ontario, du Nouveau-Brunswick, de la Nouvelle-Écosse, de l’Île-du-Prince-Édouard, de Terre-Neuve-et-Labrador, du Yukon et de Santé Canada, conseille le CEPMB sur son programme de recherche et lui suggère des sujets d’étude. Le Comité comprend également des observateurs de l’Institut canadien d’information sur la santé (ICIS) et de l’Agence canadienne des médicaments et des technologies de la santé (ACMTS).
Faits saillants
En 2013-2014, le CEPMB a publié quatre rapports au titre du SNIUMP :
L’Observateur des médicaments émergents – cinquième livraison
Le rapport présente des renseignements sur les médicaments en cours de développement qui pourraient avoir une incidence sur les dépenses éventuelles des régimes d’assurance-médicaments.
Les facteurs de coût associés aux dépenses en médicaments d’ordonnance – Un rapport méthodologique
Le rapport méthodologique présente une analyse des facteurs de coût associés aux dépenses en médicaments d’ordonnance et fournit une formule de base pour décomposer la croissance en différents facteurs relatifs aux coûts des médicaments et aux frais d’exécution des ordonnances. L’analyse de ces facteurs et de la variation de leur contribution au fil du temps peut révéler d’importants changements relativement à l’utilisation des médicaments et aux pressions sur les coûts.
Analyse éclair – Comparaisons des prix internationaux des médicaments génériques, début 2011
L’analyse éclair compare les prix des médicaments génériques au Canada avec les prix de ces mêmes médicaments dans d’autres pays industrialisés. Le rapport met en évidence les modifications qui ont été apportées à l’établissement des prix des médicaments génériques au Canada entre 2008 et le premier trimestre de 2011. On a utilisé les prix des médicaments génériques de la base de données MIDASMC d’IMS Health et du formulaire de l’Ontario pour comparer les prix.
L’utilisation des opioïdes d’ordonnance dans les régimes publics d’assurance-médicaments au Canada, de 2006-2007 à 2012-2013
L’étude fait rapport de l’utilisation des opioïdes d’ordonnance dans l’ensemble des provinces et territoires au Canada entre les exercices 2006-2007 et 2012-2013, à la lumière des questions émergentes relativement à la santé publique et à la sécurité.
Le Programme de recherche du SNIUMP fournit des descriptions sommaires des autres études du SNIUMP en cours.
En octobre 2013, le Comité consultatif du SNIUMP a tenu sa rencontre annuelle à Ottawa. La rencontre a permis de partager les résultats des études de recherche achevées et en cours, et de discuter des futures priorités de recherche.
Annexes
Pour de plus amples explications et définitions, veuillez consulter la Loi sur les brevets, le Règlement sur les médicaments brevetés, le Compendium des politiques, des Lignes directrices, et des procédures du CEPMB et le Règlement sur les aliments et drogues ou, encore, communiquer avec le CEPMB.
- ATC
-
Système de classification anatomique, thérapeutique, chimique (ATC) conçu et tenu à jour par le Centre collaborateur de l’OMS pour la méthodologie sur l’établissement des statistiques concernant les produits médicamenteux de l’Organisation mondiale de la santé (OMS). Ce système distingue les médicaments selon leur site d’action et leurs caractéristiques thérapeutiques et chimiques. Le CEPMB utilise ce système pour sélectionner les médicaments qui seront utilisés dans les comparaisons des prix.
- AVIS DE CONFORMITÉ
-
Avis donné par la Direction générale des produits de santé et des aliments de Santé Canada en vertu de l’article C.08.004.01 du Règlement sur les aliments et drogues. Cet avis confirme que le produit médicamenteux respecte les normes prescrites par Santé Canada pour une administration à des humains ou à des animaux, selon le cas, et que sa vente est autorisée au Canada.
- BREVET
-
Instrument émis par le Commissaire aux brevets sous forme de lettres patentes. Le brevet confère à son titulaire un monopole d’une durée limitée pour les allégations formulées. Le brevet confère également à son titulaire et à ses représentants légaux le droit exclusif de fabriquer, de construire, d’exploiter ou de vendre son invention.
- BREVET EN INSTANCE
-
Demande pour un brevet qui n’a pas encore été attribué.
- BREVETÉ
-
Aux termes du paragraphe 79(1) de la Loi sur les brevets, le mot « breveté » désigne « la personne ayant droit aux retombées d’un brevet pour une invention liée à un médicament, ainsi que quiconque était titulaire d’un brevet pour une telle invention ou qui exerce ou a exercé les droits d’un titulaire dans un cadre autre qu’une licence prorogée en vertu du paragraphe 11(1) de la Loi de 1992 modifiant la Loi sur les brevets ».
- CERTIFICAT DE DÉCISION PRÉALABLE
-
Certificat révocable émis à la demande du breveté en vertu du paragraphe 98(4) de la Loi sur les brevets lorsque le Conseil estime que le prix pratiqué ou proposé du produit médicamenteux ne dépasse pas le prix moyen maximal potentiel qu’autorisent ses Lignes directrices.
- CESSION DE BREVET
-
Avis donné par le breveté au Commissaire aux brevets l’informant qu’il renonce irrévocablement à ses droits de propriété à l’égard du brevet en cause et qu’il les cède au domaine public. Nota : Depuis le 30 janvier 1995, le Conseil ne reconnaît pas la cession d’un brevet lorsque le breveté utilise cette mesure pour se soustraire à sa compétence en matière d’examen du prix.
- DÉFAUT DE PRÉSENTER SES RAPPORTS
-
Défaut partiel ou complet d’un breveté de déclarer un produit médicamenteux breveté vendu conformément aux exigences réglementaires en matière de présentation de rapport prévues à la Loi sur les brevets et au Règlement sur les médicaments brevetés.
- DÉFAUT DE SOUMETTRE SES RAPPORTS
-
Défaut partiel ou complet d’un breveté de respecter les exigences réglementaires en matière de soumission de rapport prévues à la Loi sur les brevets et au Règlement sur les médicaments brevetés.
- DÉPENSES DE RECHERCHE ET DÉVELOPPEMENT
-
Aux termes du Règlement sur les médicaments brevetés, et plus particulièrement de ses articles 5 et 6, la recherche-développement s’entend des activités qui auraient été considérées admissibles au crédit d’impôt à l’investissement pour la recherche scientifique et le développement expérimental en vertu de la Loi de l’impôt sur le revenu dans sa version du 1er décembre 1987.
- DÉPENSES COURANTES DE RECHERCHE-DÉVELOPPEMENT
-
Désigne les dépenses autres qu’en capital directement liées à la recherche, dont : (a) salaires, (b) matériel direct, (c) entrepreneurs et sous-traitants, (d) autres coûts directs tels que les frais généraux de production, (e) paiements aux institutions désignées, (f) paiements aux organismes subventionnaires et (g) paiements aux autres organismes. Ces éléments sont décrits plus amplement dans le formulaire 3 présenté dans le Guide du breveté que vous trouverez dans sur notre site Web sous « Loi, Règlement et Lignes directrices ».
- ENGAGEMENT DE CONFORMITÉ VOLONTAIRE
-
Engagement écrit pris par le breveté de réduire le prix de son produit médicamenteux pour le rendre conforme aux Lignes directrices du Conseil. Le président du Conseil peut, en lieu d’un Avis d’audience, accepter un Engagement de conformité volontaire s’il sert les meilleurs intérêts du grand public. La politique du Conseil sur la conformité et l’application autorise le breveté à soumettre un Engagement de conformité volontaire même si un Avis d’audience a été émis. Toutefois, l’Engagement soumis à ce point doit être approuvé par le panel d’audience et non seulement par le président du Conseil. Le Conseil publie tous les Engagements acceptés par le président ou le Conseil.
- INDICE DES PRIX DES MÉDICAMENTS BREVETÉS (IPMB)
-
Indice établi par le CEPMB pour mesurer la variation annuelle des prix de transaction des médicaments brevetés vendus au Canada. Cet indice est établi à partir des données sur les prix et sur les ventes déclarées par les brevetés.
- LICENCE VOLONTAIRE
-
Engagement contractuel entre un breveté et un titulaire de licence permettant à ce dernier de bénéficier des retombées d’un brevet ou d’exercer des droits à l’égard de celui-ci moyennant une contrepartie financière (comme redevances sous forme de pourcentage des recettes tirées des ventes).
- MÉDICAMENT
-
Toute substance ou tout mélange de substances produites biologiquement, chimiquement ou autrement qui est appliqué ou administré in vivo pour faciliter le diagnostic, le traitement, l’atténuation ou la prévention d’une maladie, de symptômes, de troubles ou d’états physiques anormaux ou, encore, pour modifier des fonctions organiques chez les humains ou chez les animaux. Cette définition comprend les vaccins, les préparations topiques, les anesthésiques et les produits de diagnostic in vivo quel que soit le mode d’administration (p. ex. préparations transdermiques, gélules, solutions injectables, solutions pour inhalation, etc.). Cette définition exclut les appareils médicaux, les produits de diagnostic in vitro et les désinfectants qui ne sont pas utilisés in vivo.
- NUMÉRO D’IDENTIFICATION DU MÉDICAMENT (DIN)
-
Numéro d’identification que la Direction générale de la protection de la santé de Santé Canada attribue à chaque produit médicamenteux vendu sous ordonnance ou en vente libre et commercialisé en vertu du Règlement sur les aliments et drogues. Le DIN est assigné en tenant compte des éléments suivants : le fabricant du produit, le ou les ingrédients actifs, la concentration de ou des ingrédients actifs, la forme posologique, le nom de marque du produit et son mode d’administration.
- PRODUIT GÉNÉRIQUE
-
Produit médicamenteux contenant le même ingrédient actif, la même concentration et la même forme posologique que son équivalent de marque.
- PRODUIT MÉDICAMENTEUX
-
Présentation d’un médicament qui se distingue par sa forme pharmaceutique et la concentration de son ingrédient actif.
- PROGRAMME D’ACCÈS SPÉCIAL
-
Programme en vertu duquel Santé Canada permet à des praticiens d’avoir accès à des produits médicamenteux n’ayant pas encore reçu l’Avis de conformité et qui ne seraient autrement pas disponibles sur le marché canadien.
- RECHERCHE ET DÉVELOPPEMENT (R-D)
-
Recherche fondamentale ou appliquée visant à créer de nouveaux matériaux, dispositifs, produits ou procédés ou à améliorer ceux qui existent (p. ex. procédés de fabrication).
- RECHERCHE ET DÉVELOPPEMENT – AUTRE R-D ADMISSIBLE
-
Comprend les dépenses de recherchedéveloppement qui ne correspondent à aucune des catégories de R-D susmentionnées. Elle comprend les présentations sur la réglementation des produits médicamenteux, les études de biodisponibilité ainsi que les essais cliniques de phase IV.
- RECHERCHE ET DÉVELOPPEMENT – RECHERCHE APPLIQUÉE
-
Recherche et développement entrepris avec une application pratique précise en vue, notamment la recherche visant à à améliorer les procédés de fabrication ainsi que les études précliniques et cliniques.
- RECHERCHE ET DÉVELOPPEMENT – RECHERCHE FONDAMENTALE
-
Travaux qui contribuent à faire avancer la connaissance scientifique et qui sont entrepris sans aucune application pratique en vue.
- SUBSTANCE ACTIVE
-
Substance chimique ou biologique responsable de l’effet pharmacologique d’un produit médicamenteux.
Annexe 2 : Produits médicamenteux brevets ayant fait l’objet d’un premier rapport au CEPMB en 2013
| Marque de commerce |
Entreprise |
DIN |
Statut |
Niveau d’amélioration thérapeutique/ catégorie* |
|
* Vendu après l’entrée en vigueur des nouvelles Lignes directrices en 2010 :
MN Amélioration minime ou nulle
AM-S Amélioration modeste – facteurs secondaires
AM-P Amélioration modeste – facteurs principaux
AI Amélioration importante
D Découverte
Vendu avant l’entrée en vigueur des nouvelles Lignes directrices en 2010 :
Catégorie 1 Une forme posologique existante ou comparable d’un médicament existant
Catégorie 2 Une forme posologique non comparable d’un médicament existant, ou le premier DIN d’une nouvelle entité chimique constituant une découverte, sinon une amélioration importante par rapport aux DIN existants qui se prêtent à la comparaison du prix
Catégorie 3 Une forme posologique non comparable d’un médicament existant ou le premier DIN d’une nouvelle entité chimique offrant une amélioration modeste, voire même aucune amélioration par rapport aux DIN existants comparables
|
| 1 ACTIMMUNE - 100 mcg/fiole |
Vidara Therapeutics Inc. |
|
Sous examen |
|
| 2 ADEMPAS - 0,5 mg/comprimé |
Bayer Inc. |
02412764 |
Sous enquête |
MN |
| 3 ADEMPAS - 1 mg/comprimé |
Bayer Inc. |
02412772 |
Sous enquête |
MN |
| 4 ADEMPAS - 1,5 mg/comprimé |
Bayer Inc. |
02412799 |
Conforme aux Lignes directrices |
MN |
| 5 ADEMPAS - 2 mg/comprimé |
Bayer Inc. |
02412802 |
Conforme aux Lignes directrices |
MN |
| 6 ADEMPAS - 2,5 mg/comprimé |
Bayer Inc. |
02412810 |
Conforme aux Lignes directrices |
MN |
| 7 AUBAGIO - 14 mg/comprimé |
Genzyme Canada Inc. |
02416328 |
Sous examen |
MN |
| 8 AXIRON - 30 mg/dose |
Eli Lilly Canada Inc. |
02382369 |
Conforme aux Lignes directrices |
MN |
| 9 BENEFIX - 3000 unit/fiole |
Pfizer Canada Inc. |
02392984 |
Conforme aux Lignes directrices |
MN |
| 10 BREO ELLIPTA 100/25 |
GlaxoSmithKline Inc. |
02408872 |
Sous enquête |
MN |
| 11 CIPRALEX MELTZ - 10 mg/comprimé |
Lundbeck Canada Inc. |
02391449 |
Conforme aux Lignes directrices |
MN |
| 12 CIPRALEX MELTZ - 20 mg/comprimé |
Lundbeck Canada Inc. |
02391457 |
Conforme aux Lignes directrices |
MN |
| 13 CLINDOXYL ADV 10/30 |
GlaxoSmithKline Inc. |
02382822 |
Ne justifie pas une enquête |
MN |
| 14 DUODOPA 20/5 |
Abbvie |
02292165 |
Sous enquête |
AM-P |
| 15 EDARBYCLOR 40/12,5 |
Takeda Canada Inc. |
02397749 |
Conforme aux Lignes directrices |
MN |
| 16 EDARBYCLOR 40/25 |
Takeda Canada Inc. |
02397765 |
Conforme aux Lignes directrices |
MN |
| 17 EDARBYCLOR 80/12,5 |
Takeda Canada Inc. |
02397757 |
Conforme aux Lignes directrices |
MN |
| 18 ELIQUIS - 5 mg/comprimé |
Bristol-Myers Squibb Canada Co. |
02397714 |
Conforme aux Lignes directrices |
MN |
| 19 ERIVEDGE - 150 mg/capsule |
Hoffmann-La Roche Limited, Canada |
02409267 |
Ne justifie pas une enquête |
AM-P |
| 20 FLUAD - 45 mcg/dose |
Novartis Pharmaceuticals Canada Inc. |
02362384 |
Conforme aux Lignes directrices |
MN |
| 21 FYCOMPA - 2 mg/comprimé |
Eisai Limited |
02404516 |
Conforme aux Lignes directrices |
MN |
| 22 FYCOMPA - 4 mg/comprimé |
Eisai Limited |
02404524 |
Conforme aux Lignes directrices |
MN |
| 23 FYCOMPA - 6 mg/comprimé |
Eisai Limited |
02404532 |
Conforme aux Lignes directrices |
MN |
| 24 FYCOMPA - 8 mg/comprimé |
Eisai Limited |
02404540 |
Conforme aux Lignes directrices |
MN |
| 25 FYCOMPA - 10 mg/comprimé |
Eisai Limited |
02404559 |
Conforme aux Lignes directrices |
MN |
| 26 FYCOMPA - 12 mg/comprimé |
Eisai Limited |
02404567 |
Conforme aux Lignes directrices |
MN |
| 27 GALEXOS - 150 mg/capsule |
Janssen Inc. |
02416441 |
Conforme aux Lignes directrices |
MI-P |
| 28 GD-LATANOPROST/TIMOLOL 0,5/5 |
GenMed |
02373068 |
Conforme aux Lignes directrices |
MN |
| 29 GD-PREGABALIN - 25 mg/capsule |
GenMed |
02360136 |
Conforme aux Lignes directrices |
MN |
| 30 GD-PREGABALIN - 50 mg/capsule |
GenMed |
02360144 |
Conforme aux Lignes directrices |
MN |
| 31 GD-PREGABALIN - 75 mg/capsule |
GenMed |
02360152 |
Conforme aux Lignes directrices |
MN |
| 32 GD-PREGABALIN - 150 mg/capsule |
GenMed |
02360179 |
Conforme aux Lignes directrices |
MN |
| 33 GD-PREGABALIN - 225 mg/capsule |
GenMed |
02360195 |
Conforme aux Lignes directrices |
MN |
| 34 GD-PREGABALIN - 300 mg/capsule |
GenMed |
02360209 |
Conforme aux Lignes directrices |
MN |
| 35 GD-SILDENAFIL - 25 mg/comprimé |
GenMed |
02291991 |
Conforme aux Lignes directrices |
MN |
| 36 GD-SILDENAFIL - 50 mg/comprimé |
GenMed |
02292009 |
Conforme aux Lignes directrices |
MN |
| 37 GD-SILDENAFIL - 100 mg/comprimé |
GenMed |
02292017 |
Conforme aux Lignes directrices |
MN |
| 38 GELNIQUE - 100 mg/g |
Actavis Specialty Pharmaceuticals Co. |
02366150 |
Sous enquête |
MN |
| 39 GENOTROPIN - 0,6 mg/syringe |
Pfizer Canada Inc. |
02401762 |
Conforme aux Lignes directrices |
MN |
| 40 GENOTROPIN - 0,8 mg/syringe |
Pfizer Canada Inc. |
02401770 |
Conforme aux Lignes directrices |
MN |
| 41 GENOTROPIN - 1 mg/syringe |
Pfizer Canada Inc. |
02401789 |
Conforme aux Lignes directrices |
MN |
| 42 GENOTROPIN - 1,2 mg/syringe |
Pfizer Canada Inc. |
02401797 |
Conforme aux Lignes directrices |
MN |
| 43 GENOTROPIN - 1,4 mg/syringe |
Pfizer Canada Inc. |
02401800 |
Conforme aux Lignes directrices |
MN |
| 44 GENOTROPIN - 1,6 mg/syringe |
Pfizer Canada Inc. |
02401819 |
Conforme aux Lignes directrices |
MN |
| 45 GENOTROPIN - 1,8 mg/syringe |
Pfizer Canada Inc. |
02401827 |
Conforme aux Lignes directrices |
MN |
| 46 GENOTROPIN - 2 mg/syringe |
Pfizer Canada Inc. |
02401835 |
Conforme aux Lignes directrices |
MN |
| 47 GENOTROPIN - 5,3 mg/pen |
Pfizer Canada Inc. |
02401703 |
Ne justifie pas une enquête |
MN |
| 48 GENOTROPIN - 12 mg/pen |
Pfizer Canada Inc. |
02401711 |
Conforme aux Lignes directrices |
MN |
| 49 HUMALOG KWIKPEN - 100 unit/ml |
Eli Lilly Canada Inc. |
02403412 |
Sous enquête |
MN |
| 50 INTUNIV XR - 1 mg/comprimé |
Shire Canada Inc. |
02409100 |
Conforme aux Lignes directrices |
AM-S |
| 51 INTUNIV XR - 2 mg/comprimé |
Shire Canada Inc. |
02409119 |
Conforme aux Lignes directrices |
AM-S |
| 52 INTUNIV XR - 3 mg/comprimé |
Shire Canada Inc. |
02409127 |
Sous enquête |
AM-S |
| 53 INTUNIV XR - 4 mg/comprimé |
Shire Canada Inc. |
02409135 |
Sous enquête |
AM-S |
| 54 ISENTRESS - 25 mg/comprimé |
Merck Canada Inc. |
02392429 |
Conforme aux Lignes directrices |
MN |
| 55 ISENTRESS - 100 mg/comprimé |
Merck Canada Inc. |
02392437 |
Conforme aux Lignes directrices |
MN |
| 56 JAKAVI - 5 mg/comprimé |
Novartis Pharmaceuticals Canada Inc. |
02388006 |
Conforme aux Lignes directrices |
AM-P |
| 57 JAKAVI - 15 mg/comprimé |
Novartis Pharmaceuticals Canada Inc. |
02388014 |
Conforme aux Lignes directrices |
AM-P |
| 58 JAKAVI - 120 mg/comprimé |
Novartis Pharmaceuticals Canada Inc. |
02388022 |
Conforme aux Lignes directrices |
AM-P |
| 59 JAYDESS - 13 mg/unit |
Bayer Inc. |
02408295 |
Conforme aux Lignes directrices |
MN |
| 60 JENTADUETO 2.5/500 |
Boehringer Ingelheim (Canada) Ltd. |
02403250 |
Conforme aux Lignes directrices |
MN |
| 61 JENTADUETO 2,5/850 |
Boehringer Ingelheim (Canada) Ltd. |
02403269 |
Conforme aux Lignes directrices |
MN |
| 62 JENTADUETO 2,5/1000 |
Boehringer Ingelheim (Canada) Ltd. |
02403277 |
Conforme aux Lignes directrices |
MN |
| 63 JETREA - 2,5 mg/ml |
Alcon Canada Inc. |
02410818 |
Conforme aux Lignes directrices |
D |
| 64 KADCYLA - 20 mg/ml |
Hoffmann-La Roche Limited, Canada |
02412365 |
Conforme aux Lignes directrices |
MI-P |
| 65 KALYDECO - 150 mg/comprimé |
Vertex Pharmaceuticals Canada Inc. |
02397412 |
Conforme aux Lignes directrices |
SI |
| 66 KOMBOGLYZE 500/2,5 |
Bristol-Myers Squibb Canada Co. |
02389169 |
Sous enquête |
MN |
| 67 KOMBOGLYZE 850/2,5 |
Bristol-Myers Squibb Canada Co. |
02389177 |
Sous enquête |
MN |
| 68 KOMBOGLYZE 1000/2,5 |
Bristol-Myers Squibb Canada Co. |
02389185 |
Sous enquête |
MN |
| 69 LEVEMIR FLEXTOUCH - 100 unit/ml |
Novo Nordisk Canada Inc. |
02412829 |
Ne justifie pas une enquête |
MN |
| 70 MEKINIST - 0,5 mg/comprimé |
GlaxoSmithKline Inc. |
02409623 |
Sous enquête |
MN |
| 71 MEKINIST - 2 mg/comprimé |
GlaxoSmithKline Inc. |
02409658 |
Sous enquête |
MN |
| 72 METADOL - 25 mg/comprimé |
Paladin Laboratories Inc. |
02247701 |
Sous enquête |
MN |
| 73 MYRBETRIQ - 25 mg/comprimé |
Astellas Pharma Canada Inc. |
02402874 |
Conforme aux Lignes directrices |
MN |
| 74 MYRBETRIQ - 50 mg/comprimé |
Astellas Pharma Canada Inc. |
02402882 |
Conforme aux Lignes directrices |
MN |
| 75 NAGLAZYME - 1 mg/ml |
Biomarin Pharmaceutical Canada Inc. |
02412683 |
Conforme aux Lignes directrices |
Catégorie 2 |
| 76 NEUPRO - 2 mg/patch |
UCB Canada Inc. |
02403900 |
Conforme aux Lignes directrices |
AM-S |
| 77 NEUPRO - 4 mg/patch |
UCB Canada Inc. |
02403927 |
Conforme aux Lignes directrices |
AM-S |
| 78 NEUPRO - 6 mg/patch |
UCB Canada Inc. |
02403935 |
Conforme aux Lignes directrices |
AM-S |
| 79 NEUPRO - 8 mg/patch |
UCB Canada Inc. |
02403943 |
Conforme aux Lignes directrices |
AM-S |
| 80 NIMENRIX - 5 mcg/dose |
GlaxoSmithKline Inc. |
02402904 |
Conforme aux Lignes directrices |
MN |
| 81 NOCDURNA - 25 mcg/comprimé |
Ferring Pharmaceuticals Inc. |
02397927 |
Conforme aux Lignes directrices |
MN |
| 82 NUTROPIN AQ NUSPIN 5 |
Hoffmann-La Roche Limited, Canada |
02399091 |
Conforme aux Lignes directrices |
MN |
| 83 NUTROPIN AQ NUSPIN 20 |
Hoffmann-La Roche Limited, Canada |
02399083 |
Conforme aux Lignes directrices |
MN |
| 84 OLUX-E - 0,5 mg/gram |
GlaxoSmithKline Inc. |
02344408 |
Conforme aux Lignes directrices |
MN |
| 85 OMNARIS HFA - 50 mcg/dose |
Takeda Canada Inc. |
02404311 |
Ne justifie pas une enquête |
MN |
| 86 ORENCIA - 125 mg/syringe |
Bristol-Myers Squibb Canada Co. |
02402475 |
Conforme aux Lignes directrices |
AM-P |
| 87 OXY-IR - 5 mg/comprimé |
Purdue Pharma |
02231934 |
Conforme aux Lignes directrices |
Catégorie 1 |
| 88 OXY-IR - 10 mg/comprimé |
Purdue Pharma |
02240131 |
Conforme aux Lignes directrices |
Catégorie 1 |
| 89 OXY-IR - 20 mg/comprimé |
Purdue Pharma |
02240132 |
Conforme aux Lignes directrices |
Catégorie 1 |
| 90 PENTASA - 1 gm/comprimé |
Ferring Pharmaceuticals Inc. |
02399466 |
Ne justifie pas une enquête |
MN |
| 91 PERJETA - 420 mg/fiole |
Hoffmann-La Roche Limited, Canada |
02405016 |
Conforme aux Lignes directrices |
AI |
| 92 PERJETA-HERCEPTIN 420/440 |
Hoffmann-La Roche Limited, Canada |
02405024 |
Conforme aux Lignes directrices |
MN |
| 93 PICATO 0,15 - 70 mcg/tube |
Leo Pharma Inc. |
02400995 |
Conforme aux Lignes directrices |
AM-S |
| 94 PICATO 0,5 - 235 mcg/tube |
Leo Pharma Inc. |
02400995 |
Conforme aux Lignes directrices |
AM-S |
| 95 PREZISTA - 800 mg/comprimé |
Janssen Inc. |
02393050 |
Sous enquête |
MN |
| 96 SEEBRI BREEZHALER - 50 mcg/capsule |
Novartis Pharmaceuticals Canada Inc. |
02394936 |
Conforme aux Lignes directrices |
MN |
| 97 SIGNIFOR - 0,3 mg/ml |
Novartis Pharmaceuticals Canada Inc. |
02413299 |
Conforme aux Lignes directrices |
D |
| 98 SIGNIFOR - 0,6 mg/ml |
Novartis Pharmaceuticals Canada Inc. |
02413302 |
Conforme aux Lignes directrices |
D |
| 99 SIGNIFOR - 0,9 mg/ml |
Novartis Pharmaceuticals Canada Inc. |
02413310 |
Conforme aux Lignes directrices |
D |
| 100 SIMPONI - 100 mg/ml |
Janssen Inc. |
02413183 |
Conforme aux Lignes directrices |
MN |
| 101 SIMPONI - 100 mg/syringe |
Janssen Inc. |
02413175 |
Conforme aux Lignes directrices |
MN |
| 102 STELARA - 90 mg/fiole |
Janssen Inc. |
02320681 |
Conforme aux Lignes directrices |
MN |
| 103 STIBILD 150/150/200/300 |
Gilead Sciences Inc. |
02397137 |
Conforme aux Lignes directrices |
MN |
| 104 STIVARGA - 40 mg/comprimé |
Bayer Inc |
02403390 |
Conforme aux Lignes directrices |
MN |
| 105 SUBLINOX - 5 mg/comprimé |
Meda Valeant Pharma Canada Inc. |
02391678 |
Ne justifie pas une enquête |
MN |
| 106 TAFINLAR - 50 mg/capsule |
GlaxoSmithKline Inc. |
02409607 |
Sous examen |
|
| 107 TAFINLAR - 75 mg/capsule |
GlaxoSmithKline Inc. |
02409615 |
Sous examen |
|
| 108 TECFIDERA - 120 mg/capsule |
Biogen Idec Canada Inc. |
02404508 |
Conforme aux Lignes directrices |
MN |
| 109 TEFLARO - 600 mg/fiole |
Forest Laboratories Canada Inc. |
|
Conforme aux Lignes directrices |
MN |
| 110 TRETTEN - 15 mg/fiole |
Novo Nordisk Canada Inc. |
02389975 |
Conforme aux Lignes directrices |
AM-S |
| 111 TUDORZA GENUAIR - 400 mcg/dose |
Almirall Limited |
02409720 |
Conforme aux Lignes directrices |
MN |
| 112 XEOMIN - 50 unit/fiole |
Merz Pharma Canada Ltd. |
02371081 |
Conforme aux Lignes directrices |
MN |
| 113 XERESE 50/10 |
Valeant Canada |
02404044 |
Conforme aux Lignes directrices |
MN |
| 114 YAZ PLUS |
Bayer Inc. |
02387433 |
Conforme aux Lignes directrices |
MN |
| 115 ZAXINE - 500 mg/comprimé |
Salix Pharmaceuticals Inc. |
02410702 |
Sous enquête |
MN |
Annexe 3 : Recherche et développement
Tableau 21 Intervalle des ratios des dépenses de R-D par rapport aux recettes tirées des ventes, selon le nombre de brevetés ayant présenté des rapports et la valeur des recettes tirées des ventes
| Intervalle : Ratio des dépenses de R-D par rapport aux recettes tirées des ventes |
Nbre de
brevetés ayant présenté un rapport en 2013 |
Recettes
tirées
des ventes
en 2013
(millions $) |
Part en 2013 (%) |
Nbre de
brevetés ayant présenté un rapport en 2012 |
Recettes
tirées
des ventes
en 2012
(millions $) |
Part
en 2013
(%) |
|
* Il se peut que les valeurs n’équivaillent pas à 100,0 en raison de l’arrondissement.
Source : CEPMB
|
| 0% |
33 |
2 744,5 |
16,3 |
32 |
1 596,4 |
9,5 |
| £ 10 % |
37 |
13 210,3 |
78,5 |
39 |
11 794,4 |
73,2 |
| > 10 % |
11 |
863,1 |
5,1 |
14 |
3 363,6 |
17,3 |
| Total |
81 |
16 817,9 |
100,0* |
85 |
16 754,4 |
100,0 |
GRAPHIQUE 21 Dépenses courantes de R-D selon le type de recherche, 1988-2013
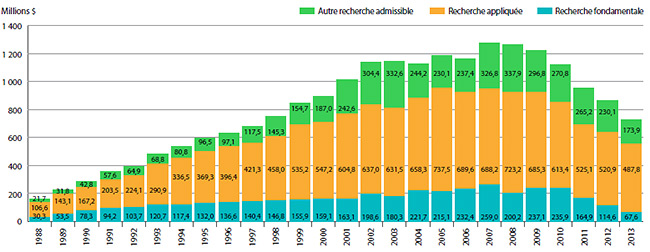
Source : CEPMB
Tableau 22 Ratios des dépenses de R-D par rapport aux recettes tirées des ventes selon les brevetés1 qui ont présenté un rapport, 2013 et 2012
| Breveté |
Ratio des dépenses de R-D par rapport aux recettes tirées des ventes
2013 |
Ratio des dépenses de R-D par rapport aux recettes tirées des ventes
2012 |
|
1 Pour éviter la double comptabilisation des recettes tirées des ventes, les recettes tirées des redevances sont incluses dans le calcul du ratio de chaque société mais non dans le calcul du ratio à l’échelle de l’industrie. Les subventions des gouvernements fédéral et provinciaux ne sont pas comptabilisées dans les dépenses utilisées pour le calcul des ratios des dépenses de R-D de chaque société, mais elles le sont dans les statistiques pour l’ensemble des brevetés. La liste des brevetés ayant soumis un rapport sur les prix n’est pas identique à celle des brevetés ayant soumis un rapport sur leurs activités de R-D en raison des différences des modalités de rapport entre les brevetés et leurs sociétés affiliées ou les détenteurs d’une licence d’exploitation. Il convient également de noter que les titulaires d’un brevet pour un médicament vétérinaire sont tenus de présenter chaque année un rapport sur leurs dépenses de R-D, mais pas nécessairement sur les prix qu’ils pratiquent et la valeur de leurs ventes.
2 Membre de Rx&D.
3 Membre de BIOTECanada.
4 Société dérivée de la division des produits de marque déposée d’Abbott en entité juridique distincte à partir du 31 octobre 2012.
5 N’était pas un breveté en 2012.
6 Anciennement connu sous le nom Fujisawa Canada Inc.
7 Anciennement connu sous le nom Aventis Pasteur Ltd.
8 Anciennement connu sous le nom Aventis Pharma Inc.
9 Anciennement connu sous le nom ICN Canada Ltd.
|
| Abbott Laboratories, Ltd.2,3 |
0,0 |
1,0 |
| AbbVie Corporation2,3,4 |
2,2 |
1,9 |
| Actavis Specialty Pharmaceuticals Co. (Watson Pharma Co.) |
0,0 |
0,0 |
| Actelion Pharmaceutiques Canada Inc.2 |
4,2 |
4,4 |
| Alcon Canada Inc. |
0,1 |
0,1 |
| Alexion Pharmaceuticals Inc.3 |
0,0 |
0,0 |
| Allergan Inc. |
3,8 |
5,3 |
| Almirall Limited2,5 |
59,2 |
– |
| Alveda Pharmaceuticals Inc. |
0,0 |
0,0 |
| Amgen Canada Inc.2,3 |
7,0 |
6,9 |
| Astellas Pharma Canada Inc.2,6 |
3,3 |
5,3 |
| AstraZeneca Canada Inc.2,3 |
1,8 |
1,8 |
| Baxter Corporation3 |
0,4 |
0,2 |
| Bayer Inc., Healthcare Division2 |
4,3 |
4,4 |
| Biogen Idec Canada Inc.3 |
10.3 |
12.5 |
| BioMarin Canada Inc.3 |
19,2 |
52,3 |
| Biovitrum AB |
0,0 |
0,0 |
| Boehringer Ingelheim (Canada) Ltd.2 |
5,8 |
11,3 |
| Bracco Diagnostics Canada Inc. |
0,0 |
0,0 |
| Bristol-Myers Squibb Pharmaceutical Group2,3 |
12,0 |
13,1 |
| Celgene Canada3 |
1,2 |
1,8 |
| CSL Behring Canada Inc. |
0,8 |
0,8 |
| Cubist Pharmaceuticals Canada, Inc. (Optimer Pharmaceuticals Canada Inc,) |
0,0 |
0,0 |
| Duchesnay Inc. |
9,4 |
6,4 |
| Eisai Limited2,3 |
12,3 |
172,5 |
| Eli Lilly Canada Inc. (includes Provel Animal Health Division)2,3 |
9,6 |
10,0 |
| EMD Serono Canada Inc.2 |
5,9 |
7,2 |
| Ferring Inc. |
0,0 |
3,1 |
| Forest Laboratories Canada Inc.2,5 |
149,5 |
– |
| Galderma Canada Inc. |
0,0 |
0,0 |
| Gilead Sciences Inc.2 |
19,5 |
24,6 |
| GlaxoSmithKline Inc.2 |
9,7 |
10,6 |
| Grifols Canada Ltd. (Talecris Biotherapeutics Ltd.)3 |
0,0 |
0,9 |
| Hoffmann-La Roche Ltd. Canada2 |
4,7 |
3,9 |
| Hospira Healthcare Corp. |
0,0 |
0,0 |
| Intermune Canada Inc.2 |
0,0 |
0,0 |
| Correvio (UK) Ltd.3 (Iroko International LP) |
0,0 |
0,0 |
| Janssen Inc.2,3 |
3,1 |
3,3 |
| Johnson & Johnson Inc. |
0,0 |
0,0 |
| Johnson & Johnson Medical Products |
0,0 |
0,8 |
| Lantheus MI Canada Inc. |
0,0 |
0,0 |
| LEO Pharma Inc.2 |
0,9 |
2,1 |
| Lundbeck Canada Inc.2 |
0,3 |
0,7 |
| McNeil Consumer Healthcare Canada |
2,9 |
2,6 |
| Meda Valeant Pharma Canada Ltd.5 |
0,0 |
– |
| Medical Futures Inc. |
0,0 |
0,0 |
| Merck Canada Inc.2,3 |
1,7 |
3,3 |
| Merus Labs5 |
0,0 |
– |
| Merz Pharma Canada Ltd. |
6,6 |
6,5 |
| Novartis Pharmaceuticals Canada Inc.2,3 |
9,4 |
12,6 |
| Novo Nordisk Canada Inc.2,3 |
1,3 |
1,7 |
| Otsuka America Pharmaceuticals2 |
39,1 |
17,4 |
| Paladin Laboratories Inc.2 |
0,0 |
0,02 |
| Pfizer Canada Inc.2,3 |
1,7 |
6,0 |
| Pharmascience Inc. |
9,8 |
8,6 |
| Purdue Pharma2 |
4,9 |
2,4 |
| Ranbaxy Pharmaceuticals Canada Inc. |
0,0 |
0,0 |
| Salix Pharmaceuticals Inc.5 |
130,7 |
– |
| sanofi pasteur Ltd.2,3,7 |
69,7 |
53,4 |
| sanofi-aventis Pharma Inc.2,8 |
3,7 |
5,8 |
| Sandoz Canada Inc. |
0,0 |
1,2 |
| Seattle Genetics Inc. |
6,6 |
14,7 |
| Sunovion (Sepracor Pharmaceuticals Canada Inc.)2 |
0,0 |
0,0 |
| Servier Canada Inc.2 |
4,6 |
5,4 |
| Shire Canada Inc.2 |
0,2 |
0,1 |
| Shire Human Genetic Therapies3 |
1,2 |
2,5 |
| Sigma Tau Pharmaceuticals Inc. |
0,0 |
0,0 |
| Sopherion Therapeutics Canada Inc. |
0,0 |
0,0 |
| Takeda Canada Inc.2,3 |
0,0 |
0,0 |
| Teva Canada Ltd. (Ratiopharm) |
0,0 |
0,0 |
| Teva Canada Innovation GP3 |
0,9 |
2,1 |
| Tribute Pharma Canada Inc. |
0,0 |
0,0 |
| Triton Pharma Inc. |
0,0 |
0,0 |
| Tyco Healthcare Group Canada Inc. |
0,0 |
0,0 |
| UCB Pharma Canada Inc.3 |
7,9 |
9,7 |
| Valeant Canada Ltd.3,9 |
0,0 |
0,0 |
| Vertex Pharma Canada Inc.3 |
21,3 |
26,4 |
| Vetoquinol Canada Inc.5 |
2,8 |
– |
| Vidara Therapeutics Inc.5 |
0,0 |
– |
| VIIV Healthcare ULC.2 |
0,0 |
0,0 |
| Warner Chilcott Canada Inc. |
0,2 |
0,1 |
Tableau 23 Dépenses courantes de R-D selon la province ou le territoire, 2013
| Province |
Dépenses par
tous les brevetés
(000 $) |
Part
régionale
(%) |
Dépenses par tous
les brevetés membres
de Rx&D (000 $) |
Part
régionale
(%) |
| Source : CEPMB |
| Terre-Neuve |
3 775,90 |
0,518 |
3 228,63 |
0,509 |
| Île-du-Prince-Édouard |
43,65 |
0,006 |
32,01 |
0,005 |
| Nouvelle-Écosse |
14 578,32 |
1,999 |
13 801,87 |
2,178 |
| Nouveau-Brunswick |
1 718,36 |
0,236 |
1 341,37 |
0,212 |
| Québec |
292 013,44 |
40,041 |
233 405,50 |
36,832 |
| Ontario |
321 955,62 |
44,147 |
293 662,89 |
46,341 |
| Manitoba |
4 525,55 |
0,621 |
3 954,54 |
0,624 |
| Saskatchewan |
1 607,98 |
0,220 |
1 063,21 |
0,168 |
| Alberta |
58 616,21 |
8,037 |
55 555,94 |
8,767 |
| Colombie-Britannique |
30 451,30 |
4,175 |
27 651,92 |
4,364 |
| Territoires |
0 |
0,000 |
0 |
0,000 |
| Canada |
729 286,33 |
100,0 |
633 697,88 |
100,0 |
Tableau 24 Dépenses courantes R-D selon le milieu de recherche et la province ou le territoire, 2013
| Province |
|
Brevetés |
Autres sociétés |
Universités |
Hôpitaux |
Autres |
|
Remarques :
- Le pourcentage figurant sous chaque catégorie de R-D correspond au pourcentage de toutes les dépenses engagées dans cette catégorie dans la province.
- Les dépenses présentées sous forme de pourcentage du total correspondent au pourcentage des dépenses de R-D par rapport à l’ensemble des dépenses de R-D faites au Canada.
- Le total des colonnes et des rangées ne correspond pas nécessairement, car certains chiffres ont été arrondis.
- Dépenses courantes plus dépenses en immobilisations (équipement + amortissement) = total des dépenses de R-D.
Source : CEPMB
|
| Terre-Neuve |
000 $ |
682,43 |
1 908,41 |
444,31 |
104,36 |
636,39 |
| % |
18,1 |
50,5 |
11,8 |
2,8 |
16,8 |
| Île-du-Prince-Édouard |
000 $ |
0,0 |
11,64 |
0,0 |
5,94 |
26,07 |
| % |
0,0 |
26,7 |
0,0 |
13,6 |
59,7 |
| Nouvelle-Écosse |
000 $ |
1 070,79 |
3 310,91 |
6 074,00 |
2 254,95 |
1 867,66 |
| % |
7,3 |
22,7 |
41,7 |
15,5 |
12,8 |
| Nouveau-Brunswick |
000 $ |
115,33 |
837,68 |
0,0 |
337,63 |
427,71 |
| % |
6,7 |
48,7 |
0,0 |
19,6 |
24,9 |
| Québec |
000 $ |
135 074,27 |
92 742,32 |
11 585,75 |
14 314,87 |
38 296,23 |
| % |
46,3 |
31,7 |
3,9 |
4,9 |
13,1 |
| Ontario |
000 $ |
172 685,52 |
70 194,20 |
13 834,96 |
34 498,05 |
30 742,89 |
| % |
53,6 |
21,8 |
4,3 |
10,7 |
9,5 |
| Manitoba |
000 $ |
794,80 |
1 601,49 |
170,04 |
1 110,99 |
848,22 |
| % |
17,6 |
35,4 |
3,8 |
24,5 |
18,7 |
| Saskatchewan |
000 $ |
95,68 |
1 051,49 |
213,48 |
133,79 |
113,54 |
| % |
6,0 |
65,4 |
13,3 |
8,3 |
7,1 |
| Alberta |
000 $ |
41 799,62 |
7 231,48 |
2 722,01 |
3 156,34 |
3 706,76 |
| % |
71,3 |
12,3 |
4,6 |
5,4 |
6,3 |
| Colombie-Britannique |
000 $ |
12 511,04 |
8 504,75 |
699,69 |
3 065,09 |
5 670,73 |
| % |
41,1 |
27,9 |
2,3 |
10,1 |
18,6 |
| Territories |
000 $ |
0,0 |
0,0 |
0,0 |
0,0 |
0,0 |
| % |
0,0 |
0,0 |
0,0 |
0,0 |
0,0 |
| Canada |
000 $ |
364 829,49 |
187 394,38 |
35 744,23 |
58 982,01 |
82 336,18 |
| % |
50,0 |
25,7 |
4,9 |
8,1 |
11,3 |
Notes
1 Les résultats statistiques présentés dans le présent chapitre se fondent sur les données que les brevetés ont soumises au CEPMB en date d’avril 2014. Il arrive que des brevetés soumettent un nouveau rapport révisant les données présentées ou contenant des données qui n’avaient pas été présentées dans un rapport antérieur. Ces données peuvent modifier d’une façon assez importante les statistiques utilisées pour la préparation du présent chapitre du rapport annuel. Pour tenir compte d’une telle éventualité, le CEPMB révise le calcul des données sur les ventes (voir la section « Ventes des produits médicamenteux brevetés »). Il fait aussi rapport du calcul révisé des indices de prix et de quantité (voir la section « Tendances observées au niveau des prix », ainsi que la section « Utilisation faite des produits médicamenteux brevetés ») et des ratios des prix pratiqués dans les pays de comparaison par rapport aux prix pratiqués au Canada (voir la section « Comparaison des prix pratiqués au Canada avec ceux pratiqués dans les pays de comparaison ») pour les cinq années précédant l’année sur laquelle porte le présent rapport annuel. Les nouvelles valeurs ainsi obtenues reflètent les données courantes disponibles. En conséquence, lorsque la révision des données a été faite, les valeurs rapportées dans le présent rapport peuvent être différentes de celles présentées dans des rapports annuels antérieurs.
2 Bien que fondés en partie sur les données obtenues en vertu d’une licence de la base de données MIDAS d’IMS, les énoncés, les constatations, les observations, les avis et les opinions exprimés dans le présent rapport annuel peuvent seulement être attribués au CEPMB et ne doivent pas être interprétés comme appartenant à IMS AG.
3 Dans le présent cas, l’« effet du retrait du médicament » correspond au montant des ventes générées en 2013 par les produits médicamenteux qui étaient assujettis à la compétence du CEPMB en 2012, mais non en 2013. L’« effet du nouveau médicament » correspond au montant des ventes générées en 2013 par les produits médicamenteux qui relevaient de la compétence du CEPMB en 2013, mais non en 2012. Les autres effets sont calculés au moyen de la relation suivante :
S p2013(i) q2013(i) - S p2012(i) q2012(i) = S [p2013 (i) - p2012(i)]q2012 (i) + S p2012 (i) [q2013 (i) - q2012 (i)] + S [p2013 (i) - p2012(i)] [q2013(i) - q2012(i)]
où py(i) correspond au prix du produit médicamenteux « i » l’année « y », qy(i) au volume physique du produit médicamenteux « i » vendu l’année « y » et S à la somme des produits médicamenteux qui relevaient de la compétence du CEPMB en matière d’examen du prix pour les années 2012 et 2013. La partie gauche de l’équation représente la variation des ventes de ces produits médicamenteux en 2013 par rapport à 2012. Du côté droit de l’équation, les trois termes définissent respectivement l’effet de volume, l’effet de prix et les effets croisés. Ces effets sont présentés dans le tableau 8.
4 Ratio annuel de la variation de la valeur monétaire des ventes des produits médicamenteux de cette catégorie thérapeutique par rapport à la variation de la valeur des ventes de tous les produits médicamenteux brevetés.
5 Ces calculs sont effectués au niveau défini par le numéro d’identification du médicament (DIN) attribué par Santé Canada. Chaque DIN représente une combinaison unique d’ingrédients actifs, de forme(s) posologique(s) et de concentration(s).
6 Les prix des produits médicamenteux (et donc l’IPMB) peuvent au cours d’une année donnée augmenter davantage que l’IPC. Cette situation peut se produire lorsque les brevetés n’ont pas appliqué les dernières augmentations annuelles de prix auxquelles ils avaient droit. Elle peut également se produire lorsque le taux prévu d’inflation/IPC se révèle plus élevé que le taux réel.
7 R représente le taux général de variation de l’IPMB et N, les groupes thérapeutiques nommés 1,2… N. R(i) représente le taux moyen de variation du prix du groupe thérapeutique principal i obtenu avec la méthodologie de l’IPMB. R étant une moyenne des variations des prix de tous les produits médicamenteux pondérée en fonction de la valeur des ventes, il devient facile de calculer la relation suivante :
R = w(1) x R(1) + w(2) x R(2) + … + w(N) x R(N)
de l’ensemble des ventes. Cette dernière équation constitue la base de la décomposition par groupe thérapeutique présentée à la dernière colonne du tableau 10. Chaque terme du côté droit multiplie le taux moyen de variation du prix pour un groupe thérapeutique donné par sa part de l’ensemble des ventes. La valeur ainsi obtenue peut facilement être considérée comme la contribution du groupe thérapeutique correspondant à la variation de l’IPMB. L’envergure de cette contribution dépend du taux de variation du prix pour le groupe thérapeutique en question et de son importance relative, mesurée par sa part de l’ensemble des ventes.
La décomposition dans le tableau 10 est approximative, étant donné que les pondérations utilisées pour calculer la contribution de chaque groupe thérapeutique sont tirées des données sur les ventes annuelles, alors que le taux de variation du prix (pour l’ensemble des groupes thérapeutiques ou pour chaque groupe thérapeutique) est calculé avec des données couvrant des périodes de six mois. L’écart obtenu est généralement minime.
8 Ce sont les grossistes qui accaparent la part de lion des ventes de produits médicamenteux brevetés avec 79,3 % de l’ensemble des ventes effectuées en 2013. Les hôpitaux sont à l’origine de 7,7 % des ventes et les ventes directes aux pharmacies de 5,1 %. Depuis 2001, les ventes aux pharmacies de produits médicamenteux brevetés ont subi une baisse draconienne, le taux étant alors de 20,1 %.
9 Les résultats pour une quatrième catégorie de clients, la catégorie « autres », ne sont pas fournis. En 2013, cette catégorie a été à la source d’environ 7,9 % de toutes les ventes de produits médicamenteux brevetés. Les acheteurs de la catégorie « autres » sont essentiellement des établissements de soins de santé autres que les hôpitaux, notamment des cliniques médicales et des centres de soins infirmiers, mais également les gouvernements. Étant donné que la composition de cette catégorie de clients varie beaucoup d’année en année, son analyse des variations de prix apparaît plus ou moins utile.
10 Ce constat fait référence au comportement des prix en général. Il peut y avoir des cas où le prix d’un produit médicamenteux a augmenté ou baissé d’une façon marquée depuis son lancement sur le marché canadien.
11 L’industrie pharmaceutique des États-Unis a fait valoir que les prix accessibles au public dans ce pays ne reflètent pas vraiment les prix réels en raison des remises et des rabais consentis sur une base confidentielle. Depuis janvier 2000, dans la foulée d’une consultation publique, le CEPMB inclut les prix figurant dans la Classification fédérale des approvisionnements (Federal Supply Schedule ou FSS) des États-Unis dans son calcul des prix moyens des médicaments brevetés pratiqués aux États-Unis. Les prix de la Classification fédérale sont négociés entre les fabricants et le département des Anciens combattants des États-Unis. Ils sont généralement moins élevés que les autres prix accessibles au public pratiqués aux États-Unis et divulgués dans les rapports des brevetés au CEPMB.
12 Le nombre de produits médicamenteux et la valeur des ventes visés par ces ratios varient, ét ant donné qu’il n’est pas toujours possible de trouver pour chaque produit médicamenteux breveté vendu au Canada les prix auxquels le médicament est vendu dans les pays de comparaison. Il convient de noter que tous les ratios moyens de prix bilatéraux indiqués au tableau 11 combinés représentent au moins 80 % des ventes effectuées au Canada en 2013, alors que les ratios multilatéraux du tableau 12 couvrent plus de 98 %.
13 Pour obtenir ces résultats, les prix dans les pays de comparaison ont été convertis en équivalents dollars canadiens à l’aide des taux de change du marché.
14 La plupart des résultats statistiques présentés dans cette section sont fondés sur les données sur les ventes provenant de MIDAS©, 2005-2013, IMS Health Incorporated ou sociétés affiliées. Tous droits réservés.16 Ces données couvrent les pharmacies et les hôpitaux.
15 Les résultats présentés dans les graphiques 13 à 16 sont fondés sur les estimations des recettes de ventes à l’usine qui comprennent les produits médicamenteux de marque brevetés et non brevetés et des produits médicamenteux génériques. Ces estimations ont été converties en équivalents dollars canadiens à l’aide des taux de change annuels moyens du marché. Les fluctuations de ces taux peuvent exercer une influence importante sur la distribution entre les grands marchés mondiaux.
16 Bien que fondés en partie sur les données obtenues en vertu d’une licence de la base de données MIDAS d’IMS, les énoncés, les constatations, les observations, les avis et les opinions exprimés dans le présent rapport annuel peuvent seulement être attribués au CEPMB et ne doivent pas être interprétés comme appartenant à IMS AG.
17 Les comparaisons faites sur cette base tiennent compte des différences aux niveaux des prix des produits médicamenteux, de l’utilisation faite des produits médicamenteux, des choix thérapeutiques habituellement faits et du revenu national.
18 Les données utilisées pour produire le tableau 15 couvrent les produits médicamenteux de marque et génériques tant brevetés que non brevetés. Par conséquent, les résultats présentés dans le tableau 15 ne peuvent être directement comparés à ceux présentés dans le tableau 9 qui, lui, couvre exclusivement les produits médicamenteux brevetés.
19 Il s’agit probablement de la situation d’une majeure partie du secteur de biotechnologie du Canada. Cependant, il convient de noter que si un breveté requiert de la recherche d’une autre société spécialisée dans la recherche en biotechnologie, le breveté devrait normalement inclure cela dans les dépenses de recherche qu’il déclare au CEPMB.
20 Le budget de 2012 a proposé des réductions au crédit d’impôt pour la recherche scientifique et le développement expérimental (RS-DE) et de nouvelles restrictions relatives aux déductions. Il a également introduit de nouvelles mesures visant à appuyer l’innovation et la R-D. Aux termes du Règlement, le CEPMB définit la R-D selon la définition de RS-DE de 1987.
21 Selon le Résumé de l’étude d’impact de la réglementation (REIR) du Règlement sur les médicaments brevetés, 1988, publié dans la partie II de la Gazette du Canada, vol. 122, no 20 – SOR/DORS/ 88-474.
22 Dans le tableau 16, les ratios des dépenses de R-D par rapport aux ventes tiennent compte des dépenses de recherche financées par le gouvernement au moyen de subventions. Si l’on exclut le financement accordé par le gouvernement, le ratio pour tous les brevetés en 2013 est de 4,4 % et celui pour les brevetés membres de Rx&D est de 5,3 %.
23 Les dépenses courantes de R-D comprennent les dépenses autres qu’en capital directement associées à la recherche, dont (a) les salaires; (b) le matériel direct, (c) les honoraires des entrepreneurs et des sous-traitants, (d) d’autres coûts directs de production tels que les frais généraux, (e) les paiements aux institutions désignées, (f) les paiements aux organismes subventionnaires et (g) les paiements à d’autres organismes. Ces éléments sont décrits plus en détail dans le formulaire 3 (Recettes et dépenses en recherche et développement), affiché sur le site Web du CEPMB. Les dépenses courantes de R-D représentent 96,8 % de l’ensemble des dépenses de R-D de 2013. Les coûts en immobilisations représentent 1,7 % des dépenses courantes de R-D et les frais d’amortissement admissibles, 1,4 %.
24 La « recherche fondamentale » désigne les travaux qui contribuent à faire avancer la connaissance scientifique et qui sont entrepris sans aucune application scientifique en vue. La « recherche appliquée » désigne les travaux qui contribuent à faire avancer la connaissance scientifique et qui sont entrepris avec une application pratique en vue. Elle peut viser à créer de nouveaux produits ou procédés, à améliorer ceux qui existent à l’aide de procédés de fabrication ou, encore, d’études précliniques ou cliniques. Enfin, l’expression « Autre R-D admissible » désigne les présentations sur la réglementation des médicaments, les études de biodisponibilité ainsi que les essais cliniques de phase IV.
25 Dans le graphique 20, les ventes sont celles effectuées exclusivement au pays. Elles ne comprennent pas les ventes à l’exportation.