Working Group to Inform the Patented Medicine Prices Review Board (PMPRB) Steering Committee on Modernization of Price Review Process Guidelines
Final Report - March 2019
PDF version (4.17 MB)
Table of Contents
-
Section 1: Criteria for classifying medicines as ‘Category 1’
-
Section 2: Supply-side cost effectiveness thresholds
-
Section 3: Multiple indications
-
Section 4: Accounting for uncertainty
-
Section 6: Market size factor
-
Appendix 1: Conceptual Framework
-
Appendix 2: Materials Presented at Meetings of the Working Group
-
Appendix 3: ‘On The Record’ Comments
-
Appendix 4: Terms of Reference
-
Appendix 5: Policy Intent
-
Appendix 8: External Review of Draft Report
Purpose
The purpose of this report is to summarise the deliberations and recommendations of the Working Group to Inform the Patented Medicine Prices Review Board (PMPRB) Steering Committee on Modernization of Price Review Process Guidelines.
Introduction
In June 2018, the PMPRB established a Steering Committee on Modernization of Price Review Process Guidelines (hereafter the ‘Steering Committee’). Its mandate was to assist the PMPRB in synthesizing stakeholder views on key technical and operational modalities of the PMPRB’s new draft Guidelines.
In July 2018, the PMPRB established the Working Group to Inform the Patented Medicine Prices Review Board (PMPRB) Steering Committee on Modernization of Price Review Process Guidelines (hereafter the ‘Working Group’). Its mandate was to inform the Steering Committee on certain issues that the Steering Committee believed would benefit from the review of experts in health technology assessment and other economic and scientific matters.
This report provides a summary of the Working Group’s deliberations and recommendations.
Membership
The chair of the Working Group was Dr Mike Paulden (University of Alberta).
Twelve individuals sat as members of the Working Group (listed alphabetically):
- Sylvie Bouchard (INESSS)Footnote 1 [represented by Patrick Dufort and Marie-Claude Aubin];
- Dr Chris Cameron (Dalhousie University and Cornerstone Research Group);
- Dr Doug Coyle (University of Ottawa);
- Don Husereau (University of Ottawa);
- Dr Peter Jamieson (University of Calgary);
- Dr Frédéric Lavoie (Pfizer Canada) (Industry Representative);
- Karen Lee (University of Ottawa and CADTH)Footnote 2;
- Dr Christopher McCabe (University of Alberta and Institute of Health Economics);
- Dr Stuart Peacock (Simon Fraser University and BC Cancer Agency);
- Maureen Smith (Patient);
- Geoff Sprang (Agmen) (Industry Representative);
- Dr Tania Stafinski (University of Alberta).
Two individuals sat as observers of the Working Group:
- Edward Burrows (Innovation, Science and Economic Development);
- Nelson Millar (Health Canada).
One individual acted as an external reviewer of the Working Group’s draft report:
- Dr Mark Sculpher (University of York).
An additional individual from CADTH, Dr Tammy Clifford, accepted an invitation to sit as a member of the Working Group but did not participate in the Working Group’s deliberations. Dr Clifford also did not contribute towards, or vote on, the Working Group’s recommendations.
Terms of Reference
The Terms of Reference (Appendix 4) required that the Working Group examine and make recommendations with respect to specific considerations and questions within the following six ‘areas of focus’:
- Options for determining what medicines fall into ‘Category 1’
- A Category 1 medicine is one for which a preliminary review of the available clinical, pharmacoeconomic, market impact, treatment cost and other relevant data would suggest is at elevated risk of excessive pricing.
- The following criteria have been identified as supporting a Category 1 classification:
- The medicine is ‘first in class’ or a ‘substantial’ improvement over existing options
- The medicine’s opportunity cost exceeds its expected health gain
- The medicine is expected to have a high market impact
- The medicine has a high average annual treatment cost
- Should other criteria be considered? What are the relevant metrics for selecting medicines that meet the identified criteria and what options exist for using these metrics?
- Application of supply-side cost effectiveness thresholds in setting ceiling prices for Category 1 medicines
- Potential approaches for implementing a price ceiling based on a medicine’s opportunity cost.
- Potential approaches for allowing price ceilings above opportunity cost for certain types of medicines (e.g. pediatric, rare, oncology, etc.)
- Medicines with multiple indications
- Options for addressing medicines with multiple indications (e.g. multiple price ceilings or a single ceiling reflecting one particular indication).
- Accounting for uncertainty
- Options for using the CADTH and/or INESSS reference case analyses to set a ceiling price.
- Options for accounting for and/or addressing uncertainty in the point estimate for each value-based price ceiling.
- Perspectives
- Options to account for the consideration of a public health care system vs societal perspective, including the option of applying a higher value-based price ceiling in cases where there is a ‘significant’ difference between price ceilings under each perspective.
- How to define a ‘significant’ difference in price ceilings between each perspective.
- Application of the market size factor in setting ceiling prices
- Approaches to derive an appropriate affordability adjustment to a medicine’s ceiling price based on an application of the market size and GDP factors (e.g. based on the US ‘ICER’ [Institute for Clinical and Economic Review] approach).
Under the Terms of Reference, the Steering Committee had the opportunity to specify additional areas of focus for the Working Group. The Steering Committee did not identify any additional areas of focus for the Working Group to consider.
Objections
The industry members (Frédéric Lavoie and Geoff Sprang) repeatedly raised objections to what they regarded as the “very narrow boundaries” established by the Terms of Reference.
Among these objections was a concern that the Working Group was not permitted to examine whether the PMPRB should be considering economic factors as part of the proposed reforms, nor any logistical or operational issues associated with implementation of the proposed reforms.
The industry members also stated that, as representatives of BIOTECanada and Innovative Medicines Canada (IMC), they “do not support the inclusion of proposed economic factors in a quasi-judicial price ceiling regulatory methodology given the uncertainty these factors would introduce, their practical challenges and complexity of implementation”, arguing that “the government’s regulatory objectives can be achieved by much simpler, more transparent and predictable mechanisms that will ensure access to necessary prescription medications while achieving the regulatory “bright lines” which PMPRB has recognized as a key consideration”.
The industry members submitted a number of ‘on the record’ comments to the chair regarding these and other matters, all of which are reproduced verbatim in Appendix 3.1 to 3.5.
The patient member (Maureen Smith) also submitted ‘on the record’ comments regarding these and other matters, which are reproduced verbatim in Appendix 3.6.
Policy intent
The PMPRB provided the Working Group with a copy of the Regulations Amending the Patented Medicines Regulations, as published in Canada Gazette Part I: Vol 151 (2017).
This document includes a Regulatory Impact Analysis Statement and the Proposed Regulatory Text and is reproduced in Appendix 5.1.
The Working Group was instructed by the PMPRB to make its considerations and recommendations on the assumption that the Regulations Amending the Patented Medicines Regulations will remain unchanged in their final publication.
The Working Group therefore considered the Regulatory Impact Analysis Statement and Proposed Regulatory Text as providing a definitive statement of the policy intent with respect to the proposed regulations.
In addition, the PMPRB provided three supporting documents to aid the Working Group in understanding the policy intent:
- PMPRB Guidelines Scoping Paper (Appendix 5.2);
- PMPRB Framework Modernization Presentation (Appendix 5.3);
- PMPRB Short Primer (Appendix 5.4).
The chair sought clarity from the PMPRB in cases where the Working Group was not clear about any aspect of the policy intent.
Process and procedure
The Working Group was convened in July 2018 and met three times in-person and multiple times via teleconference between July 2018 and February 2019:
- 26 July 2018 (all day in-person meeting);
- 22 and 24 August 2018 (1 hour teleconference for each of six areas of focus);
- 24 August 2018 (2 hour teleconference);
- 25 September 2018 (2 hour teleconference);
- 12 October 2018 (all day in-person meeting);
- 28 November 2018 (2 hour teleconference);
- 5 February 2019 (all day in-person meeting).
The Working Group was originally intended to report in October 2018, but this timeline was extended until March 2019.
Detailed meeting notes were taken by PMPRB staff and emailed to the chair following each meeting. A draft summary of these notes was circulated among Working Group members. In order to encourage a frank and open discussion, the chair committed to not identifying members alongside their comments in the Working Group’s report, unless requested to by the member. Members were permitted to provide ‘on the record’ comments regarding any matters of concern.
One week prior to the final in-person meeting on 5 February 2019, the chair circulated a draft ‘Conceptual Framework’ to all members. A revised version is reproduced in Appendix 1.
The purpose of this ‘Conceptual Framework’ was to guide members in making consistent recommendations across all six areas of focus, while respecting the policy intent and the range of views expressed by members throughout the Working Group’s deliberations.
On 7 February 2019, the chair circulated a set of ‘draft potential recommendations’. Members were invited to submit comments or suggested modifications until 15 February 2019.
On 18 February 2019, the chair circulated a draft report of the Working Group’s deliberations to all members and the external reviewer, including a final set of ‘potential recommendations’.
Under the Terms of Reference, recommendations were determined by a vote of the members, with the chair having the casting vote in the event of a tie. Members were asked to vote on the potential recommendations using an online form, and the full results of the vote were shared with all members. The chair committed not to identify members who voted ‘in favour’ or ‘against’ each potential recommendation in the Working Group’s final report.
Comments on the draft report, and votes on the potential recommendations, were accepted until 1 March 2019. The final report was submitted to the PMPRB on 6 March 2019.
1: Criteria for classifying medicines as ‘Category 1’
1.1 Terms of Reference
Within this area of focus, the Terms of Reference required the Working Group to examine and make recommendations with respect to the following considerations and questions:
A Category 1 medicine is one for which a preliminary review of the available clinical, pharmacoeconomic, market impact, treatment cost and other relevant data would suggest is at elevated risk of excessive pricing.
The following criteria have been identified as supporting a Category 1 classification:
- The medicine is ‘first in class’ or a ‘substantial’ improvement over existing options;
- The medicine’s opportunity cost exceeds its expected health gain;
- The medicine is expected to have a high market impact;
- The medicine has a high average annual treatment cost.
Should other criteria be considered? What are the relevant metrics for selecting medicines that meet the identified criteria and what options exist for using these metrics?
The chair clarified with the PMPRB whether the Terms of Reference permitted the Working Group to consider whether any of the criteria should be omitted. The PMPRB confirmed that such a consideration was within the purview of the Working Group.
1.2 Policy Intent
The PMPRB Guidelines Scoping Paper includes the following statement which provides context regarding the policy intent with respect to this area of focus:
“The second part of the framework consists of a screening phase which would classify new patented drugs as either high or low priority based on their anticipated impact on Canadian consumers, including individual patients and institutional payers (e.g., public and private drug plans). At this stage in the process, the PMPRB would consider whether the drug is first in class, has few or no therapeutic alternatives, provides significant therapeutic improvement over existing treatment options, is indicated for a condition that has a high prevalence in Canada, is a high cost drug (i.e. an average annual cost higher than a GDP-based threshold) or is classified as a high priority drug by other agencies/regulators in the health care system (such as the Canadian Agency for Drugs and Technologies in Health (CADTH) or Health Canada) because of unmet medical need. Drugs that appear to be high priority based on these screening factors would be subject to automatic investigation and a comprehensive review to determine whether their price is potentially excessive.”
(p.6, emphasis added)
The PMPRB Framework Modernization Presentation includes the following slide which provides context regarding the policy intent with respect to this area of focus:
Proposed PRICE Review Schematic
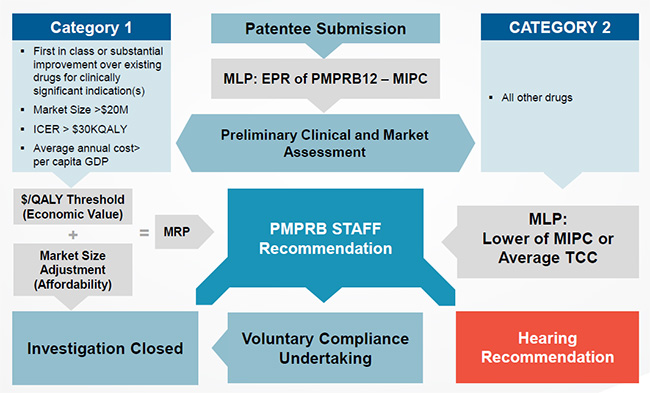
Figure description
Slide 11 is a work-flow diagram of how the proposed PRICE review process will function.
The first step in the process is the receipt of a Patentee submission for a new patented drug which is represented the box in the center of the diagram.
From there, a Maximum List Price (MLP) is established. This is done through an external price review based on the PMPRB12 countries, as proposed in the amendments to the Regulations. The MLP is established as the median of an international price comparison (MIPC) of the countries in the PMPRB12. This is represented in the box below the receipt of the Patentee submission.
In next step in the process, represented by the box below, a Preliminary Clinical and Market Assessment is conducted. Based on the results of this assessment the drug is categorised as one of two categories:
- A Category 1 drug, the attributes of which are included in the box to the left, indicated by an arrow pointing to the box.
- A Category 2 drug, all other drugs that do not have a Category 1 classification. This is represented by the box to the right.
Starting from the top left, Category 1 drugs are those that are either:
- First in class or offer a substantial improvement over existing drugs for clinically significant indication(s)
- Have a Market Size greater than $20M
- Expected to diminish population health ($/QALY>$30K)
Once a drug is classified as a Category 1 drug, it moves down to the box that represents an Economic Value test. To pass this test, the drug’s cost effectiveness value must be below a certain $/QALY threshold.
If the drug passes the economic value test, it moves to the box below which represents an affordability test, based on a market size adjustment. If the drug fails either the economic value test or affordability test it moves to a central box. The central box represents the PMPRB Staff Recommendation stage.
If the a drug passes both the economic value test and affordability test, it then moves down to the last box on the left and the Investigation is closed.
At the top right is the starting box for Category 2 drugs. For this category the Median International Price is compared to the Average of the Therapeutic Class Comparison. The MLP becomes the lower of the two tests as indicated in the second box on the right. From here, the drug moves to the centre box, PMPRB Staff Recommendation stage.
At the PMPRB Staff Recommendation stage, staff review the merits of each drug on a case-by-case basis and make a recommendation to the Board.
At this stage, PMPRB Staff make one of the following recommendations: Close Investigation as indicated in the box at the bottom left; proceed to a Hearing as indicated by the box at the bottom right; or agree to a Voluntary Compliance Undertaking as indicated in the bottom center box.
From the Voluntary Compliance Undertaking box, the drug moves to the Investigation Closed stage on the bottom left once the VCU has been signed.
1.3 Summary of Deliberations
There was widespread agreement among members of the Working Group that not all medicines require the same extent of review, and that a ‘risk-based’ approach is desirable.
However, there was debate among the Working Group regarding the criteria that should be used by the PMPRB to identify medicines at elevated risk of excessive pricing (‘Category 1’).
1.3.1 No other criteria considered
Under the Terms of Reference, the Working Group was required to examine and make recommendations regarding whether “other criteria” should be considered by the PMPRB.
No members of the Working Group proposed that any other criteria be considered beyond those specified in the Terms of Reference.
The following potential recommendation was put to a vote of the Working Group:
1.1: The Working Group does not recommend any additional criteria beyond those specified in the Terms of Reference.
Members voted 12 in favour and 0 against this potential recommendation.
This was therefore adopted as a formal recommendation of the Working Group.
1.3.2 ‘Substantial improvement over existing options’
A number of members expressed concern about the wording of Criterion A (‘The medicine is ‘first in class’ or a ‘substantial’ improvement over existing options’).
Although there was general agreement that ‘first in class’ medicines should be classified as ‘Category 1’, many members questioned why medicines that offer “a ‘substantial’ improvement over existing options” should be classified as ‘Category 1’ if none of the other criteria are met.
Concern was raised by some members that inclusion of this term might penalize manufacturers for producing medicines that offer ‘substantial improvement’, disincentivizing their development. Some members questioned whether this would, in turn, undermine the policy intent.
The chair asked the PMPRB to clarify the policy intent behind the inclusion of this term. The PMPRB responded that medicines that offer a ‘substantial’ improvement over existing options are more likely to dominate their respective market, increasing the risk of ‘excessive pricing’.
Some members argued that, even if a medicine dominates its market, if the medicine does not have ‘high’ market impact or a ‘high’ average annual treatment cost then the number of patients affected will be relatively small. Within a ‘risk based’ approach to classifying medicines, this might justify excluding the ‘substantial improvement’ term from Criteria A. One member dissented from this position, arguing that the PMPRB has a mandate to protect consumers from ‘excessive prices’, even if the number of patients affected is small.
Members of the Working Group were unable to identify examples of medicines which offer a ‘substantial’ improvement over existing options but would not be considered ‘first in class’ and would not have ‘high’ market impact or a ‘high’ average annual treatment cost. Even if inclusion of the ‘substantial improvement’ term is consistent with the policy objective, this raises the question as to whether its inclusion is redundant, given the presence of these other criteria.
The following potential recommendation was put to a vote of the Working Group:
1.2: The Working Group recommends that the PMPRB consider whether the wording “substantial improvement over existing options” within Criterion A is redundant or inconsistent with the policy intent, and, if so, remove this from consideration.
Members voted 11 in favour and 1 against this potential recommendation.
This was therefore adopted as a formal recommendation of the Working Group.
1.3.3 ‘Opportunity cost’ criterion
There was widespread agreement that Criterion B (‘The medicine’s opportunity cost exceeds its expected health gain’) should not be considered when classifying medicines as ‘Category 1’.
Some members cited the logistical difficulty of establishing cost-utility estimates for all newly launched medicines, rather than only those classified as Category 1. However, since logistical issues were not within scope of the Terms of Reference, these issues were not considered by the Working Group.
The industry members argued that the PMPRB’s proposed $30,000 per quality-adjusted life year (QALY) threshold is sufficiently low as to capture over 90% of all new medicines, such that classification as ‘Category 1’ would not serve as a useful screening mechanism. A potential response to this specific concern would be to raise the threshold used for screening to a sufficiently high level that a manageable number of new medicines are classified ‘Category 1’.
Another reason for excluding Criterion B, given by some members and consistent with the Conceptual Framework, is that this criterion may be redundant in the presence of the other criteria. If a medicine does not satisfy any of the other criteria - that is, it does not have a ‘high’ average annual cost, does not have ‘high’ market impact, is not ‘first in class’ and does not offer a ‘substantial improvement’ over existing treatment - then the potential loss in consumer surplus that might result from its adoption is limited, regardless of the incremental cost-effectiveness ratio (ICER). Under a risk-based approach, it may therefore be better to focus the resources available for assessing ‘Category 1’ medicines on medicines with ‘high’ average annual treatment cost, ‘high’ market impact and/or the potential to dominate their respective market.
The following potential recommendation was put to a vote of the Working Group:
1.3: The Working Group recommends that Criterion B be removed from consideration.
Members voted 11 in favour and 1 against this potential recommendation.
This was therefore adopted as a formal recommendation of the Working Group.
1.3.4 ‘High average annual treatment cost’
There was disagreement amongst the Working Group regarding Criterion D (‘The medicine has a high average annual treatment cost’), specifically whether ‘high average annual treatment cost’ should be considered in absolute terms or as incremental upon existing treatment.
It was noted that a new medicine could have ‘high average annual treatment cost’, but might replace an existing treatment that also has ‘high average annual treatment cost’, such that the incremental average annual treatment cost is not ‘high’.
Some members noted that, if the existing treatment has ‘high average annual treatment cost’, this increases the risk that the existing treatment is itself considered to be ‘excessively priced’. In such cases, the new medicine may also be considered to be ‘excessively priced’, even if the incremental average annual treatment cost is not ‘high’.
As noted in the Conceptual Framework, the opportunity cost of adopting a new medicine is a function of its incremental cost compared to existing treatment. All else equal, the risk that adopting a new medicine will result in negative consumer surplus would therefore be expected to be greater for a medicine with high incremental average annual treatment cost, compared to a medicine with high absolute average annual treatment cost but low incremental average annual treatment cost. For this reason, the PMPRB may wish to consider ‘average annual treatment cost’ within Criterion D as being incremental upon existing treatment.
There are several considerations that would need to be made when calculating this incremental cost. The relevant treatment comparator would need to be established and the cost of treatment with the comparator estimated over the relevant time horizon. If the comparator is itself a patented medicine, then consideration would also need to be given to any expected reduction in the cost of the comparator should generic alternatives to the comparator become available during the patent life of the new medicine.
The following potential recommendation was put to a vote of the Working Group:
1.4: The Working Group recommends that “average annual treatment cost” within Criterion D be considered as incremental upon existing treatment.
Members voted 11 in favour and 1 against this potential recommendation.
This was therefore adopted as a formal recommendation of the Working Group.
1.3.5 Relevant metrics
The Terms of Reference required that the Working Group examine and make recommendations regarding the “relevant metrics for selecting medicines that meet the identified criteria”. The chair interpreted this as referring to the measures and definitions used for each criteria. For example, if the term ‘substantial improvement’ is retained in Criterion A, how would ‘improvement’ be measured and how would a ‘substantial improvement’ be defined?
There was general agreement that the most appropriate metrics for each criterion would be those already used in Canadian practice. For example, if the PMPRB retains consideration of the ‘substantial improvement’ term in Criterion A, then the definition of ‘substantial improvement’ could be based upon the definition already adopted by the PMPRB. Other potential sources for definitions suggested by members included health technology assessment (HTA) and regulatory agencies in Canada.
The following potential recommendation was put to a vote of the Working Group:
1.5: The Working Group recommends that the measures and definitions used for each criterion reflect existing Canadian practice.
Members voted 10 in favour and 2 against this potential recommendation.
This was therefore adopted as a formal recommendation of the Working Group.
1.3.6 Determining a threshold for each criterion
In addition to identifying “relevant metrics”, the Terms of Reference required that the Working Group examine and make recommendations regarding “options” for using these metrics.
There was some discussion regarding how to determine an appropriate ‘threshold’ to adopt for each criterion, building upon some potential thresholds proposed by the PMPRB.
At the first meeting of the Working Group, the PMPRB proposed that, in considering Criterion B (‘The medicine’s opportunity cost exceeds its expected health gain’), the ICER could potentially be compared to a threshold of $30,000 per quality-adjusted life year (QALY). This was based on an estimate by Ochalek et al. (2018) of the opportunity cost of funding new medicines within Canada’s public health care systems (considered further in Topic 2).1 Some members raised concern that a $30,000 per QALY threshold would be sufficiently low as to capture a substantial proportion of all new medicines considered by the PMPRB, such that categorization as ‘Category 1’ might not serve as a useful ‘screening’ mechanism. However, in light of the general consensus among the Working Group that Criterion B should not be considered by the PMPRB, no further discussion of this threshold took place.
The PMPRB also proposed a potential ‘market impact’ threshold of either $20m or $40m, and proposed that a medicine could be considered to be of ‘high market impact’ if it reached this threshold in any one of either the first 3 years or 5 years after launch. The PMPRB provided the Working Group with estimates of the proportion of all medicines that would be classified as ‘Category 1’ under each combination of these potential thresholds (based solely on Criterion C):
- $20m market size in any one of the first 3 years: 22% of all medicines
- $20m market size in any one of the first 5 years: 27% of all medicines
- $40m market size in any one of the first 3 years: 17% of all medicines
- $40m market size in any one of the first 5 years: 20% of all medicines
Finally, the PMPRB proposed a potential ‘average annual treatment cost’ threshold of $50,000. The PMPRB estimated that this threshold would result in 4% of all medicines being classified as ‘Category 1’ (based solely on Criterion D).
The Working Group noted that the sensitivity of each criterion as a ‘screen’ is dependent upon the threshold adopted. The Working Group did not have the necessary data to calculate how many medicines would be classified as ‘Category 1’ under different combinations of thresholds across the criteria. Furthermore, it was noted that the ‘ideal’ number of medicines to classify as ‘high risk’ depends upon the PMPRB’s capacity for assessing ‘Category 1’ medicines (which was unknown to the Working Group), while the ‘ideal’ types of medicines to classify as ‘high risk’ depend upon the policy intent.
The Working Group was therefore not in a position to make specific recommendations regarding the threshold to adopt for each criterion. Instead, the chair proposed that the PMPRB should determine the threshold for each criterion, taking into account its capacity for assessing ‘Category 1’ medicines, the technical considerations of the Working Group, and the policy intent.
The following potential recommendation was put to a vote of the Working Group:
1.6: The Working Group recommends that a threshold for each criterion be determined by the PMPRB, taking into account its capacity for assessing ‘Category 1’ medicines, the technical considerations of the Working Group, and the policy intent.
Members voted 10 in favour and 2 against this potential recommendation.
This was therefore adopted as a formal recommendation of the Working Group.
1.3.7 Clear specification of the threshold for each criterion
The two industry members on the Working Group emphasized the importance of the PMPRB clearly specifying the threshold to be used for each criterion, so as to provide a “clear bright line” to manufacturers.
A technical justification for this request is that a clear specification of the threshold for each criterion reduces uncertainty. The Conceptual Framework outlines how uncertainty in a medicine’s pharmacoeconomic value may result in an expected loss in economic surplus, such that there may be value in reducing this uncertainty. Similarly, uncertainty in whether a medicine may be subject to ‘Category 1’ classification may impose an expected loss on manufacturers and other stakeholders.
The following potential recommendation was put to a vote of the Working Group:
1.7: The Working Group recommends that the threshold for each criterion be clearly specified, so as to reduce uncertainty for stakeholders.
Members voted 12 in favour and 0 against this potential recommendation.
This was therefore adopted as a formal recommendation of the Working Group.
1.3.8 Other considerations
There was some discussion as to whether ‘high market impact’ should be considered as incremental upon existing treatment (similar to the consideration of ‘high average annual treatment cost’ in section 1.3.4). Some members argued that a medicine with high market size may replace an existing treatment which also has high market size, such that the net market impact is relatively small.
However, it was apparent from the PMPRB Guidelines Scoping Paper, as well as the proposed ‘market size adjustment’ (section 6), that there is a policy concern regarding medicines with high absolute market impact. The PMPRB confirmed to the chair that this was the case. Given this policy intent, the Working Group did not consider any potential recommendation to modify the wording of the ‘high market impact’ criterion so that it is incremental upon existing treatment.
2: Supply-side cost effectiveness thresholds
2.1 Terms of Reference
Within this area of focus, the Terms of Reference required the Working Group to examine and make recommendations with respect to the following considerations and questions:
Potential approaches for implementing a price ceiling based on a medicine’s opportunity cost.
Potential approaches for allowing price ceilings above opportunity cost for certain types of medicines (e.g. pediatric, rare, oncology, etc).
2.2 Policy Intent
The Regulatory Impact Analysis Statement includes the following statements which provide context regarding the policy intent with respect to this area of focus:
“Information regarding pharmacoeconomic value: patentees would be required to provide the PMPRB with all published cost-utility analyses that express the value in terms of the cost per quality-adjusted life year (QALY). Cost-utility analyses are viewed by experts as the “gold standard” approach to considering the economic value of new medicines.”
(p.10, emphasis added)
“Without the proposed amendments, it is estimated that public health care systems from across Canada will spend an additional $3.9 billion (PV) for the same quantity of patented medicine. This represents a significant opportunity cost for the Canadian public health care system, as these funds could have been used in other areas of the health care system to better the health of Canadians.”
(p.16, emphasis added)
The PMPRB Guidelines Scoping Paper includes the following statement which provides context regarding the policy intent with respect to this area of focus:
“The first part of the test would assess the incremental cost per quality-adjusted life year (QALY) of the drug, as determined by CADTH’s health technology assessment process, against an explicit cost effectiveness threshold. The threshold would be based on the opportunity cost associated with displacing the least cost effective health technology in the Canadian health system, otherwise understood as the marginal cost of a QALY, as calculated by expert health economists and revised periodically to reflect changing market conditions. Drugs that prolong life or provide significant QALY gains could be subject to a more generous threshold, as Canadian payers have demonstrated a higher willingness to pay for these types of drugs”.
(p.6, emphasis added)
The PMPRB Framework Modernization Presentation includes the following slide which provides context regarding the policy intent with respect to this area of focus:
Part III: MRP for Category 1 drugs
Step 1: application of pharmacoeconomic factor
- Empirical work undertaken by Karl Claxton at the University of York suggests a $30K/QALY opportunity cost threshold for Canada.
- PMPRB will use this estimate at the screening phase to determine whether a drug should go in Category 1 or Category 2.
- Category 1 drugs will then be subject to a baseline maximum value-based price ceiling of $60K/QALY, for reasons of practicality and efficiency.
- Drugs that meet certain clinical characteristics (e.g., high burden of disease or significant absolute gain in QALY) may be subject to a higher $/QALY ceiling.
2.3 Summary of Deliberations
The Working Group’s deliberations on this topic were informed by two documents commissioned by the PMPRB prior to establishment of the Working Group:
- A white paper prepared by the Institute of Health Economics (IHE) titled “Theoretical models of the cost-effectiveness threshold, value assessment, and health care system sustainability”, hereafter referred to as the ‘IHE report’.2
- A report prepared by Jessica Ochalek and colleagues from the University of York titled “Assessing health opportunity costs for the Canadian health care systems”, hereafter referred to as ‘Ochalek et al. (2018)’.1
2.3.1 Appropriateness of using a supply-side threshold
As noted in the IHE report, a supply-side threshold can be used to estimate the ‘health opportunity cost’ associated with adopting a new medicine within a public health care system. This health opportunity cost is measured in units of health benefit (typically QALYs) and reflects the estimated health ‘forgone’ by other patients within the health care system if limited resources are used to adopt the new medicine.
For example, Ochalek et al. (2018) estimated a supply-side threshold of $30,000 per QALY for Canada as a whole, with some variation across provinces and territories (considered further in section 2.3.4). This estimate implies that every additional $30,000 spent on a new medicine results in one forgone QALY by other patients across Canada’s public health care systems. A higher estimate of the supply-side threshold would imply that fewer QALYs are displaced at any given incremental cost associated with a new medicine, and conversely a lower supply-side threshold would imply that more QALYs are displaced for any given incremental cost.
Additional explanation and examples are provided in the Conceptual Framework.
There was debate amongst Working Group members as to whether a supply-side threshold is always the most appropriate means for estimating the opportunity cost of new medicines. Specifically, consideration was given as to whether a ‘demand-side threshold’ might be more appropriate than a supply-side threshold in some cases.
As noted in the IHE report, a demand-side threshold reflects Canadians’ ‘willingness-to-pay’ for health benefits. Some members argued that a demand-side threshold might therefore be a more appropriate threshold for private insurers and patients who pay out-of-pocket.
Nevertheless, in light of the PMPRB’s clarification that the policy intent is to adopt the perspective of the Canadian public health care system (section 5.2), the focus of the Working Group’s deliberations was on a supply-side approach to estimating the threshold.
Since the policy intent is to adopt the perspective of Canada’s public health care systems, and since the Regulatory Impact Analysis Statement views the QALY, as used in cost-utility analysis, as the “gold standard” approach to considering the economic value of new medicines, it follows that the most relevant measure of the opportunity cost of a new medicine, given this policy intent, is an estimate of the QALYs forgone by patients within Canada’s public health care systems. As noted in the Conceptual Framework, this may be estimated using an estimate of the incremental cost of the new medicine and an estimate of a supply-side cost-effective threshold, expressed in terms of cost per QALY.
The following potential recommendation was put to a vote of the Working Group:
2.1: The Working Group regards the use of a supply-side cost-effectiveness threshold, as a means for estimating the opportunity cost of adopting new medicines within Canada’s public health care systems, as consistent with the policy intent.
Members voted 10 in favour and 2 against this potential recommendation.
This was therefore adopted as a formal recommendation of the Working Group.
2.3.2 Uncertainty in the empirical evidence base
The Working Group was unanimous in considering the empirical evidence base with respect to Canadian estimates of supply-side thresholds to be uncertain.
The only existing estimate of a supply-side threshold for Canada is that provided by Ochalek et al. (2018). This work reported estimates of supply-side thresholds for each province and territory in terms of cost per disability adjusted life year (DALY) averted. Based on these estimates, the authors argued that “a cost per DALY threshold is likely to be less than $50,000 for Canada as a whole”. The authors further argued that “a cost per QALY threshold is likely to be similar or lower than a cost per DALY averted threshold”, concluding that “a cost per QALY threshold of $30,000 per QALY would be a reasonable assessment of the health effects of changes in health expenditure for Canada as a whole and is likely to be similar across most provinces”.
The authors acknowledged that this research was not primarily based upon Canadian data, noting that “further research to provide Canadian and/or province specific elasticity estimates using within country and within province data should be regarded as a priority”.
Some members of the Working Group expressed concerns with the instrumental variables (IVs) used by Ochalek et al. (2018).
One member noted that the authors employed two specific IVs that are potentially problematic:
- Military expenditure per capita of neighbouring countries;
- A measure of institutional quality, captured using:
- The level of infrastructure (proxied by ‘paved roads per square km’);
- Shock in ‘donor funding’ (absolute deviation from the historical mean).
This member viewed the appropriateness of these IVs as questionable in the Canadian context. Canada’s neighbor is the United States, which is an outlier in terms of military expenditure per capita in the sample of countries used in the Ochalek et al. (2018) study. Canada is also an outlier in terms of ‘paved roads per square km’, ranking 90th out of 125 countries.3 Since relatively few high income countries receive ‘donor funding’, this member noted that ‘paved roads per square km’ is effectively the sole IV for infrastructure quality.
These potentially ‘weak’ IVs raise concerns about the parameter estimates from the authors’ regression model. Specifically, if the IVs are only weakly correlated with the endogenous regressors, parameter estimates may be biased, estimates may be inconsistent, tests of significance may have incorrect size, and confidence intervals may be wrong.4–6
The following potential recommendation was put to a vote of the Working Group:
2.2: The Working Group regards the current evidence base with respect to Canadian estimates of supply-side cost-effectiveness thresholds, including the empirical research by Ochalek et al. (2018), as uncertain.
Members voted 12 in favour and 0 against this potential recommendation.
This was therefore adopted as a formal recommendation of the Working Group.
2.3.3 Direction and magnitude of bias in the $30,000 per QALY estimate
Given the Working Group’s concern with the IVs used in the Ochalek et al. (2018) research, members considered the potential direction and magnitude of bias in the $30,000 per QALY estimate.
At a public seminar, the chair asked the corresponding author of the Ochalek et al. (2018) research, Dr Karl Claxton, for his views on the implications of any weakness in the IVs.7 Dr Claxton’s response was that any weakness in the IVs would be expected to weaken the relationship between health expenditures and health outcomes, in turn resulting in an overestimate of the cost-effectiveness threshold.
The implication of Dr Claxton’s remarks is that a re-estimate of the supply-side threshold with stronger IVs would be expected to be below $30,000 per QALY. However, the Working Group member who initially questioned the strength of the IVs in the Ochalek et al. (2018) research disagreed, arguing that the direction of bias as a result of weak IVs is unknown.
The following potential recommendation was put to a vote of the Working Group:
2.3: The Working Group regards the direction and magnitude of any bias in the $30,000 per QALY estimate by Ochalek et al. (2018) to be unknown.
Members voted 12 in favour and 0 against this potential recommendation.
This was therefore adopted as a formal recommendation of the Working Group.
2.3.4 Differences across provinces and territories
Several members noted that a different supply-side threshold would be expected for each Canadian public health care system.
Theoretically, the supply-side threshold is affected by the budget of the health care system in question, among other considerations.8 Since each provincial and territorial health care system has its own budget, a different supply-side threshold would be expected for each.
This is consistent with the results of the work by Ochalek et al. (2018), which found a different supply-side threshold (in terms of cost per DALY averted) in each province and territory.1
The Working Group considered several potential approaches for setting a single ceiling price across all provinces and territories, including:
- A ceiling price at which the medicine is ‘just’ cost-effective in the province or territory with the highest supply-side threshold;
- A ceiling price at which the medicine is ‘just’ cost-effective in the province or territory with the lowest supply-side threshold;
- A ceiling price at which the medicine is ‘just’ cost-effective across Canada as a whole.
A consideration of the implications of each approach is provided in the Conceptual Framework. In summary, each approach results in a different allocation of the total ‘economic surplus’ among ‘consumers’ (patients) and ‘producers’ (manufacturers). The first approach results in negative overall consumer surplus, the second approach results in positive overall consumer surplus, while the third approach results in zero overall consumer surplus.
Since the preferred allocation of the economic surplus is a matter for policy makers, the Working Group does not advocate for any specific approach. Instead, the Working Group recommends that any single threshold used for the purpose of informing a ceiling price be consistent with the policy intent, given the technical considerations outlined by the Working Group.
The following potential recommendation was put to a vote of the Working Group:
2.4: The Working Group recognizes that each provincial and territorial public health care system has a unique supply-side cost-effectiveness threshold, and recommends that any single threshold used for the purpose of informing a ceiling price be consistent with the policy intent.
Members voted 12 in favour and 0 against this potential recommendation.
This was therefore adopted as a formal recommendation of the Working Group.
2.3.5 Medicines with large net budget impact
In theory, adopting medicines with a large net budget impact into a budget constrained public health care system would be expected to result in a disproportionately large opportunity cost.8,9 (Note that “net budget impact” is distinct from the “market size” consideration in section 6.)
One approach for dealing with this is to use a progressively lower supply-side threshold for medicines with progressively larger net budget impact. One member cited the empirical work by James Lomas, which estimated how the supply-side threshold for the English NHS would fall as the net budget impact of a new health technology increases.9 For new hepatitis C treatments, which had an estimated net budget impact of £772m in the first year of use, Lomas found that the supply-side threshold would need to be adjusted down from £12,936 per QALY (the supply-side threshold for marginal changes in health care expenditure) to £12,452 per QALY.9,10
The Working Group was unaware of any other attempts internationally to estimate supply-side thresholds associated with non-marginal changes in health expenditures. Since no equivalent empirical estimates are available for Canada, there is no data to inform such a downwards adjustment to the Canadian supply-side threshold at the present time.
The following potential recommendation was put to a vote of the Working Group:
2.5: The Working Group recognizes that, in principle, a downwards adjustment should be applied to the supply-side cost-effectiveness threshold for medicines with substantial net budget impact, but notes that there is no Canadian empirical evidence to inform the magnitude of such an adjustment at the present time.
Members voted 10 in favour and 2 against this potential recommendation.
This was therefore adopted as a formal recommendation of the Working Group.
2.3.6 Equity weights
The Terms of Reference tasked the Working Group with considering “Potential approaches for allowing price ceilings above opportunity cost for certain types of medicines (e.g. pediatric, rare, oncology, etc)”.
The Working Group noted that, under CADTH’s ‘reference case’ requirements, all QALYs are assigned equal value. A justification of this position is provided in CADTH’s ‘Guidelines for the Economic Evaluation of Health Technologies: Canada’ (4th Edition; pp.59-60).11 CADTH’s reference case therefore reflects an equity position under which a ‘weight’ of 1 is applied to all QALYs, regardless of any characteristics of the patients, disease or technology in question.
Critically, a weight of 1 on all QALYs does not permit a ceiling price “above opportunity cost” for “certain types of medicines” but not others. The Working Group therefore considered the potential for applying different weights to some QALYs, and hence departing from CADTH’s reference case assumption that all QALYs have equal value.
There is a small but growing empirical literature on the types of characteristics for which society may assign greater or lesser weight when valuing health gains.12–18 One member provided the Working Group with a brief summary of this literature. Characteristics that are often found to be important in empirical studies include severity of illness (particularly the presence or otherwise of life threatening or progressively chronically debilitating illness), the availability of active treatment alternatives, the prevalence of disease, the type of health gain (such as a reduction in pain), and the magnitude of health gain. These factors are often found to interact with one another, and so should not be considered independently. In the opinion of this member, greater empirical work is needed to fully understand these interactions and the ‘weights’ that would be put on each characteristic.
Members also discussed theoretical issues associated with applying weights to some QALYs but not others. One member expressed concern that some important conceptual problems have not yet been addressed in the literature - for example, would a greater weight on QALYs for ‘cancer’ apply to all QALYs gained by a patient with cancer (including those gained through treatment for other diseases) or only the QALYs gained through cancer treatment (such that other QALY gains for the same patient for other diseases would be assigned different weight). There is also an ongoing and unresolved debate regarding whether weights should be applied directly to QALYs or to the cost-effectiveness threshold. The latter approach has been used by NICE in the UK but has received criticism for resulting in ‘inconsistencies’ in its consideration of social value.19
As a result of these limitations in the empirical and theoretical literature, the predominant view of members was that equity weights other than 1 should not be implemented at the present time.
There was some discussion by the Working Group regarding the potential implications of this recommendation for medicines for rare diseases. As noted in the Conceptual Framework, medicines with small market size may be expected to have a higher supply curve (at the respective quantity) than medicines with large market size. Such medicines may therefore be less profitable at a given ceiling price compared to medicines with larger market size. This issue is considered further in section 6.
The following potential recommendation was put to a vote of the Working Group:
2.6: The Working Group does not recommend the implementation of ‘equity weights’ other than 1, as would be required to allow price ceilings above opportunity cost for some medicines but not others, due to limitations in the existing theoretical and empirical evidence base.
Members voted 9 in favour and 3 against this potential recommendation.
This was therefore adopted as a formal recommendation of the Working Group.
2.3.7 Clear specification of the supply-side threshold
In common with the request that any thresholds used for classifying ‘Category 1’ medicines be clearly specified (section 1.3.7), the two industry members emphasized the desirability that any supply-side threshold used for the purposes of informing a price ceiling be clearly specified.
As noted in the Conceptual Framework, the supply-side threshold is a key determinant of the location of the ‘demand curve’ for a new medicine. A technical justification for requesting that the supply-side threshold be clearly specified is that it reduces uncertainty for manufacturers regarding the location of this demand curve, and hence the producer surplus if the ceiling price is informed by this demand curve.
There was general agreement among the Working Group about the desirability of specifying the supply-side threshold, and hence providing greater clarity to manufacturers and other stakeholders regarding the location of the demand curve.
Nevertheless, as noted in the Conceptual Framework, there is also considerable uncertainty about the location of the manufacturer’s ‘supply curve’. This increases uncertainty regarding the set of possible ceiling prices at which consumer and producer surplus are both positive, potentially resulting in a loss of economic surplus for both consumers and producers. To minimize this uncertainty, efforts should be made to better understand the location of the supply curve for new medicines. This would complement efforts to provide greater certainty regarding the location of the demand curve through a clear specification of the supply-side threshold.
The following potential recommendation was put to a vote of the Working Group:
2.7: The Working Group recommends that any estimate of the supply-side threshold adopted by the PMPRB for the purposes of informing a price ceiling be clearly specified, so as to reduce uncertainty for stakeholders.
Members voted 12 in favour and 0 against this potential recommendation.
This was therefore adopted as a formal recommendation of the Working Group.
2.3.8 Further empirical research
Given the uncertainties in the existing empirical evidence base regarding Canadian supply-side cost-effectiveness thresholds (sections 2.3.2 and 2.3.3), there was broad support among members of the Working Group for conducting further empirical research.
Since differences in supply-side thresholds across provinces and territories are predicted by theoretical work and were observed by Ochalek et al. (2018) (section 2.3.4), there was also agreement that any future Canadian empirical studies should consider potential variation in estimates of supply-side cost-effectiveness thresholds across jurisdictions within Canada.
The following potential recommendation was put to a vote of the Working Group:
2.8: The Working Group recommends that the PMPRB support further empirical research to estimate a supply-side cost-effectiveness threshold for Canada. This research should consider and report on variation in estimates of supply-side cost-effectiveness thresholds across jurisdictions within Canada.
Members voted 12 in favour and 0 against this potential recommendation.
This was therefore adopted as a formal recommendation of the Working Group.
2.3.9 Specifying an ‘interim’ threshold
Since the existing empirical evidence on Canadian supply-side thresholds was considered to be uncertain, and since further empirical research will take time to conduct and report, members discussed how a threshold might be specified by the PMPRB in the interim.
Existing Canadian policy thresholds
One potential interim approach considered by the Working Group is for the PMPRB to specify a threshold in line with existing ‘policy thresholds’ used by Canadian HTA agencies.
The Working Group observed that no Canadian HTA agencies currently specify an explicit cost per QALY policy threshold. However, one member noted that INESSS uses an informal policy threshold of $50,000 to $100,000 per QALY, with other members providing anecdotal evidence of similar policy thresholds being used informally by other HTA agencies in Canada (with higher policy thresholds used in some cases, such as for cancer).
Another member suggested that it may be useful to understand what policy threshold is informally used by the the pan-Canadian Pharmaceutical Alliance (pCPA) in its negotiations.
One member cited a 2016 article in the Hamilton Spectator, which reported that “the pan-Canadian Oncology Drug Review has set an unofficial threshold of $100,000 per quality-adjusted life year for new cancer medications”, and also a 2009 letter by the Deputy Minister of Health and Long-Term Care for Ontario, which noted that the Committee to Evaluate Drugs “typically considers a range of $40-60,000 [per] QALY as an acceptable range”.20,21
Taken together, this evidence suggests that informal policy thresholds used by HTA agencies in Canada are in the region of $50,000 to $100,000 per QALY, with oncology medicines assessed at the higher end of this range and other medicines assessed relatively lower within this range.
It should be noted that none of these policy thresholds is based on an empirical assessment of the opportunity cost of adopting new medicines within Canada’s public health care systems, as would be required to specify a ‘supply-side’ threshold.
Supply-side thresholds from other jurisdictions
Another potential interim approach is to consider empirical estimates of supply-side thresholds for other jurisdictions with similar wealth and medicine market characteristics as Canada.
The IHE report summarized three existing published estimates of supply-side thresholds for other jurisdictions:2
- The work by Claxton et al. (2015), which estimated a supply-side threshold of
£12,936 per QALY for the public health care system in the UK.10
- The work by Vallejo-Torres et al. (2017), which estimated a supply-side threshold of between €21,000 and €25,000 per QALY for the public health care system in Spain.22
- The work by Edney et al. (2017), which estimated a supply-side threshold of
AU$28,033 per QALY for the public health care system in Australia.23
The chair noted that the $30,000 per QALY estimate from Ochalek et al. (2018) is broadly in line with these estimates, and that all three of these countries are on the proposed PMPRB12 list of countries with “reasonably comparable economic wealth” and “similar medicine market size characteristics” as Canada. Absent reasons why Canada would be considered an ‘outlier’ among PMPRB12 countries, one might therefore reasonably expect a future Canadian estimate of a supply-side threshold to be similar to the estimates reported in these countries. Nevertheless, given the various determinants of the supply-side threshold, some variation in estimates across countries would be expected.8
The following potential recommendation was put to a vote of the Working Group:
2.9: The Working Group recommends that any ‘interim’ threshold specified by the PMPRB prior to completion of further Canadian empirical work should be informed by a comprehensive consideration of existing thresholds used by Canadian HTA agencies and empirical estimates of supply-side thresholds from other relevant jurisdictions.
Members voted 10 in favour and 2 against this potential recommendation.
This was therefore adopted as a formal recommendation of the Working Group.
3: Multiple indications
3.1 Terms of Reference
Within this area of focus, the Terms of Reference required the Working Group to examine and make recommendations with respect to the following considerations and questions:
Options for addressing medicines with multiple indications (e.g. multiple price ceilings or a single ceiling reflecting one particular indication).
3.2 Policy Intent
The Regulatory Impact Analysis Statement includes the following statements which provide context regarding the policy intent with respect to this area of focus:
“The price paid for a medicine should take into consideration the value it produces.”
(p.8, emphasis added)
The PMPRB Guidelines Scoping Paper includes the following statement which provides context regarding the policy intent with respect to this area of focus:
“The fifth and final part of the new framework would involve the periodic “re-benching” of drugs to ensure that previous determinations of potential excessive pricing and/or price ceilings remain relevant in light of new indications (resulting in a change of market size) or changes in market conditions. Depending on the nature of the change, the re-benching process could result in a decrease or increase in ceiling price.”
(p.7, emphasis added)
3.3 Summary of Deliberations
Two broad approaches were considered by the Working Group: a separate ceiling price for each indication (‘indication-specific pricing’), or a single ceiling price across all indications.
There was general agreement that indication-specific pricing is the more appealing approach in principle. As noted in the Conceptual Framework, the incremental effectiveness of any medicine generally differs across indications. Indication-specific pricing would permit the ceiling price of the medicine to reflect this differing value for each indication. This would appear to closely align with the policy intent, as stated in the Regulatory Impact Analysis Statement, that “the price paid for a medicine should take into consideration the value it produces”.
However, although one member was of the view that multi-indication pricing may be feasible for some ‘Category 1’ medicines, several members expressed concern that indication-specific pricing is not possible in Canada, given current limitations in data capture and reporting.
It was noted that indication-specific pricing requires an IT infrastructure for collecting data on volume per indication. An informal review conducted by one member identified a number of different approaches internationally.24,25 France, Germany and Australia all use indication-specific pricing, based on expected patient volumes for each indication. Italy engages in risk-sharing arrangements using indication-specific patient registries. Express Scripts in the United States is using indication-specific pricing for cancer medicines, and the UK piloted the feasibility of this approach using the Systemic Anti-Cancer Therapy Dataset (SACT) data set. Belgium and Spain have also used indication-specific pricing for expensive medicines and hospital-based medicines, respectively.
Since logistical and implementation issues were out of the scope of the Terms of Reference, Working Group members did not give detailed consideration to the feasibility of implementing indication-specific pricing in Canada. Instead, the Working Group’s deliberations focused exclusively on options for specifying a single ceiling price across multiple indications.
3.3.1 Specifying a single ceiling price across all indications
The Working Group considered several potential approaches for setting a single ceiling price across multiple indications, including:
- A ceiling price at which the medicine is ‘just’ cost-effective in the most cost-effective indication;
- A ceiling price at which the medicine is ‘just’ cost-effective in the least cost-effective indication;
- A ceiling price at which the medicine is ‘just’ cost-effective across all indications;
- A ceiling price at which the medicine is ‘just’ cost-effective in the first indication considered by the PMPRB.
A consideration of the implications of each approach is provided in the Conceptual Framework.
In common with the different potential approaches for setting a ceiling price across provinces and territories (section 2.3.4), each approach results in a different allocation of the total economic surplus among consumers and producers. The first approach results in negative overall consumer surplus, the second approach results in positive overall consumer surplus, the third approach results in zero overall consumer surplus, while the fourth approach results in zero expected consumer surplus if manufacturers do not behave strategically when launching medicines or negative expected consumer surplus if manufacturers do behave strategically.
At the final in-person meeting, the PMPRB asked the chair to consider a fifth potential approach for setting a single ceiling price across multiple indications:
- 5. A ceiling price at which the medicine is ‘just’ cost-effective in one specific ‘key’ indication identified by the PMPRB.
This approach has similarities to the fourth approach considered above, insofar as the ceiling price would be based upon the cost-effectiveness of the new medicine in one indication only. It would also share an advantage that the fourth approach has over the first three approaches, insofar as the ceiling price would not need to be rebenched over time as new indications are launched (unless the ‘key’ indication were to change).
The implications for the allocation of the total economic surplus with this fifth approach depend upon whether the ‘key’ indication is more or less cost-effective than other indications. If this ‘key’ indication is the most cost-effective, then the implications are the same as for the first approach, with negative overall consumer surplus. Alternatively, if the ‘key’ indication is the least cost-effective, then the implications are the same as for the second approach, with positive overall consumer surplus. In both cases consumer surplus in the ‘key’ indication is zero.
As with the consideration of different potential approaches for setting a ceiling prices across provinces and territories (section 2.3.4), the Working Group does not advocate for any specific approach since the preferred allocation of the economic surplus is a matter for policy makers.
Instead, the Working Group recommends that any single threshold used for the purpose of informing a ceiling price be consistent with the policy intent, given the technical considerations outlined by the Working Group.
The following potential recommendation was put to a vote of the Working Group:
3.1: The Working Group recommends that the PMPRB specify a single ceiling price for each medicine that applies across all indications and is consistent with the policy intent.
Members voted 12 in favour and 0 against this potential recommendation.
This was therefore adopted as a formal recommendation of the Working Group.
4: Accounting for uncertainty
4.1 Terms of Reference
Within this area of focus, the Terms of Reference required the Working Group to examine and make recommendations with respect to the following considerations and questions:
Options for using the CADTH and/or INESSS reference case analyses to set a ceiling price.
Options for accounting for and/or addressing uncertainty in the point estimate for each value-based price ceiling.
4.2 Policy Intent
The Regulatory Impact Analysis Statement includes the following statements which provide context regarding the policy intent with respect to this area of focus:
“In recognition of the significant expertise that can be necessary to prepare and validate cost-utility analyses, reporting would be limited to those that have been prepared by a publicly funded Canadian organization, such as the Canadian Agency for Drugs and Technologies in Health (CADTH) or the Institut national d’excellence en santé et services sociaux (INESSS). These organizations have dedicated expertise, and they generally conduct pharmacoeconomic analyses for medicines seeking to be reimbursed by public insurers. The PMPRB would consider these analyses in its evaluation of price excessiveness. It would not duplicate the work conducted by CADTH and INESSS as part of reimbursement processes.”
(pp.10-11, emphasis added)
None of the documents provided to the Working Group by the PMPRB included any statement regarding the policy intent with respect to “options for accounting for and/or addressing uncertainty in the point estimate for each value-based price ceiling”.
4.3 Summary of Deliberations
4.3.1 Using the CADTH and/or INESSS reference case analyses
The Terms of Reference tasked the Working Group with considering “options for using the CADTH and/or INESSS reference case analyses to set a ceiling price”.
Members discussed how the results of pharmacoeconomic analyses of a medicine reported by CADTH, INESSS and other Canadian HTA agencies generally differ from those reported by the manufacturer and also from each other. The industry members argued that cost-utility estimates by CADTH and INESSS “often exhibit differences in their estimates pertaining to heterogeneous assumptions and expert opinions”, and that this variability is “a function of the analyst that produces the assessment and the peer reviewers that challenge the analyses”.
Members also discussed whether the assumptions adopted by CADTH and INESSS in their ‘reference case’ analyses are appropriate for use by the PMPRB when setting ceiling prices. Some members suggested that the PMPRB might wish to establish its own ‘reference case’, clearly specifying the requirements and any necessary assumptions for pharmacoeconomic analyses used to inform ceiling prices. Although the policy intent is to “not duplicate the work conducted by CADTH and INESSS”, possible departures from existing CADTH and INESSS reference case assumptions include a clear specification of a supply-side cost-effectiveness threshold and a potential departure from the assumption of risk-neutrality (see section 4.3.3).
Since matters of process were beyond the remit given by the Terms of Reference, the Working Group did not consider what specific processes might be established by the PMPRB to arrive at a single set of pharmacoeconomic results from which to inform a ceiling price. Nevertheless, there was a widespread view among Working Group members that clarity is required in whatever processes are established by the PMPRB.
The following potential recommendation was put to a vote of the Working Group:
4.1: The Working Group recognizes that there is variation in the results of pharmacoeconomic analyses reported by CADTH and INESSS, and recommends that the PMPRB establish clear processes for identifying how these analyses will be used to inform a ceiling price.
Members voted 12 in favour and 0 against this potential recommendation.
This was therefore adopted as a formal recommendation of the Working Group.
4.3.1 Ensuring unbiased estimates
The Working Group noted that the most recent edition of CADTH’s ‘Guidelines for the Economic Evaluation of Health Technologies: Canada’ (4th Edition) includes specific recommendations for addressing uncertainty in pharmacoeconomic analysis.11 These include an assessment of parameter uncertainty (through probabilistic analysis), structural uncertainty (through scenario analysis), and methodological uncertainty (through a comparison of ‘reference case’ and ‘non-reference case’ analyses). INESSS has similar requirements for considering uncertainty.26
Some members expressed concern that not all pharmacoeconomic analyses currently satisfy these recent CADTH guidelines, and that better enforcement of these guidelines is needed to ensure that uncertainty is appropriately addressed in all pharmacoeconomic analyses considered by the PMPRB when informing ceiling prices.
Members also noted that current HTA processes at CADTH and INESSS are undertaken for the purpose of assisting public payers in making decisions related to funding and informing pricing negotiations, rather than to inform ceiling prices set by the PMPRB. There was broad agreement that the PMPRB should engage with CADTH and INESSS, and any other relevant stakeholders, regarding modifications that may be required to these processes given the proposed change in the context of their use.
While considerations of the specific processes adopted by CADTH and INESSS are beyond the scope of the Working Group, the key technical principle is that all pharmacoeconomic analyses should satisfy the same basic set of requirements, including a comprehensive and unbiased assessment of parameter uncertainty, structural uncertainty and methodological uncertainty.
The following potential recommendation was put to a vote of the Working Group:
4.2: The Working Group recommends that all pharmacoeconomic analyses used for the purpose of informing a ceiling price should satisfy the requirements of the most recent edition of CADTH’s ‘Guidelines for the Economic Evaluation of Health Technologies: Canada’, including an unbiased assessment of parameter uncertainty, structural uncertainty and methodological uncertainty.
Members voted 12 in favour and 0 against this potential recommendation.
This was therefore adopted as a formal recommendation of the Working Group.
4.3.2 Addressing uncertainty in the point estimate
The Terms of Reference also required the Working Group to consider “options for accounting for and/or addressing uncertainty in the point estimate for each value-based price ceiling”.
It was agreed that there are a number of sources of uncertainty in any pharmacoeconomic analysis. One member noted that clinical uncertainty is typically the primary source of uncertainty when CADTH considers new medicines, particularly for rare conditions. There are also uncertainties in the incremental costs associated with new medicines.
Furthermore, since the supply-side threshold requires empirical estimation, it will inevitably be uncertain. For example, the UK research which estimated a supply-side threshold reported a probability distribution in addition to a point estimate.10
As noted in the Conceptual Framework, any uncertainty in the incremental costs and benefits results in uncertainty in the ICER. The price at which the ICER is equal to the supply-side threshold is also uncertain, resulting in uncertainty in the true location of the demand curve. This, in turn, results in uncertainty in the ceiling price that is consistent with the policy objective regarding the allocation of the economic surplus between consumers and producers.
Some members noted that CADTH does not always report a point estimate for the ICER, but that a point estimate would be required for the purposes of informing a ceiling price.
Members discussed how a ceiling price might be informed when there is uncertainty around the ICER. The standard approach for considering uncertainty in economic evaluations is to use the expected values of the incremental costs and incremental benefits in order to calculate an ICER. This is the approach adopted in CADTH’s ‘Guidelines for the Economic Evaluation of Health Technologies: Canada’ (4th Edition).11 This approach implicitly assumes ‘risk neutrality’, which is typically justified on the basis of the Arrow-Lind principle.27
Members also debated using the upper bound of the credible interval around the ICER. Concern was raised that this approach would provide a disincentive for manufacturers to conduct research that reduces uncertainty around the ICER, since additional uncertainty would be rewarded with a higher ceiling price. It would also result in negative expected consumer surplus.
As noted in the Conceptual Framework, if the standard approach is adopted and a ceiling price is specified at which the ICER (calculated by dividing the expected incremental costs by the expected incremental QALYs) equals the expected value of the supply-side cost-effectiveness threshold, then the expected consumer surplus would be zero. (Note that the actual consumer surplus may be positive or negative, but the expected consumer surplus would be zero).
If the policy intent is to ensure that expected consumer surplus is non-negative, and if a risk-neutral position is adopted, then this would be the highest ceiling price consistent with this policy objective. Alternatively, if a risk-adverse position is adopted, then a higher or lower ceiling price is required to mitigate this risk. Raising the ceiling price may reduce the risk that a medicine is not launched, while lowering the ceiling price may reduce the risk that a medicine results in negative consumer surplus.
Since the PMPRB’s risk attitude is not known, the Working Group cannot specify the most appropriate option for informing a ceiling price. Instead, the Working Group recommends that the PMPRB adopt an approach for considering uncertainty that is consistent with its risk attitude. If the PMPRB is ‘risk-neutral’, this requires that the ceiling price be informed by the expected values of the incremental costs and QALYs for the medicine and the expected value of the supply-side cost-effectiveness threshold. If the PMPRB is not ‘risk-neutral’, then consideration should be given to setting a ceiling price that is higher or lower than that under risk neutrality, given the policy intent.
The following potential recommendation was put to a vote of the Working Group:
4.3: The Working Group recommends that the PMPRB adopt an approach for considering uncertainty that is consistent with its risk attitude. If the PMPRB is ‘risk-neutral’, this requires that the ceiling price be informed by the expected values of the incremental costs and QALYs for the medicine and the expected value of the supply-side cost-effectiveness threshold. If the PMPRB is not ‘risk-neutral’, then consideration should be given to setting a ceiling price that is higher or lower than that under risk neutrality, given the policy intent.
Members voted 10 in favour and 2 against this potential recommendation.
This was therefore adopted as a formal recommendation of the Working Group.
4.3.3 Value of information analysis
In conventional pharmacoeconomics, the expected loss in consumer surplus that results from uncertainty is estimated using ‘value of information’ (VOI) analysis.
Since the focus of conventional pharmacoeconomic analysis is making a yes/no decision regarding adoption of a new medicine, conventional VOI analysis considers the expected loss associated with making the ‘wrong’ decision (e.g. approving a medicine that would otherwise have been rejected, or vice versa).
In the context of the PMPRB using ‘pharmacoeconomic value’ as a factor when considering the ceiling price for a new medicine, the expected loss as a result of uncertainty comes not from making the ‘wrong’ yes/no decision, but from setting the ‘wrong’ ceiling price. One member of the Working Group (Dr Christopher McCabe) circulated a technical note (Appendix 2.2) and gave a presentation (Appendix 2.4) outlining how uncertainty can be considered in this context. The Conceptual Framework built upon a number of the ideas outlined by Dr McCabe.
As noted in the Conceptual Framework, in many cases the expected impact upon consumer surplus of setting the ‘wrong’ ceiling price as a result of uncertainty is zero. This is the case if the medicine is still launched at a ceiling price coinciding with the expected demand curve, or if the medicine would not have launched even at a ceiling price coinciding with the actual demand curve. However, in cases where the medicine would have launched at a ceiling price coinciding with the actual demand curve, but does not launch at a ceiling price coinciding with the expected demand curve, uncertainty results in an expected loss in economic surplus.
In principle, the PMPRB could use VOI analysis to estimate this expected loss in economic surplus, and hence the value associated with obtaining additional sample information for one or more uncertain parameters. The results of these analyses could then be used to apply a reduction to a medicine’s ceiling price to reflect the diminished expected pharmacoeconomic value as a result of uncertainty.
Conducting such VOI analyses would require an understanding of the location of the supply curve, since this is required to estimate the expected loss in economic surplus. As noted in the Conceptual Framework, in practice the location of the supply curve is unknown. Although the supply curve could be modelled with a probability distribution in order to permit VOI analysis to take place, methods for estimating the parameters of such a distribution are undeveloped.
As a result of these unresolved challenges, the Working Group does not make a recommendation on whether to use VOI analysis at the present time.
5: Perspectives
5.1 Terms of Reference
Within this area of focus, the Terms of Reference required the Working Group to examine and make recommendations with respect to the following considerations and questions:
Options to account for the consideration of a public health care system vs societal perspective, including the option of applying a higher value-based price ceiling in cases where there is a ‘significant’ difference between price ceilings under each perspective.
How to define a ‘significant’ difference in price ceilings between each perspective.
5.2 Policy Intent
Two months into the Working Group’s deliberations, the PMPRB informed the Working Group that a public health care system perspective “needs to be used to meet the policy objective of the [Regulatory Impact Analysis Statement]”.
5.3 Summary of Deliberations
5.3.1 Acknowledgement of policy intent
Two months into the Working Group’s deliberations, the PMPRB informed the Working Group that it had come to the view that a public health care system perspective “needs to be used to meet the policy objective of the [Regulatory Impact Analysis Statement]”.
The PMPRB noted that, in coming to this view, it had benefited from the Working Group’s discussions with respect to this area of focus.
Given this intervention from the PMPRB, the Working Group did not vote on any potential recommendations for this area of focus. Instead, the Working Group acknowledges that the policy intent is to adopt the perspective of Canada’s public health care systems.
5.3.2 Considerations on the choice of perspective
Prior to the PMPRB’s intervention to clarify the policy intent, the Working Group discussed some of the differences between a ‘public health care system’ perspective and a ‘societal’ perspective and some of the possible implications of these differences when setting ceiling prices.
Differences between perspectives
As noted in CADTH’s ‘Guidelines for the Economic Evaluation of Health Technologies: Canada’ (4th Edition, pp.29-31), there are differences between the costs and outcomes considered under a ‘public health care system’ and those considered under a ‘societal’ perspective.11
A ‘public health care system’ perspective only considers costs borne by the public health care payer, and the only outcomes considered are health effects relevant to patients and caregivers.
A ‘societal’ perspective also considers costs that fall on private insurers (e.g. medicines that are not covered by the public payer), other government sectors (e.g. social services and affordable housing), and patients or caregivers (e.g. out-of-pocket payments and travel costs). In addition, a societal perspective considers productivity costs (e.g. due to reduced working capacity or absence from work) and broadens the consideration of outcomes to include non-health effects relevant to patients and caregivers (e.g. better educational achievements).
Private insurers and out-of-pocket payers
Industry members on the Working Group expressed concern that, under a health care system perspective, costs borne by private insurers and out-of-pocket payers would not be taken into account. These members also argued that the willingness-to-pay of some private payers is higher than that of public payers, which would not be taken into account through consideration of a supply-side cost-effectiveness threshold. It was further argued that savings to private payers through a lower ceiling price may not be passed on to individuals or employers.
In support of this position, some members noted that the willingness-to-pay of private payers may be better reflected by estimates of a ‘demand-side’ cost-effectiveness threshold rather than a supply-side threshold. As outlined in the IHE report, there are reasons to expect that a demand-side cost-effectiveness threshold would be higher than a supply-side threshold.
In response, one member argued that it is not meaningful to consider the willingness-to-pay of private payers in isolation from the willingness-to-pay of public payers, on the basis that the market for private payers could not exist in its present state without a sustainable public health care system. According to this member, it is therefore reasonable for the PMPRB to set a ceiling price that ensures the sustainability of the public health care system, even if this is lower than a ceiling price based on the willingness-to-pay of private payers.
One member supported a societal perspective on the basis that the PMPRB should account for “the many rare disease patients who rely on alternatives to the public health care system”.
Problems with a societal perspective
A number of members discussed problems with the consideration of a societal perspective.
One member suggested that adopting a societal perspective, rather than a public health care system perspective, results in “increased uncertainty with no real impact”. Another member argued that adopting a societal perspective implies that policy makers are willing to trade health benefits for other societal benefits, which may not be the case.
Several members expressed concern with the consideration of productivity costs that would be made under a societal perspective. Some cited the technical difficulty of estimating productivity costs and the additional uncertainty that results. Other pointed out ethical concerns, including the potential for productivity to be valued less for those with lower earning power, including women and the retired, which may be considered discriminatory.
6: Market size factor
6.1 Terms of Reference
Within this area of focus, the Terms of Reference required the Working Group to examine and make recommendations with respect to the following considerations and questions:
Approaches to derive an appropriate affordability adjustment to a medicine’s ceiling price based on an application of the market size and GDP factors (e.g. based on the US ‘ICER’ approach).
6.2 Policy Intent
The Regulatory Impact Analysis Statement includes the following statements which provide context regarding the policy intent with respect to this area of focus:
“The addition of this [market size] factor in the Regulations could enable the PMPRB to develop market impact tests for medicines that are likely to pose affordability challenges for insurers due to the market size for the medicine. The impact of an excessive price is a function of both price and volume; the larger the size of the market for the medicine in Canada, the greater the impact of its price”.
(p.8, emphasis added)
“The introduction of GDP in Canada and GDP per capita in Canada as a price regulatory factor would provide the PMPRB with measures of ability to pay for medicines at the national and individual level. The inclusion of this factor would allow the PMPRB to assess the impact of a medicine’s price on the finances of consumers and insurers. It could also enable the PMPRB to develop market impact tests for medicines that are likely to pose affordability challenges for insurers due to the market size for the medicine.”
(p.9, emphasis added)
The Proposed Regulatory Text includes the following text:
“4.4 For the purposes of paragraph 85(1)(e) of the Act, the other factors that the Board must take into consideration to determine whether a medicine that is sold in any market in Canada after December 31, 2018 is being or has been sold at an excessive price are the following:
- the pharmacoeconomic value in Canada of the medicine and that of other medicines in the same therapeutic class;
- the size of the market for the medicine in Canada and in countries other than Canada; and
- the gross domestic product in Canada and the gross domestic product per capita in Canada.”
(p.24, emphasis added)
The PMPRB Guidelines Scoping Paper includes the following statement which provides context regarding the policy intent with respect to this area of focus:
“The second part of the test would assess whether a drug that meets the cost effectiveness threshold should have its price further adjusted because of its expected impact on payers within the first three to five years from launch (assuming appropriate clinical utilization and no rationing of care). This test would consider the anticipated market size of the new drug against GDP growth, with the latter serving as a rough proxy for how much Canadian consumers can afford to pay for the new patented drugs that come to market on an annual basis. The test could also be used to allow a price adjustment upward in instances where a drug has a very high opportunity cost but very small market impact due to the extreme rarity of the condition it is indicated to treat.”
(p.6, emphasis added)
“The fifth and final part of the new framework would involve the periodic “re-benching” of drugs to ensure that previous determinations of potential excessive pricing and/or price ceilings remain relevant in light of new indications (resulting in a change of market size) or changes in market conditions. Depending on the nature of the change, the re-benching process could result in a decrease or increase in ceiling price.”
(p.7, emphasis added)
The PMPRB Framework Modernization Presentation includes the following statements which provide context regarding the policy intent with respect to this area of focus:
“Drugs that are expected to have a significant market size and impact on the healthcare system will have a lower ceiling price to deter rationing.”
(p.7, emphasis added)
The PMPRB Framework Modernization Presentation includes the following slides which provide context regarding the policy intent with respect to this area of focus:
Part III: MRP for Category 1 drugs (continued)
Step 2: application of market size and GDP factors
- A Category 1 drug that meets the applicable $/QALY ceiling may still face an adjustment in price if the application of the market size and GDP factors raise affordability concerns.
- Using new drug contribution to GDP and GDP growth over the last five years, the PMPRB is estimating a threshold of $20M per new drug.
- New Category 1 drugs with an estimated market size that exceeds this threshold within any of its first five years of sale will require further price adjustments.
- The adjustment would see the MRP reduced by a certain percentage discount which would increase as the expected market size increases.
- The $20M threshold would also increase annually based on GDP growth and/or CPI.
Application of new factors to Category 1 drugs -
potential thresholds
| Type of review |
$/QALY target to set MRP |
Market impact adjustment |
| Baseline New Drug (market size up to $20M) |
$60K |
N/A |
| “Premium” New Drug (e.g. high burden, EDRD, significant absolute QALY gain) |
$90K to $150K |
N/A |
| High Impact New Drug (market size over $20M) |
$60K |
10% reduction on MRP for each additional $10M market size (to 50% maximum) |
6.3 Summary of Deliberations
The Working Group noted that the Proposed Regulatory Text includes separate consideration of the pharmacoeconomic value, market size, and GDP factors. The ‘affordability adjustment’ that the Working Group was tasked with considering would therefore be applied separately from the consideration of ‘pharmacoeconomic value’.
6.3.1 Implications for consumer and producer surplus
The proposed market size adjustment includes a potential upwards ceiling price adjustment for medicines with small market size and, independently, a potential downwards ceiling price adjustment for medicines with large market size.
As shown in the Conceptual Framework, the first of these adjustments would have the effect of increasing the producer surplus, at the expense of consumer surplus, for medicines with small market size. The second of these adjustments would increase the consumer surplus, at the expense of producer surplus, for medicines with large market size.
An additional implication of the first adjustment is that, by increasing the profitability of medicines with small market size, this might result in greater access to such medicines. The potential for this is demonstrated in Figure 13B of the Conceptual Framework. This adjustment may therefore provide a means for mitigating the concerns expressed by one member regarding the potential impact of a lower ceiling price on access to orphan drugs (see section 2.3.7).
Since the desired allocation of the economic surplus among consumers and producers is a matter for policy makers, the Working Group does not take a position on the appropriate magnitude of any proposed market size adjustments. Instead, the Working Group recommends that the PMPRB consider the implications of any proposed market size adjustments for the allocation of the economic surplus, and ensure that these are consistent with the policy intent.
The following potential recommendation was put to a vote of the Working Group:
6.1: The Working Group recommends that the PMPRB consider the implications of any market size adjustments for the allocation of consumer and producer surplus, and ensure that these are consistent with the policy intent.
Members voted 12 in favour and 0 against this potential recommendation.
This was therefore adopted as a formal recommendation of the Working Group.
6.3.2 Potential incentives and disincentives
The Working Group discussed several potential incentives and disincentives associated with implementation of a market size adjustment.
It was noted that the estimated market size of a medicine at launch is uncertain. A market size adjustment based on a medicine’s estimated market size might therefore result in a downwards adjustment to the ceiling price for a medicine which does not ultimately achieve a large market size. Conversely, a downwards adjustment might not be applied to a medicine that unexpectedly achieves a large market size. To minimize any resulting disincentives, the market size adjustment would ideally be applied to actual market size rather than expected market size.
If the reduction in ceiling price for medicines with large market size is large, then manufacturers may be incentivized to reduce the quantity supplied so as to avoid the reduction in the ceiling price. As demonstrated in the Conceptual Framework, this risk may be particularly acute if the medicine in question has multiple indications, and if pricing across all indications is based upon the least cost-effective indication. This is because this pricing approach may already provide an incentive for manufacturers to avoid launching in one or more indications, and the addition of a market size adjustment may exacerbate this risk.
By providing a higher ceiling price for medicines with low market size, a market size adjustment might also relatively incentivize the development of such medicines. Over time, a reduction in medicines with large market size and an increase in medicines with small market size might result in progressively smaller gains and progressively larger losses in consumer surplus.
The following potential recommendation was put to a vote of the Working Group:
6.2: The Working Group recommends that the PMPRB consider the potential incentives and disincentives that might result from the application of any market size adjustments.
Members voted 12 in favour and 0 against this potential recommendation.
This was therefore adopted as a formal recommendation of the Working Group.
6.3.3 GDP and GDP per capita
US ‘ICER’ approach
The Terms of Reference cited the Institute for Clinical and Economic Review (ICER) as providing a potential approach to inform an ‘affordability adjustment’.
(Note that the acronym ‘ICER’ has been used elsewhere in this report to refer to a medicine’s ‘incremental cost-effectiveness ratio’, which is the more common usage of this acronym. Within this section only, ‘ICER’ will be used to refer to the Institute for Clinical and Economic Review).
One member reached out to Dr Dan Ollendorf, former Chief Scientific Officer at ICER, who provided a copy of his submission to Health Canada during the consultation period on the proposed amendments. On behalf of ICER, Dr Ollendorf noted that “we are supportive of the PMPRB’s efforts to better align pricing of pharmaceuticals with value”, and that “we applaud the PMPRB for considering amendments that provide additional focus on pricing innovative medicines according to the value they bring to individual patients, families, and the overall health system”. However, Dr Ollendorf raised a note of caution regarding the proposed ‘affordability’ criteria, noting “several technical challenges with implementing the market size factor for price setting at ICER”. Among these, “there was a challenge in interpreting an explicit linkage of budget impact results to a price”, “it proved difficult for individual decision-makers to make sense of a national budget threshold”, and “any explicit linkage of a threshold to price-setting required ICER to estimate what ‘unmanaged’ uptake would look like, which was extraordinarily difficult”.
UK approach
During the Working Group’s deliberations, it was announced that the UK’s new five-year ‘Voluntary Pricing and Access Scheme’ for branded medicines, which came into force on 1 January 2019, includes a 2% cap on nominal annual growth of the total medicines bill.
Using GDP to update thresholds
The Working Group discussed how any thresholds specified for the criteria used to classify medicines as ‘Category 1’, as well as any supply-side threshold specified by the PMPRB, may need to be periodically revised in response to changes in GDP and GDP per capita over time.
It was noted that the supply-side threshold for any specific province or territory is a function of the budget for the respective health care system, in addition to a number of other factors.8 A change in GDP or GDP per capita over time would therefore be expected to have an indirect impact upon the supply-side threshold through a change in the size of the health care budget. It follows that the supply-side threshold should not be adjusted directly to account for changes in GDP or GDP per capita; rather, it should be recalculated periodically to reflect changes in the size of provincial and territorial health care budgets and the marginal productivity of health care services that face displacement from the adoption of new medicines.
The following potential recommendation was put to a vote of the Working Group:
6.3: The Working Group recommends that the PMPRB periodically reconsider any specified thresholds in response to changes in GDP and GDP per capita over time, including the supply-side cost-effectiveness threshold and any thresholds for criteria used to classify medicines as ‘Category 1’.
Members voted 12 in favour and 0 against this potential recommendation.
This was therefore adopted as a formal recommendation of the Working Group.
6.3.4 Considerations beyond ‘pharmacoeconomic value’
The chair noted that application of both the ‘market size’ and ‘gross domestic product’ factors require considerations beyond those made in assessments of ‘pharmacoeconomic value’.
Since the Working Group was primarily composed of experts in pharmacoeconomics, there may be important technical considerations for the application of these two factors that are beyond the expertise of the Working Group.
Appendix 1: Conceptual Framework
A1.1 Foreword
This Conceptual Framework was drafted by the chair prior to the final meeting of the Working Group. Its purpose was to guide the Working Group in making consistent recommendations across all six areas of focus, while respecting the policy intent and the range of views expressed by members of the Working Group throughout their deliberations.
A1.1.1 Policy intent
This framework incorporates the following components of the policy intent:
During the Working Group’s deliberations, the PMPRB stated that the most appropriate perspective to adopt when considering the ‘pharmacoeconomic value’ factor described in Amendment 4.4(a) in the Regulations Amending the Patented Medicines Regulations is that of Canada’s publicly funded health care systems.
The Regulatory Impact Analysis Statement (Appendix 5.1) states that the quality-adjusted life year (QALY), as used in cost-utility analysis, is regarded as the “gold standard” approach to considering the economic value of new medicines.
In a July 2018 document prepared for the Working Group (Appendix 5.4), the PMPRB clarified that the purpose of the PMPRB is to ensure that patentees do not charge excessive prices during the statutory monopoly period.
The PMPRB clarified to the Working Group that its mandate is to protect consumers from excessive pricing, and not to ensure that products are launched into the market.
A1.1.2 Deliberations of the Working Group
This framework reflects the following considerations from the Working Group’s deliberations:
The Terms of Reference required the Working Group to consider potential approaches for allowing higher ceiling prices for some medicines on the basis of specific characteristics. This would require departing from the position that all QALYs have equal value, allowing for ‘equity weights’ (other than 1) to be applied to some QALYs but not others. Although there is an emerging body of empirical evidence, it was agreed by the Working Group that methods to apply equity weights (other than 1) are undeveloped at the present time. For the purposes of this conceptual framework, QALYs are therefore assigned equal value.
The Working Group considered several approaches for setting a single ceiling price across provinces and territories, including:
- A ceiling price at which the medicine is ‘just’ cost-effective in the province or territory with the highest k (such that the ICER equals this highest k );
- A ceiling price at which the medicine is ‘just’ cost-effective in the province or territory with the lowest k (such that the ICER equals this lowest k );
- A ceiling price at which the medicine is ‘just’ cost-effective across Canada as a whole (such that the ICER equals a ‘weighted average’ of k across Canada).
Although the Working Group agreed that a different ceiling price should be specified for each indication in principle, concerns were raised about the feasibility of doing this in Canada at the present time. The Working Group therefore considered various approaches for setting a single ceiling price across multiple indications, including:
- A ceiling price at which the medicine is ‘just’ cost-effective in the most cost-effective indication (such that the ICER equals k in this indication);
- A ceiling price at which the medicine is ‘just’ cost-effective in the least cost-effective indication;
- A ceiling price at which the medicine is ‘just’ cost-effective across all indications (such that a ‘weighted average’ of the ICER across all indications equals k );
- A ceiling price at which the medicine is ‘just’ cost-effective in the first indication considered by the PMPRB (such that the ICER equals k in this indication).
A1.2 Economic principles
When considering how the price of any good ought to be determined, it is informative to consider some fundamental economic principles.
At any given price, the ‘economic surplus’ from a good is the sum of two parts:
- The ‘consumer surplus’, which is the benefit obtained by consumers because they are able to purchase the good at a price lower than their ‘willingness-to-pay’;
- The ‘producer surplus’, which is the benefit obtained by producers because they are able to sell the good at a price higher than their ‘willingness-to-accept’.
A1.2.1 Standard models
Mainstream economics has a number of standard models which describe how consumers and producers behave under different market conditions, and the implications of this for the allocation of the economic surplus between consumers and producers. Among many possible models, the following are of particular relevance for the Working Group’s deliberations:
- In a perfectly competitive market, an equilibrium price arises at which there is positive consumer surplus and positive producer surplus. This is because most consumers pay a price lower than their maximum willingness-to-pay (as represented by a downwards sloping demand curve), while most producers receive a price higher than their minimum willingness-to-accept (as represented by an upwards sloping supply curve). The overall economic surplus is positive and allocated between consumers and producers.
- In a monopolistic market with a single price, the single producer reduces output and raises its price so as to maximize the producer surplus. Consumer surplus is diminished but remains positive, since some consumers still pay a price below their willingness-to-pay. However, the overall economic surplus is diminished because reducing output results in a ‘deadweight loss’: some consumers are willing to pay a price above the producer’s supply curve, but the producer would prefer not to supply to those consumers since greater profits arise by supplying fewer consumers at a higher price.
- In a monopolistic market with perfect price discrimination, the producer charges a different price to each consumer so as to extract the entire economic surplus. Consumer surplus is zero, since all consumers pay a price equivalent to their willingness-to-pay. The entire overall economic surplus is retained by the producer.
In order to consider the consumer and producer surplus that might arise from the PMPRB setting a ceiling price on a new medicine, we must first specify demand and supply curves.
A1.2.2 Demand curve for a medicine
The demand curve reflects society’s willingness-to-pay for the medicine in question.
It is for the PMPRB, rather than members of the Working Group, to define the components of this demand curve. The Working Group therefore defers to the policy intent when considering the relevant components of the demand curve.
During the Working Group’s deliberations, the PMPRB stated that the most appropriate perspective to adopt when considering the ‘pharmacoeconomic value’ factor described in Amendment 4.4(a) in the Regulations Amending the Patented Medicines Regulations is that of Canada’s publicly funded health care systems.
The Regulatory Impact Analysis Statement (Appendix 5.1) states that the quality-adjusted life year (QALY), as used in cost-utility analysis, is regarded as the “gold standard” approach to considering the economic value of new medicines.
In a July 2018 document prepared for the Working Group (Appendix 5.4), the PMPRB clarified that the purpose of the PMPRB is to ensure that patentees do not charge excessive prices during the statutory monopoly period.
In light of this policy intent, a reasonable specification of the demand curve for a new medicine is based upon the net impact upon the lifetime health of patients associated with adopting the medicine within Canada’s publicly funded health care systems for the duration of the statutory monopoly period, where health is measured in QALYs and discounted to a present value.
The net impact of a new medicine upon patient health is a function of two components:
- The gain in health experienced by patients who receive the new medicine; and
- The loss in health experienced by other patients whose health care subsequently receives less funding than it would have done in the absence of the new medicine.
The Terms of Reference required the Working Group to consider potential approaches for allowing higher ceiling prices for some medicines on the basis of specific characteristics. This would require departing from the position that all QALYs have equal value, allowing for ‘equity weights’ (other than 1) to be applied to some QALYs but not others. Although there is an emerging body of empirical evidence, it was agreed by the Working Group that methods to apply equity weights (other than 1) are undeveloped at the present time. For the purposes of this conceptual framework, QALYs are therefore assigned equal value.
The gain in health for patients who receive the medicine is routinely calculated by CADTH and INESSS as part of their existing methods for conducting economic evaluations, and is typically denoted as ΔH (where the delta refers to ‘incremental’ and H refers to ‘health benefit’).
The loss in health experienced by other patients is commonly referred to as the ‘opportunity cost’ of funding the new medicine, although it has been argued that the true opportunity cost is greater than just this ‘displaced health’.28,29
Since the patients who incur these health losses are typically unidentifiable, the standard approach for estimating the magnitude of this health loss is to divide the incremental costs of the new medicine, commonly denoted as ΔC , by a parameter reflecting the ‘shadow price’ of the relevant health care budget constraint, typically denoted as k . This latter parameter is also commonly referred to as the ‘supply-side cost-effectiveness threshold’.
For example, in the case studies provided to the Working Group by the PMPRB, it was assumed that k is $60,000 per QALY (the empirical evidence for specifying k is considered elsewhere in this report). This implies that every additional $60,000 in cost imposed by a new medicine upon Canada’s public health care systems results in a health loss of 1 QALY.
Assuming that there is only one indication for the new medicine, and assuming a single value of k that applies regardless of the quantity of medicine supplied (assumptions reconsidered later),
the demand curve for the new medicine is a perfectly elastic horizontal line that plots the ceiling price at which the health gain from the medicine is exactly offset by the health loss, such that the net health benefit is zero. That is, the demand curve plots the ceiling price at which
ΔH = ΔC/k. (1)
Rearranging equation (1), it follows that the demand curve plots the ceiling price at which the incremental cost-effectiveness ratio (ICER) of the new medicine is equal to k :
ΔC/ΔH = k. (2)
For the hypothetical medicine in Figure 1, the ICER is equal to k at a ceiling price of P1, such
that the demand curve is also plotted at this ceiling price.
Figure 1
Figure 1: Demand curve for a hypothetical medicine (D1)
The PMPRB provided the Working Group with a number of case studies (Appendix 6). For each of these case studies, the reported ‘PV Threshold Price’ is the ceiling price at which the medicine has an ICER of $60,000 per QALY. Since $60,000 per QALY is the PMPRB’s assumed value of k , it follows that every additional $60,000 spent on each new medicine at the ‘PV Threshold Price’ would provide 1 additional QALY but is assumed to displace 1 additional QALY in other patients, such that the net health benefit is zero. It follows that the demand curve for the new medicine considered in each case study would be plotted at the ‘PV Threshold Price’.
Since the ceiling price at which the ICER is equal to k varies across medicines, each medicine
has a different demand curve. The more cost-effective a medicine is, and hence the more QALYs produced at a given ceiling price, the higher the ceiling price at which the ICER is equal
to k and the higher the demand curve (Figure 2A). Conversely, the less cost-effective a
medicine is, the lower the demand curve (Figure 2B).
It follows that the developers of future medicines have two mechanisms by which they can raise the demand curve for their medicine upon launch: improve the effectiveness of the medicine (and the resulting health gain) or reduce the price (and the resulting health loss).
Figure 2A
Figure 2A: Demand curve for a more cost-effective medicine (D2)
Figure 2B
Figure 2B: Demand curve for a less cost-effective medicine (D3)
A1.2.3 Supply curve for a medicine
The supply curve plots the lowest price that a manufacturer would be willing to accept for a medicine. This is sometimes referred to as the ‘reservation’ (or ‘reserve’) price of the medicine.
The supply curve is a function of a number of potential considerations, including the initial costs associated with developing the medicine, the marginal costs of production, and the potential implications for pricing in other jurisdictions as a result of ‘reference pricing’.
It is important to note that the supply curve in a specific jurisdiction does not necessarily reflect only the marginal costs of production or the required return on investment for the manufacturer. Due to the possibility of reference pricing, a manufacturer might be unwilling to accept a price in a specific jurisdiction, even if this covers the marginal costs of production and provides a sufficient return on investment in that jurisdiction, if this results in a lower price in one or more other jurisdictions. One means of mitigating this possibility is through the use of confidential pricing arrangements, such that the price actually paid in a specific jurisdiction is lower than the ‘list price’ used by other jurisdictions for the purpose of reference pricing. Confidential pricing arrangements may therefore be expected to lower the supply curve, since the implications for reference pricing in other jurisdictions are no longer a relevant factor.
Regardless of whether reference pricing is a relevant factor, the components of the supply curve are complex. Furthermore, compared to the components of the demand curve (such as k ), relatively little empirical research has been conducted into the components of the supply curve, with existing research focused primarily on estimating the costs associated with research and development (rather than the expected reservation price). As a result of this asymmetry, the supply curve for each new medicine is highly uncertain. For the purposes of this framework, the medicine’s supply curve will therefore be treated as unknown (and plotted as a dashed line).
Despite being unknown, we may reasonably expect the supply curve for a medicine to have the following basic properties:
- A relatively high intercept on the vertical axis, reflecting the substantial initial costs associated with researching and developing the medicine;
- A downwards slope, reflecting a declining per-patient cost of supplying the medicine as the quantity supplied increases. This declining per-patient cost arises from the ability to spread the initial costs of research and development across a greater number of patients, and also potential economies of scale in the production of the medicine.
Since initial research and development costs and production costs vary across medicines, each medicine would be expected to have a different supply curve.
For example, recent empirical work found that the initial development costs for an orphan drug (with a small patient population, resulting in a relatively small quantity supplied) are $291m USD (average capitalized clinical cost), compared to $412m USD for a non-orphan drug.30
Other recent work has found that the research and development costs associated with a new orphan drug are smaller than those for a non-orphan drug, but, given the smaller patient population, a higher per-patient price is required for orphan drugs to sustain a similar return on investment than non-orphan drugs.31
Figure 3 plots possible supply curves for two hypothetical medicines. Although both medicines are assumed to have large development costs, the first medicine (represented by supply curve S1) will be supplied at a lower price, for any given quantity, than the second medicine (represented by supply curve S2). For example, at a given quantity, Q1, the manufacturer of the first medicine is willing to accept a price of P4, whereas the manufacturer of the second medicine requires a higher price of P5. Among many other possible reasons, this might be due to the first medicine having relatively lower marginal costs of production.
Figure 3
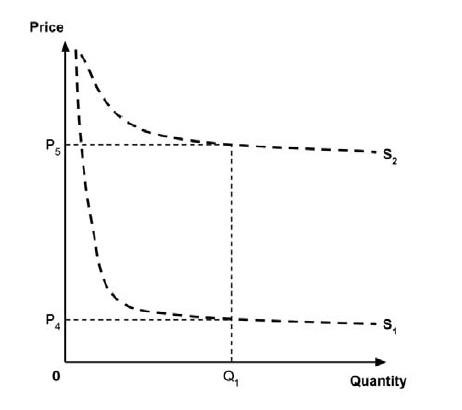
Figure 3: Supply curves for two hypothetical medicines, with relatively low (S1) and high (S2) marginal costs of production
A1.2.4 Economic surplus
The demand and supply curves may be used to consider the ‘economic surplus’ that results from adoption of a new medicine and, at any given ceiling price, the distribution of this economic surplus between consumers (patients) and producers (the manufacturers of new medicines).
When demand and supply curves are plotted on the same figure, the economic surplus is illustrated by the area of the region below the demand curve and above the supply curve, minus any area above the demand curve but below the supply curve, and bounded between the vertical axis and the quantity of medicine adopted.
For example, Figure 4A plots the demand (D1) and supply (S1) curves for a medicine with a relatively low supply curve. At a quantity of Q1, the economic surplus is positive and illustrated by the area of region 2 minus the area of region 1.
Figure 4B, by contrast, plots the demand (D1) and supply (S2) curves for a medicine with a relatively high supply curve. Since the supply curve lies entirely above the demand curve, adopting this medicine at a quantity of Q1 would result in a negative economic surplus, as illustrated by the area of region 3.
Figure 4A
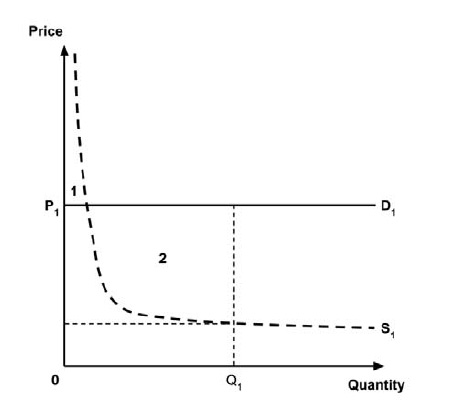
Figure 4A: Demand and supply curves for a medicine with a relatively low supply curve, resulting in a positive total economic surplus
Figure 4B
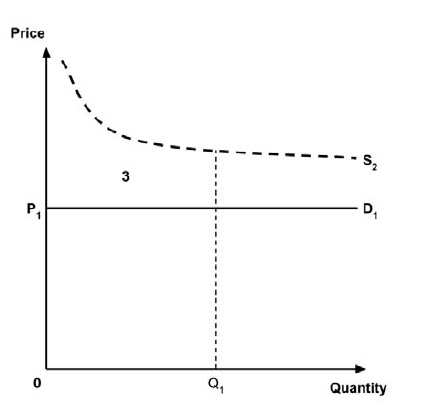
Figure 4B: Demand and supply curves for a medicine with a relatively high supply curve, resulting in a negative total economic surplus
A1.2.5 Defining consumer and producer surplus
Given the policy intent, the ‘consumer surplus’ arising from adoption of a new medicine reflects the net health benefit (in QALYs) for patients within Canada’s public health care systems.
The ‘producer surplus’, meanwhile, reflects profits for the manufacturers of new medicines.
A1.2.6 Allocating a positive economic surplus
How the economic surplus might be allocated among ‘consumers’ (patients) and ‘producers’ (manufacturers) depends upon whether this overall economic surplus is positive or negative.
If the economic surplus is positive, as in Figure 5A, then there is a range of possible ceiling prices at which consumer and producer surplus are both positive, such that adoption of the new medicine would provide a net benefit to patients and also the manufacturer.
The upper bound of this range is a ceiling price corresponding to the demand curve (P1 in Figure 5A), at which the ICER is k . At this ceiling price, the entirety of the economic surplus (illustrated by the area of region 2 minus the area of region 1) is allocated to the producer, such that the consumer surplus is zero. This is analogous to the allocation of consumer and producer surplus that would arise in a conventional model of monopoly with perfect price discrimination (in which the producer extracts the entire economic surplus).
The lower bound of this range is a ceiling price at which producer surplus is zero (P6 in Figure 5B). At this ceiling price, the ICER is below k and consumer surplus is positive, illustrated by the combined area of regions 4 and 5. Producer surplus is zero, illustrated by the area of region 6 minus the combined area of regions 1 and 4. Note that the overall economic surplus remains the same as in Figure 5A, and is equivalent to the combined area of regions 1, 5 and 6 only (since area 4 constitutes both a benefit for consumers and a loss for producers).
A ceiling price above P1 (so the ICER exceeds k ) would result in negative consumer surplus (such that the new medicine would diminish population health), and a ceiling price below P6 would result in negative producer surplus (such that the new medicine is not profitable).
It follows that only a ceiling price between P1 and P6 in Figure 5B would result in both positive consumer surplus and positive producer surplus. At any ceiling price within this range, the ICER of the new medicine is lower than k . Compared to the allocation of consumer and producer
surplus which arises when the ceiling price corresponds to the demand curve (such that the ICER is exactly k ), this allocation is closer to that which would arise in a conventional model of a competitive market (in which consumer and producer surplus are both positive).
Figure 5A
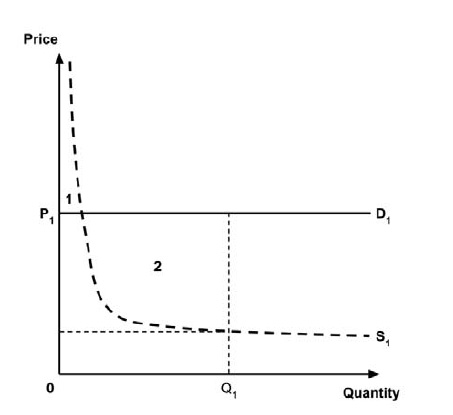
Figure 5A: At a price of P1, the entire economic surplus is allocated to the producer (region 2 minus region 1)
Figure 5B
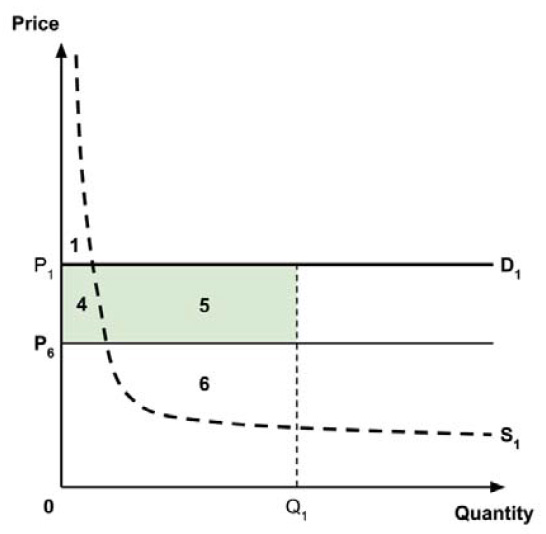
Figure 5B: At a price of P6, the entire economic surplus is allocated to the consumer (regions 4 and 5)
A1.2.7 Allocating a negative economic surplus
If the economic surplus is negative, as in Figure 4B, then there are no possible ceiling prices at which both consumer and producer surplus are positive.
Figure 6
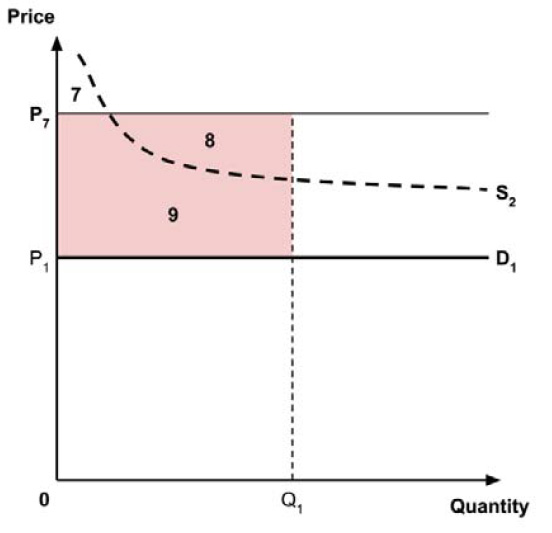
Figure 6: Where the supply curve lies above the demand curve, producer surplus cannot be positive unless consumer surplus is negative
Although a higher ceiling price can be sought for the medicine at which producer surplus is positive, this will result in negative consumer surplus. The consequence of a negative consumer surplus is that other patients will incur a greater loss in health than will be gained by the patients who receive the new medicine, in turn diminishing population health.
For example, in Figure 6, a ceiling price of P7 results in a positive producer surplus, as illustrated by the area of region 8 minus the area of region 7. However, consumer surplus is negative, as illustrated by the combined area of regions 8 and 9. Attempts to avoid this negative consumer surplus by lowering the ceiling price will also result in negative producer surplus.
The potential for the supply curve to lie above the demand curve is a particularly important consideration for medicines that are supplied to relatively few patients, such as orphan drugs, for which the supply curve may be more likely to be higher than the demand curve at the relevant quantity.
A1.3 Pricing across provinces and territories
The IHE report considered the various determinants of k .2
A key determinant is the size of the relevant health care budget, with larger per-capita health
care budgets resulting in higher values of k (all else equal).
Other determinants include the marginal productivity of existing health care activities that might experience reduced funding or displacement if a new medicine is adopted.8
A1.3.1 Variations in ‘k’ across provinces and territories
Since provinces and territories in Canada have some autonomy in setting health care budgets
and prioritizing spending, it follows that k would be expected to vary by province and territory.
This is supported by the empirical work by Ochalek et al. (2018), which reported varying estimates of the marginal ‘cost per DALY averted’ across provinces and territories.1 Using data from Claxton et al. (2017), this report found higher estimates in the territories (ranging from
$30,633 per DALY averted in Yukon to $52,191 per DALY averted in the Northwest Territories), and lower estimates across the provinces (ranging from a low of $16,425 per DALY averted in Prince Edward Island to a high of $26,060 per DALY averted in Alberta).
Note that, although these estimates were reported in terms of marginal ‘cost per DALY averted’, similar variation in estimates would be expected if these were instead reported in terms of marginal ‘cost per QALY gained’, which is how k should be specified given the policy intent.
A1.3.2 Implications for the opportunity cost of new medicines
An important implication of this variation in k is that the opportunity cost of adopting a new
medicine would be expected to differ across provinces and territories. The lower k is in any
province or territory, the greater the expected opportunity cost associated with adopting a new medicine (in terms of health forgone by other patients).
For example, based upon the report by Ochalek et al. (2018), every additional $1m spent on new medicines in Prince Edward Island would have an opportunity cost of approximately 60 DALYs, but every additional $1m spent on new medicines in Alberta would have a smaller opportunity cost of approximately 40 DALYs. All else equal, the net health benefit of any new medicine would therefore be expected to be smaller in Prince Edward Island than in Alberta.
A1.3.3 Implications for the demand curve
Since the demand curve plots the ceiling price at which the ICER of the new medicine is equal
to k (equation 2), it follows that the demand curve will be higher in provinces and territories with
larger estimates of k .
For example, based on the empirical work by Ochalek et al. (2018), we might expect the lowest demand curve in Prince Edward Island, the highest provincial demand curve in Alberta, and the highest demand curve overall in the Northwest Territories.
The width of each demand curve (the quantity demanded) would also be expected to differ across provinces and territories, since the number of patients receiving each new medicine will vary due to differences in population size and demographics.
A1.3.4 Approaches for setting a single ceiling price
The Working Group considered several approaches for setting a single ceiling price across provinces and territories, including:
- A ceiling price at which the medicine is ‘just’ cost-effective in the province or territory with the highest k (such that the ICER equals this highest k );
- A ceiling price at which the medicine is ‘just’ cost-effective in the province or territory with the lowest k (such that the ICER equals this lowest k );
- A ceiling price at which the medicine is ‘just’ cost-effective across Canada as a whole (such that the ICER equals a ‘weighted average’ of k across Canada).
Figures 7A to 7D demonstrate the implications of each of these approaches using a simplified model of a new medicine provided to patients across two provinces. In this model, ‘Province A’ has a higher k than ‘Province B’, such that the ceiling price at which the ICER of the medicine equals k in ‘Province A’ is P8. Given its size and demographics, ‘Province A’ demands a quantity of medicine Q2. ‘Province B’ demands a smaller quantity, Q3 - Q2, and has a lower k than ‘Province A’, such that the ICER would equal k for this province at a ceiling price of P9.
Figure 7A
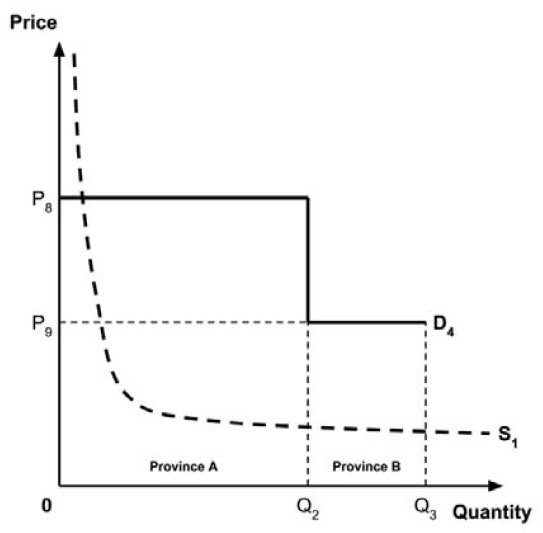
Figure 7A: The demand curve for a medicine across two provinces
Figure 7B
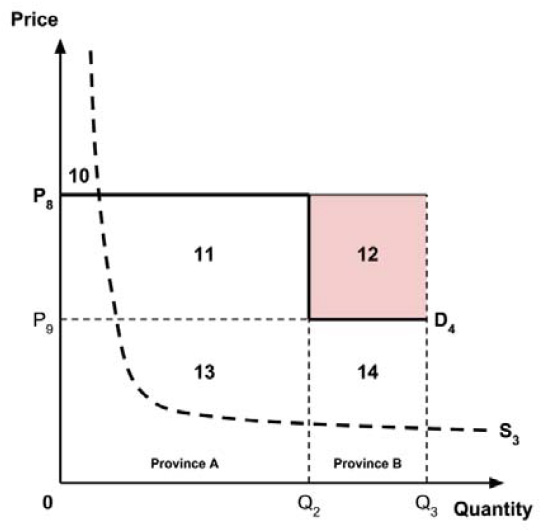
Figure 7B: Under the first approach (ceiling price P8), consumer surplus is negative in ‘Province B’ and zero in ‘Province A’, so negative overall
Figure 7C
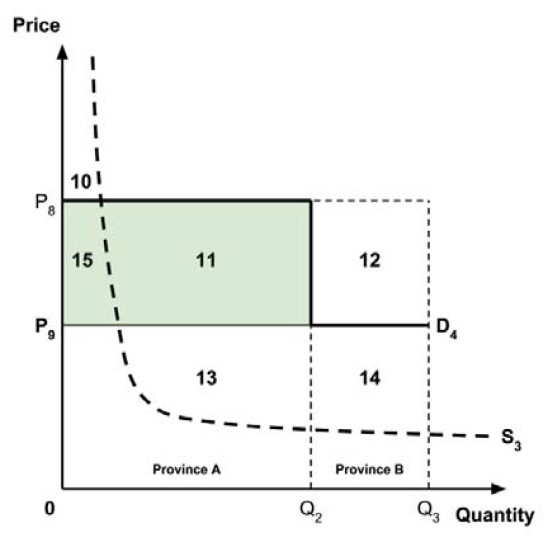
Figure 7C: Under the second approach (ceiling price P9), consumer surplus is positive in ‘Province A’ and zero in ‘Province B’, so positive overall
Figure 7D
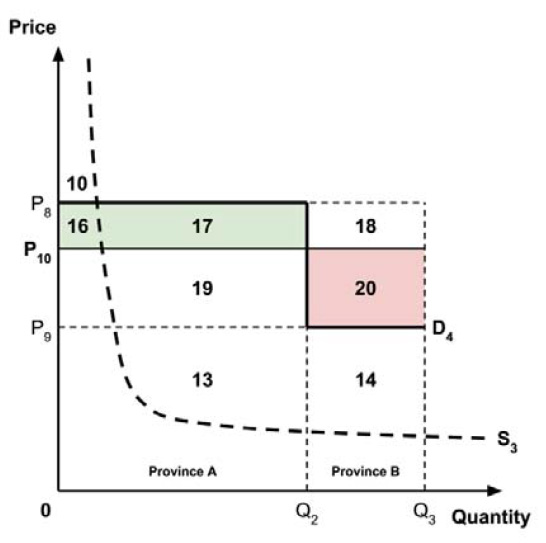
Figure 7D: Under the third approach (ceiling price P10), consumer surplus is positive in ‘Province A’), negative in ‘Province B’, and zero overall
Approach 1: Set a ceiling price according to the highest k
Under the first approach considered by the Working Group, the ceiling price of the new medicine would be set at P8 in both provinces (Figure 7B).
This would result in no consumer surplus in ‘Province A’ (since the ceiling price corresponds exactly to the demand curve), but negative consumer surplus in ‘Province B’ (as illustrated by the area of region 12) since the ceiling price lies above the demand curve. It follows that the total consumer surplus (across both provinces) would be negative, such that population health is diminished.
At this ceiling price, the producer surplus is illustrated by the combined area of regions 11, 12, 13 and 14, minus the area of region 10.
Approach 2: Set a ceiling price according to the lowest k
Under the second approach, the ceiling price would be set at P9 in both provinces (Figure 7C).
This would result in a positive consumer surplus in ‘Province A’ (the combined area of regions 11 and 15), and no consumer surplus in ‘Province B’ (since the ceiling price corresponds exactly to the demand curve), such that the total consumer surplus is positive.
The producer surplus would be lower than under the first approach, as illustrated by the combined area of regions 13 and 14, minus the combined area of regions 10 and 15.
Approach 3: Set a ceiling price according to a weighted average of k
Under the third approach, the ceiling price would be set between P8 and P9 such that the total consumer surplus (across both provinces) is zero. In this example, this requires setting a ceiling price of P10 (Figure 7D).
At a ceiling price of P10, the positive consumer surplus in ‘Province A’ (combined area of regions 16 and 17) is exactly offset by the negative consumer surplus in ‘Province B’ (area of region 20).
The producer surplus is lower than under the first approach but greater than under the second approach, as illustrated by the combined area of regions 13, 14, 19 and 20, minus the combined area of regions 10 and 16.
A1.3.5 Implications of a supply curve above the demand curve
In Figures 7A to 7D, the supply curve for the new medicine was plotted such that producer surplus is positive at all ceiling prices between P8 and P9. However, for medicines with a higher supply curve, it is possible that negative producer surplus might arise at some ceiling prices within this range.
For example, Figure 8A plots a supply curve (S4) which lies entirely above P9. Under the first approach considered above (pricing at P8), producer surplus would be positive (the combined area of regions 22 and 23, minus the area of region 21), but the consumer surplus would be negative (as in Figure 7B). However, under the second approach (pricing at P9), the medicine would have negative producer surplus (the combined area of regions 21, 24 and 25).
For medicines with a particularly high supply curve, negative producer surplus might arise at all ceiling prices between P8 and P9.
For example, Figure 8B plots a supply curve (S5) which lies entirely above P8. It follows that the medicine would have negative producer surplus under all of the approaches considered above, including under the first approach with a ceiling price of P8 (where the negative producer surplus is illustrated by the area of region 26). In this case, no ceiling price exists which provides both positive consumer and producer surplus, as would arise in a competitive market.
Figure 8A
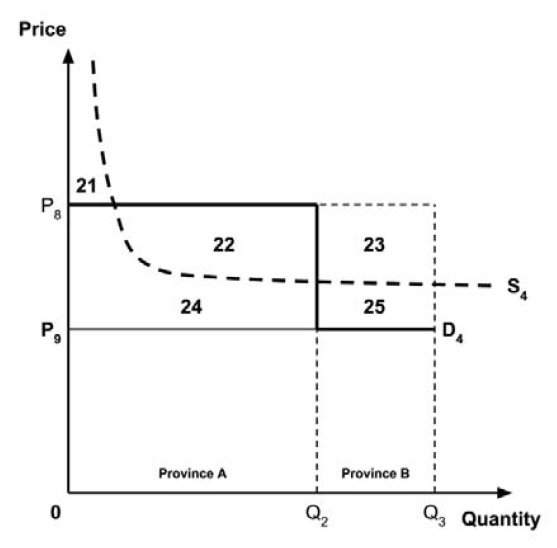
Figure 8A: With a higher supply curve (S4), the medicine is profitable at price P8 but unprofitable at price P9
Figure 8B
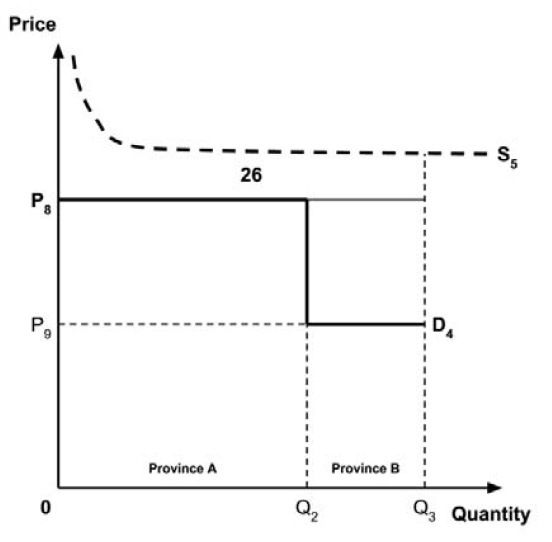
Figure 8B: With an even higher supply curve (S5), the medicine is unprofitable even at price P8
A1.3.6 Policy implications
The most desirable approach for setting a single ceiling price across Canada depends upon the policy intent.
Note that it is not the role of the Working Group to specify the policy intent. While the implications of some potential policy objectives are considered below, this should not be construed as an endorsement by the Working Group of any particular policy objective. Also note that this analysis is not exhaustive: there are other potential policy objectives and approaches for setting a ceiling price across provinces and territories.
Potential policy objective 1
If the policy maker desires that new medicines do not diminish population health across Canada as a whole, such that overall consumer surplus is at least zero, then the first approach considered above is inconsistent with this policy objective. This is because this approach results in diminished population health (negative consumer surplus) in all provinces and territories
except that with the highest k (in which consumer surplus is zero), resulting in diminished
population health (negative consumer surplus) overall.
The second approach comfortably satisfies this policy objective (since it results in positive overall consumer surplus), while the third approach only just satisfies this policy objective (since it results in an overall consumer surplus of zero).
It follows that the ceiling price that arises under the third approach (P10 in Figure 7D) is the maximum ceiling price that would be consistent with this policy objective. At this ceiling price, overall consumer surplus is zero, analogous to the consumer surplus arising in a standard model of a monopoly with perfect price discrimination.
Potential policy objective 2
If the policy maker instead desires that new medicines do not diminish population health within any province or territory, then both the first and third approaches are inconsistent with this policy objective. This is because both approaches result in diminished population health (negative consumer surplus) in at least one province or territory.
The second approach would only just satisfy this policy objective, since consumer surplus is zero in the province or territory with the lowest k .
It follows that the ceiling price that arises under the second approach (P9 in Figure 7C) is the maximum ceiling price that would be consistent with this policy objective. Provided that producer surplus is positive, such that the new medicine is launched, overall consumer surplus is also positive.
Note that if producer surplus is negative at P9 then it is not possible to set a ceiling price which satisfies this policy objective and provides for positive producer surplus.
Potential policy objective 3
If the policy maker wishes to set ceiling prices for new medicines so as to maximize population health across Canada as a whole, then consideration should be given to the location of the supply curve. Since the location of the supply curve is uncertain, this is challenging in practice.
A key assumption in the analysis below is that a medicine will not be launched if producer surplus is negative. If a medicine is not launched, the pharmacoeconomic value is zero since there is no resulting net gain in QALYs. For the pharmacoeconomic value to be positive, the medicine must be launched at a ceiling price that results in positive consumer surplus.
The PMPRB clarified to the Working Group that its mandate is to protect consumers from excessive pricing, and not to ensure that products are launched into the market.
If the supply curve is understood to be sufficiently high that the medicine would not be profitable at the ceiling price arising under the third approach (P10 in Figure 7D), then it is not possible to specify a ceiling price at which the medicine is profitable and improves population health.
Alternatively, if the medicine is profitable at the ceiling price arising under the third approach (P10 in Figure 7D), but is not profitable at the ceiling price arising under under the second approach (P9 in Figure 7C), then maximizing population health requires specifying a ceiling price somewhere between P9 and P10, such that consumer surplus is maximized subject to producer surplus being non-negative.
Finally, if the supply curve is understood to be sufficiently low that the medicine would be profitable at the ceiling price arising under the second approach (P9 in Figure 7C), then maximizing population health requires setting a ceiling price below P9, so as to maximize consumer surplus subject to producer surplus being non-negative. However, since the true location of the supply curve is uncertain, any reduction in the ceiling price carries a risk that producer surplus might become negative, such that the medicine would not launch at all. In such circumstances, consumer surplus would be zero, whereas at a higher ceiling price of P9 the new medicine would have launched and consumer surplus would have been positive.
A1.4 Pricing across indications
Where a medicine is available for multiple indications, this has implications for specification of the demand curve for a new medicine.
If the per-patient health gain from the new medicine is different in each indication, then the
ceiling price at which the ICER is equal to k will also differ across indications.
It follows that the demand curve will generally be different for each indication, with a relatively higher ceiling price corresponding to an ICER of k for those indications in which the medicine has a relatively greater per-patient health gain.
A1.4.1 Approaches for setting a single ceiling price across indications
Although the Working Group agreed that a different ceiling price should be specified for each indication in principle, concerns were raised about the feasibility of doing this in Canada at the present time. The Working Group therefore considered various approaches for setting a single ceiling price across multiple indications, including:
- A ceiling price at which the medicine is ‘just’ cost-effective in the most cost-effective indication (such that the ICER equals k in this indication);
- A ceiling price at which the medicine is ‘just’ cost-effective in the least cost-effective indication;
- A ceiling price at which the medicine is ‘just’ cost-effective across all indications (such that a ‘weighted average’ of the ICER across all indications equals k );
- A ceiling price at which the medicine is ‘just’ cost-effective in the first indication considered by the PMPRB (such that the ICER equals k in this indication).
Figure 9A
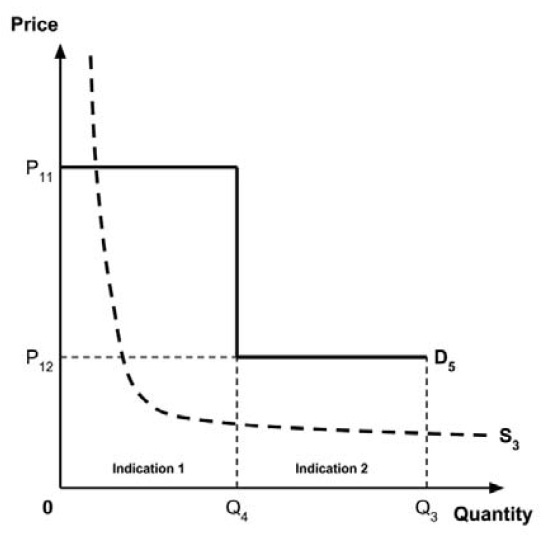
Figure 9A: The demand curve for a medicine across two indications
Figure 9B
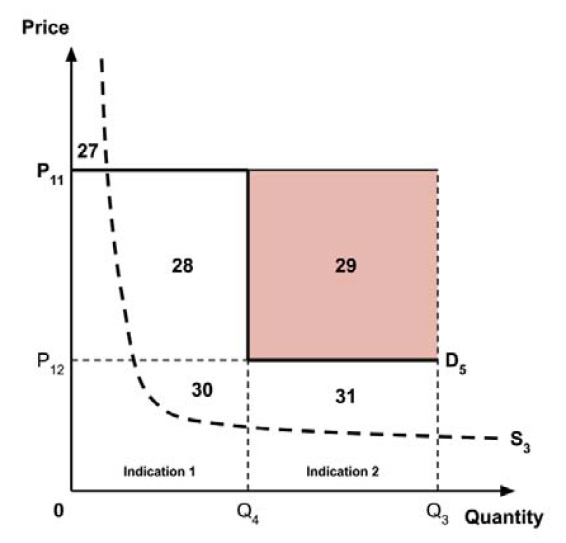
Figure 9B: Under the first approach (ceiling price P11), consumer surplus is negative in ‘Indication 2’ and zero in ‘Indication 1’, so negative overall
Figure 9C
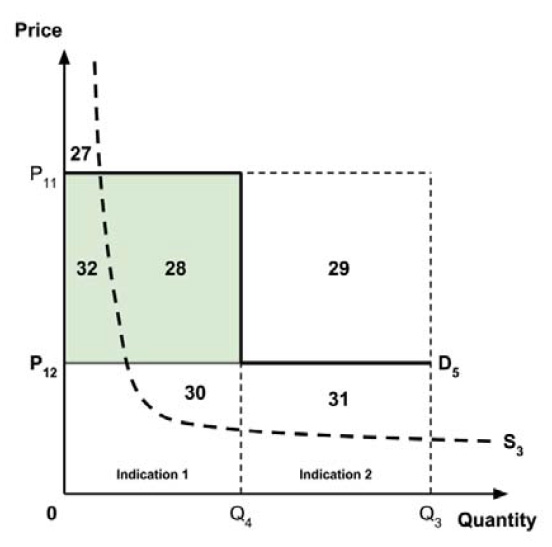
Figure 9C: Under the second approach (ceiling price P12), consumer surplus is positive in ‘Indication 1’ and zero in ‘Indication 2’, so positive overall
Figure 9D
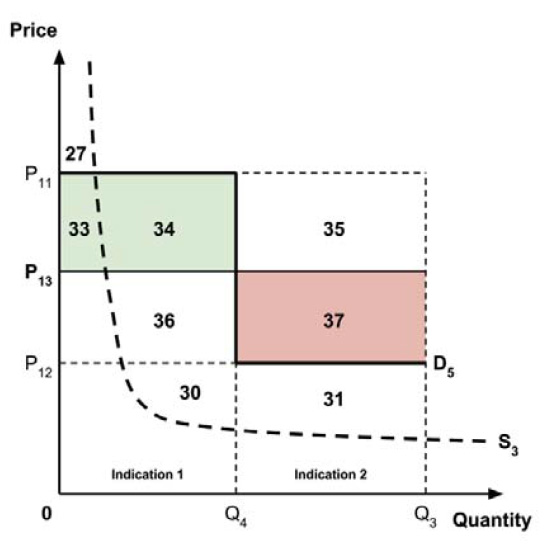
Figure 9D: Under the third approach (ceiling price P13), consumer surplus is positive in ‘Indication 1’, negative in ‘Indication 2’, and zero overall
Figures 9A to 9D demonstrate the implications of each of these approaches using a simplified model of a new medicine provided to patients across two indications. In this model, the medicine is relatively more effective for patients in ‘Indication 1’, resulting in a larger health gain.
Given this effectiveness, the ceiling price at which the ICER equals k for patients in ‘Indication 1’ is P11. The quantity of medicine demanded by patients in ‘Indication 1’ is Q4. The medicine is relatively less effective for patients in ‘Indication 2’, such that the ICER equals k at a lower ceiling price of P12. The quantity of medicine demanded by patients in ‘Indication 2’ is Q3 - Q4.
In the analysis below, it is assumed that the medicine is always launched in both indications (i.e. the manufacturer does not strategically limit launch of the medicine to only one indication). The possible implications of such strategic behaviour are considered later in this section.
Approach 1: Set a ceiling price based on the most cost-effective indication
Under the first approach considered by the Working Group, the ceiling price of the new medicine would be set at P11 across both indications (Figure 9B).
This would result in no consumer surplus in ‘Indication 1’ (since the ceiling price corresponds exactly to the demand curve), but negative consumer surplus in ‘Indication 2’ (as illustrated by the area of region 29) since the ceiling price lies above the demand curve. It follows that the total consumer surplus across both indications would be negative, such that population health is diminished.
At this ceiling price, the producer surplus is illustrated by the combined area of regions 28 to 31, minus the area of region 27.
Approach 2: Set a ceiling price based on the least cost-effective indication
Under the second approach, the ceiling price would be set at P12 in both indications (Figure 9C).
This would result in a positive consumer surplus in ‘Indication 1’ (regions 28 and 32), and no consumer surplus in ‘Indication 2’ (since the ceiling price corresponds exactly to the demand curve), such that the total consumer surplus is positive.
The producer surplus would be lower than under the first approach, as illustrated by the combined area of regions 30 and 31, minus the combined area of regions 27 and 32.
Approach 3: Set a ceiling price based on a ‘weighted average’ of all indications
Under the third approach, the ceiling price would be set between P11 and P12 such that the total consumer surplus (across both indications) is zero. In this example, this requires setting a ceiling price of P13 (Figure 9D).
At this ceiling price, the positive consumer surplus in ‘Indication 1’ (combined area of regions 33 and 34) is exactly offset by the negative consumer surplus in ‘Indication 2’ (area of region 37).
The producer surplus is lower than under the first approach but greater than under the second approach (as illustrated by the combined area of regions 30, 31, 36 and 37, minus the combined area of regions 27 and 33).
Approach 4: Set a ceiling price based on the first indication considered
Under the fourth approach, the ceiling price would be set at either P11 or P12, depending upon which indication is first considered by the PMPRB.
This approach is the simplest to administer, since it does not require rebenching of ceiling prices in future if and when additional indications are launched.
However, because producer surplus is unambiguously greater at a ceiling price of P11 than P12, this approach provides an incentive for the manufacturer to launch in the most cost-effective indication first (in this case ‘Indication 1’) to secure a higher ceiling price for future indications.
(If the manufacturer instead launches in ‘Indication 2’ first, then the loss in producer surplus is illustrated by the combined area of regions 28, 29 and 32 on Figure 9C.)
If manufacturers act upon this incentive and prioritize launch of the most cost-effective indication first, then overall consumer surplus will be zero within the initial indication and become negative once additional indications are launched. If manufacturers are perfectly strategic, then this approach would have the same implications for consumer surplus as Approach 1.
If manufacturers do not act upon this incentive, then in some cases consumer surplus from additional indications will be positive (if a less cost-effective indication is launched first) and in other cases consumer surplus from additional indications will be negative (if a more
cost-effective indication is launched first). If the decision as to which indication to launch first is truly random, then a reasonable expectation would be that the expected consumer surplus associated with additional indications is zero, since it is equally likely to be positive or negative. This would have equivalent implications for consumer surplus as Approach 3.
It follows that this approach may be considered as lying somewhere between Approach 1 and Approach 3, with expected consumer surplus ranging between negative (if manufacturers are in any way strategic) to zero (if manufacturers are not strategic at all).
A1.4.2 Similarities to pricing across multiple provinces and territories
There are several similarities between the Working Group’s considerations regarding pricing across multiple indications and those regarding pricing across multiple provinces and territories.
In both cases, the demand curve for a medicine differs across subsets of patients who receive a medicine, whether on the basis of province or territory or on the basis of disease indication. In both cases, the Working Group considered pricing according to the highest or lowest of these demand curves, or pricing according to a ‘weighted average’ approach. And in both cases, these various approaches resulted in very different implications for the allocation of consumer and producer surplus (with one approach resulting in negative consumer surplus, another resulting in positive consumer surplus, and a third approach resulting in zero consumer surplus).
There are, however, some distinctions. First, the reason why demand curves differ across
provinces or territories (because k varies for a given ΔH ) is different from why demand curves
differ across indications (because ΔH varies for a given k ). Second, the manufacturer may
have an opportunity to behave strategically regarding the order in which indications are launched, or may choose not to launch in a specific indication at all, in order to maximize profits.
A1.4.3 Potential for strategic behaviour by manufacturers
Since manufacturers may choose not to launch in one or more indications, any approach for setting ceiling prices across indications can potentially induce strategic behaviour by manufacturers, with implications for the allocation of consumer and producer surplus.
In the earlier analysis of each of the four approaches for pricing across indications, it was assumed that the new medicine would always be launched in both indications. Under the second approach, launch of the medicine in ‘Indication 2’ resulted in a lower ceiling price in ‘Indication 1’, in turn leading to positive consumer surplus. However, if this approach were to be adopted in practice, manufacturers might strategically choose not to launch in ‘Indication 2’, resulting in a higher ceiling price for ‘Indication 1’, in turn leading to zero consumer surplus.
The reasons for this can be seen by considering Figure 10, which is adapted from Figure 9C.
If the manufacturer launches in both indications then the ceiling price is P12 (based on the demand curve for ‘Indication 2’, the least cost-effective indication). The producer surplus is then the combined area of regions 30 and 31 minus the combined area of regions 27 and 32.
Figure 10
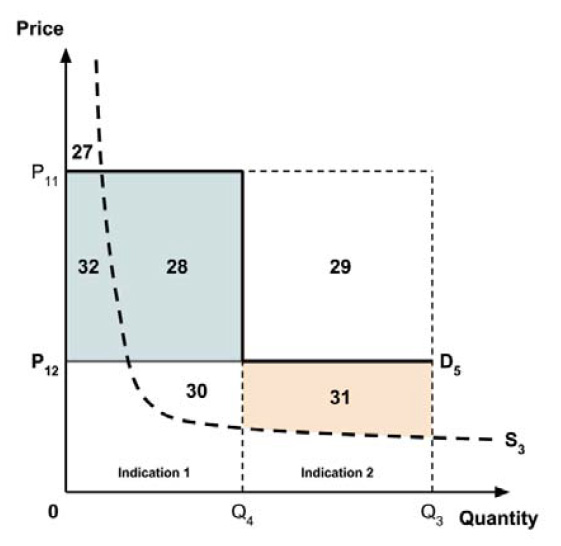
Figure 10: Under Approach 2, the manufacturer may strategically choose not to launch in Indication 2, thereby increasing producer surplus by the area of regions 28 and 32 minus region 31
However, if the manufacturer instead launches only in ‘Indication 1’, then this would now be the least cost-effective indication and so the ceiling price would be P11. The producer surplus would now be the combined area of regions 28 and 30 minus the area of region 27.
It follows that, by avoiding launching in ‘Indication 2’, the manufacturer forgoes the producer surplus in region 31 but gains additional producer surplus in regions 28 and 32. In the example given in Figure 10, this gain in producer surplus outweighs the loss. A manufacturer wishing to maximize producer surplus would therefore strategically launch in ‘Indication 1’ only.
This strategic behaviour has several implications. First, it increases the producer surplus. Second, it reduces the consumer surplus (in this case to zero, although in an example with many indications the consumer surplus may be positive if the medicine still launches in two or more indications). Third, it results in a ‘deadweight loss’, represented by the area of region 31.
This deadweight loss arises because there is a demand for the medicine for patients in ‘Indication 2’, with a willingness-to-pay of P12, and the manufacturer is willing to supply to these patients at a ceiling price lower than this. It follows that there is an economic surplus to be realized by providing the medicine to patients in ‘Indication 2’. However, the manufacturer is unwilling to supply to these patients because, by doing so, the total surplus allocated to the manufacturer falls (since the ceiling price would fall from P11 to P12 in both indications). The potential economic surplus in region 31 is therefore not realized.
There are several potential ways to address this issue, each with positives and negatives. Applying a different ceiling price to each indication, such that the ceiling price for ‘Indication 1’ is independent of the ceiling price for ‘Indication 2’, would remove the incentive not to supply to ‘Indication 2’. However, as noted earlier, members of the Working Group expressed concerns about the feasibility of implementing indication-specific pricing at the present time. Also, unless a ceiling price below the respective demand curve was applied in each indication, overall consumer surplus would be zero (analogous to that in a standard model of a monopoly with perfect price discrimination).
The policy maker might also consider applying a ceiling price higher than P12 if the medicine is launched in both indications. In order for this to result in positive consumer surplus overall, this ceiling price would have to be lower than P13 in Figure 9D (the ceiling price that arises under the third approach, in which consumer surplus is zero). Although a ceiling price between P12 and P13 would result in negative consumer surplus in ‘Indication 2’, there would still be positive consumer surplus overall, which might be considered preferable to the situation where the medicine is launched only in ‘Indication 1’ and consumer surplus is zero.
Another potential solution would be to ‘penalize’ the manufacturer for choosing not to launch in ‘Indication 2’ by setting a ceiling price below P11 if the medicine is launched only in ‘Indication 1’. This might result in producer surplus being maximized by launching in both indications, incentivising the manufacturer to also launch in ‘Indication 2’. However, if the overall producer surplus becomes negative as a result of this lower ceiling price, the manufacturer might choose not to launch the medicine in any indication, resulting in zero consumer surplus.
Regardless of the approach taken, if the policy maker attempts to mitigate this strategic behaviour by raising or lowering the ceiling price then a key challenge is determining how much higher or lower the ceiling price should be. Since the supply curve is uncertain in practice, it is difficult to provide guidance on how much to raise or lower the ceiling price in any given case.
A1.4.4 Policy implications
In common with the considerations made earlier regarding the setting of a single ceiling price across provinces and territories, the most desirable approach for setting a single ceiling price across indications depends upon the policy intent.
Note that it is not the role of the Working Group to specify the policy intent. While the implications of some potential policy objectives are considered below, this should not be construed as an endorsement by the Working Group of any particular policy objective. Also note that this analysis is not exhaustive: there are other potential policy objectives and approaches for setting a ceiling price across indications.
Potential policy objective 1
If the policy maker desires that new medicines do not diminish population health across Canada as a whole, such that overall consumer surplus is at least zero, then the first approach considered above is inconsistent with this policy objective. This is because this approach results in diminished population health (negative consumer surplus) in all indications except that which is the most cost-effective (in which consumer surplus is zero), resulting in diminished population health (negative consumer surplus) overall.
The second approach comfortably satisfies this policy objective (since it results is positive overall consumer surplus), while the third approach only just satisfies this policy objective (since it results in an overall consumer surplus of zero). The fourth approach might satisfy this policy objective if manufacturers are not strategic, but if manufacturers behave strategically then the expectation would be that consumer surplus is negative overall, in which case this approach would not satisfy this objective.
It follows that the ceiling price that arises under the third approach (P13 in Figure 9D) is the maximum ceiling price that would be consistent with this policy objective. At this ceiling price, overall consumer surplus is zero, analogous to the consumer surplus arising in a standard model of a monopoly with perfect price discrimination.
Potential policy objective 2
If the policy maker instead desires that new medicines do not diminish population health within any specific indication, then both the first and third approaches are inconsistent with this policy objective. This is because both approaches result in diminished population health (negative consumer surplus) in at least one indication. Unless manufacturers consistently launch in the least-effective indication first, the fourth approach is also inconsistent with this objective.
The second approach would only just satisfy this policy objective, since consumer surplus is zero in the least cost-effective indication.
It follows that the ceiling price that arises under the second approach (P12 in Figure 9C) is the maximum ceiling price that is consistent with this policy objective.
Potential policy objective 3
If the policy maker wishes to set ceiling prices for new medicines so as to maximize population health across Canada as a whole, then (in common with the earlier consideration of this policy objective when pricing across provinces and territories) consideration should be given to the location of the supply curve.
As before, a key assumption is that a medicine will not be launched if producer surplus is negative. If a medicine is not launched, the pharmacoeconomic value is zero since there is no resulting net gain in QALYs. For the pharmacoeconomic value to be positive, the medicine must be launched at a ceiling price that results in positive consumer surplus.
Also as before, the most desirable ceiling price under this policy objective is the lowest ceiling price at which producer surplus is non-zero. Depending upon the location of the supply curve, this might be at a ceiling price below P12 in Figure 9D, leading to greater consumer surplus than that resulting from any of the four approaches considered above. However, as before, lowering the ceiling price to extract additional consumer surplus carries a risk that producer surplus may become negative, such that the medicine is not launched and consumer surplus is zero.
The highest ceiling price that should be considered under this objective is that which arises under the third approach, P13 in Figure 9D, since consumer surplus is zero at this ceiling price (analogous to a standard model of a monopoly with perfect price discrimination).
A1.5 Uncertainty
This framework has so far assumed that ΔH , ΔC and k are known with certainty, such that a demand curve can be plotted at a fixed ceiling price within each province/territory and indication.
In practice, the estimates of ΔH and ΔC arising from probabilistic analyses conducted by CADTH and INESSS are uncertain, and hence the ICER of the new medicine is uncertain. Furthermore, since k is subject to empirical estimation, this will also be uncertain.
A1.5.1 Implications for the demand curve
Since both the ICER and k are uncertain, the ceiling price at which the ICER is equal to k , and
hence the location of the demand curve, is also uncertain.
Nevertheless, since CADTH now mandates the use of probabilistic analysis, the analysis output may be used to assign probability distributions to ΔH and ΔC . Similarly, empirical work should allow for a probability distribution to be assigned to k(see, for example, Claxton et al. 2015).10 It follows that it should be possible to assign a probability distribution to the demand curve.
Figure 11 reproduces the demand curve from Figure 1 with a 95% credible interval. In this
example, given uncertainty in ΔH , ΔC and k , the net health benefit (consumer surplus) of the
medicine is expected to be zero at a ceiling price of P1 (illustrated by the ‘mean’ demand curve). Given this uncertainty, there is a 95% probability that the net health benefit is actually zero at a ceiling price between P14 and P15 (illustrated by the ‘U 95%’ and ‘L 95%’ demand curves).
Figure 11
Figure 11: Demand curve subject to a 95% credible interval
A1.5.2 Expected loss in economic surplus
Since the ceiling price at which the net health benefit (consumer surplus) of the medicine is actually zero is uncertain, there is a possibility that the ceiling price at which a new medicine is expected to provide zero consumer surplus (P1 in Figure 11) will actually result in positive consumer surplus, and similarly there is a possibility that this ceiling price will actually result in negative consumer surplus. There is also a possibility that this ceiling price will actually result in zero consumer surplus, which would arise if P1 lies below the supply curve (such that the medicine is not launched).
Consider Figure 12A. In this example, the actual ceiling price at which net health benefit (consumer surplus) is zero is P16. This is the ceiling price at which the demand curve would be plotted if ΔH , ΔC and k were known with certainty. Since these parameters are uncertain, the true location of this actual demand curve is unknown (and is plotted with a dashed line). Instead, we have an estimate of the expected ceiling price at which net health benefit is zero (P1), and also an estimate of the 95% credible interval (between P14 and P15).
Suppose the PMPRB specifies a ceiling price of P1, based on the expected (mean) demand curve. Because P1 is lower than the (unknown) actual demand curve, but above the (unknown) supply curve at quantity Q1, it follows that a ceiling price of P will result in a positive consumer surplus (illustrated by the combined area of regions 34, 35 and 36). Producer surplus will also be positive (illustrated by the combined area of regions 37 and 38, minus the combined area of regions 33 and 34), but lower than it would have been if the ceiling price were set according to the actual demand curve (with the reduction in producer surplus equal to the gain in consumer surplus). Critically, because producer surplus is positive at P1, the medicine is still launched. It follows that, in this example, uncertainty has resulted in a positive consumer surplus.
Now consider Figure 12B. In this example, the actual demand curve (P17) lies below the expected (mean) demand curve (P1). It follows that, if the medicine is adopted at a ceiling price P1, then consumer surplus will be negative (illustrated by the combined area of regions 40, 41 and 42), since a higher ceiling price is paid than that at which consumer surplus is zero.
Producer surplus is greater than it would have been in the absence of uncertainty (illustrated by the combined area of regions 41 to 44, minus the area of region 39), with this gain in producer surplus equal to the reduction in consumer surplus (illustrated by the combined area of regions 40, 41 and 42).
This brings us to a key result. Provided that the medicine is launched at a ceiling price coinciding with the expected demand curve (a crucial requirement considered further below), the expected consumer surplus is zero (analogous with a model of a monopoly with perfect price discrimination). The actual consumer surplus may be positive (as in Figure 12A) or negative (as in Figure 12B), but the expected consumer surplus is zero.
Figure 12A
Figure 12A: Example where the actual demand curve (P16) lies above the expected demand curve (P1) and the medicine is launched
Figure 12B
Figure 12B: Example where the actual demand curve (P17) lies below the expected demand curve (P1) and the medicine is launched
Figure 12C
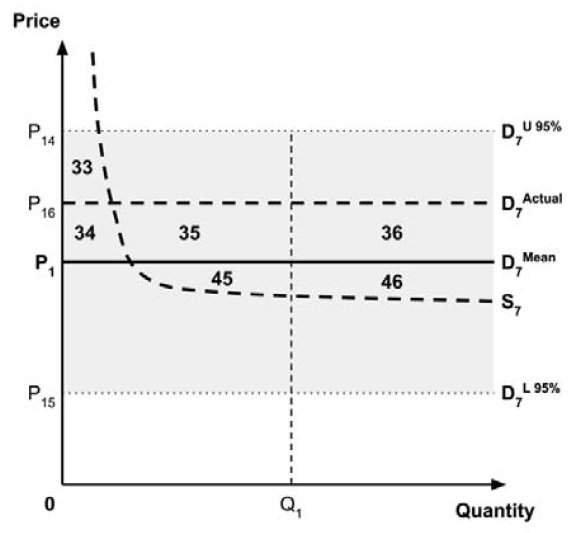
Figure 12C: Example where the actual demand curve (P16) lies above the expected demand curve (P1) and the medicine is not launched
Figure 12D
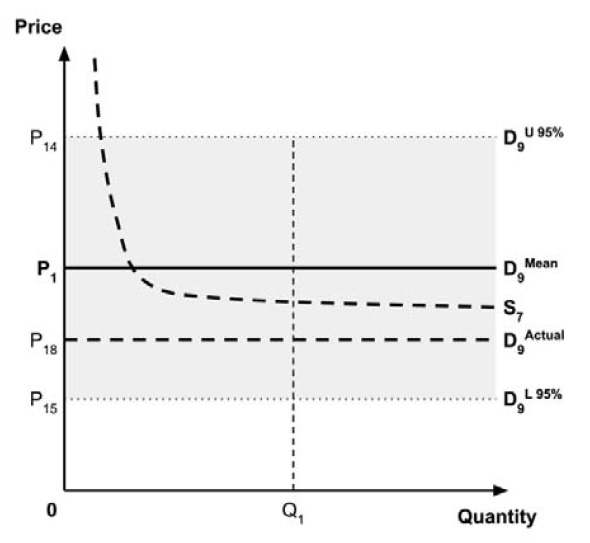
Figure 12D: Example where the actual demand curve (P18) lies below the expected demand curve (P1) and the medicine is not launched
However, this result does not hold if the medicine is not launched as a result of uncertainty.
Consider Figure 12C. In this example, the actual and expected demand curves are identical to those in Figure 12A, but the supply curve is now higher (S7). If the ceiling price coinciding with the actual demand curve (P16) were known in practice and offered to the manufacturer, then the medicine would be launched since the producer surplus would be positive (illustrated by the combined area of regions 35, 36, 45 and 46, minus the area of region 33). However, this is not possible because the actual demand curve is unknown. If the manufacturer is instead offered the ceiling price coinciding with the expected demand curve (P1), then the manufacturer will choose not to launch the medicine, because the producer surplus would now be negative (illustrated by the combined area of regions 45 and 46, minus the combined area of regions 33 and 34). Since the producer surplus would be negative, and so the medicine is not launched, it follows that both the consumer surplus and producer surplus are zero. Compared to Figure 12A, in which both consumer and producer surplus were positive since the medicine still launched, in this example the uncertainty results in a loss of economic surplus (with the total loss illustrated by the combined area of regions 35, 36, 45 and 46, minus the area of region 33).
Finally, consider Figure 12D. The expected demand curve and supply curve are identical to those in Figure 12C, so again the medicine is not launched because it would have negative producer surplus. However, in this example the actual demand curve (P18) is lower than the expected demand curve (P1). As a result, the medicine would not have launched anyway in the absence of uncertainty, such that the uncertainty does not result in a loss in economic surplus (since there would have been none anyway).
To summarize the results from the examples above:
- If the medicine is launched at a ceiling price coinciding with the expected demand curve then the expected consumer and producer surplus is zero.
- If the medicine is unprofitable at a ceiling price coinciding with the expected demand curve, and is also unprofitable at a ceiling price coinciding with the actual demand curve, then the consumer surplus is zero.
- If the medicine is unprofitable at a ceiling price coinciding with the expected demand curve, but would have been profitable at a ceiling price coinciding with the actual demand curve, then the impact of uncertainty is to diminish the total economic surplus such that expected consumer surplus at a ceiling price coinciding with the expected demand curve is negative.
It follows from this third result that uncertainty is associated with an expected loss in consumer surplus, such that reducing uncertainty results in an expected gain in consumer surplus.
A1.6 Market size
The PMPRB has proposed that a ‘market size adjustment’ may be applied to the ceiling price for some Category 1 medicines. This includes a potential upwards ceiling price adjustment for medicines with small market size and, independently, a potential downwards ceiling price adjustment for medicines with large market size.
The first of these would have the effect of increasing the producer surplus (at the expense of consumer surplus) for medicines with small market size. The second would increase the consumer surplus (at the expense of producer surplus) for medicines with large market size.
Consider Figure 13A, which reproduces the demand and supply curves for a hypothetical new medicine from Figure 4A.
For simplicity, it is assumed that the medicine has a single indication and there are no
differences in k across provinces and territories, such that there is a single horizontal demand
curve (D1) at a ceiling price of P1. It is also assumed that the ceiling price of the medicine is P1, such that consumer surplus is zero (in the absence of a market size adjustment).
Figure 13A
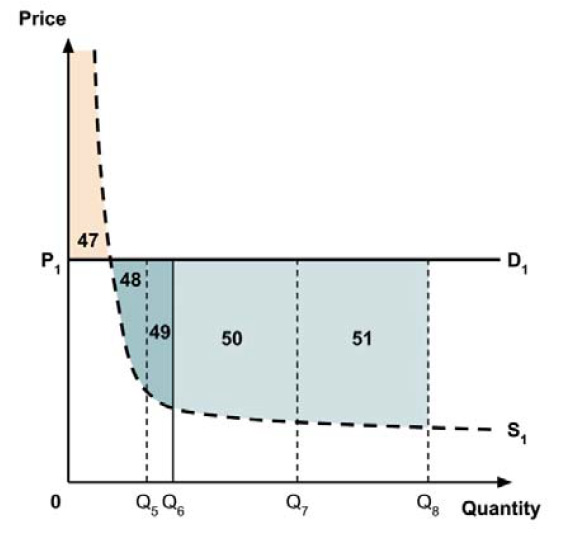
Figure 13A: Without any ‘market size adjustment’
Figure 13B
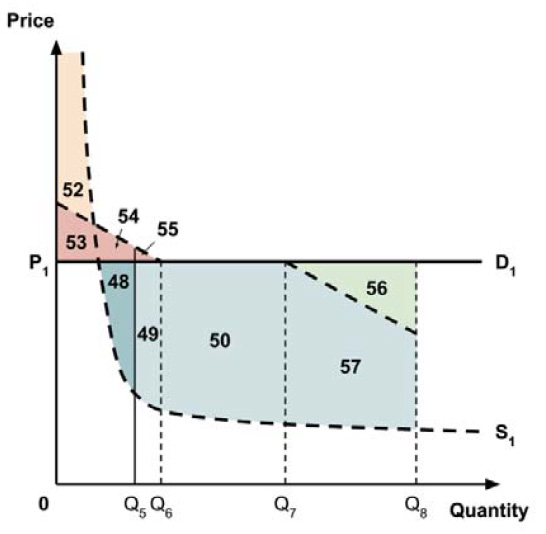
Figure 13B: With a hypothetical ‘market size adjustment’
If this medicine has very small market size (quantity Q5), then it will have negative producer surplus (as illustrated by the area of region 48 minus the area of region 47), such that it would not be profitable to launch. If the medicine has slightly larger market size (Q6), then the producer surplus increases (by the area of region 49) but is now zero, such that the manufacturer is ambivalent about launching the medicine. With even larger market size (Q7), the producer surplus increases further (by the area of region 50), such that the medicine is now profitable.
And with the largest market size (Q8), the medicine has an even greater producer surplus (as illustrated by the combined area of regions 48, 49, 50 and 51, minus the area of region 47).
Note that Q6 is the minimum market size at which the medicine is profitable. A smaller market size results in negative producer surplus, while a larger market size results in increasingly positive producer surplus.
A1.6.1 Implications of a market size adjustment
Now consider Figure 13B, which illustrates a hypothetical ‘market size adjustment’. Following this market size adjustment, medicines with market size below Q6 receive a higher ceiling price, while medicines with market size above Q7 receive a lower ceiling price.
In order to allow for comparisons between medicines with small and large market size, it will now be assumed that there are many new medicines, each with identical demand and supply curves as plotted in Figure 13B, with these medicines differing in terms of their market size.
This hypothetical market size adjustment has a number of implications.
Implication 1: Increased consumer surplus from medicines with large market size
The reduction in the ceiling price for medicines with large market size results in an increase in consumer surplus (as illustrated by the area of region 56 for a medicine with market size Q8).
Producer surplus for medicines with large market size is reduced by an equivalent amount, but remains positive because it was sufficiently large prior to the reduction in ceiling price.
Since the market size adjustment did not cause the demand curve to cross the supply curve, the producer surplus for a medicine with market size Q8 remains larger than the producer surplus at any smaller market size (as illustrated by the combined area of regions 48, 49, 50 and 57, minus the combined area of regions 52 and 53).
Implication 2: Reduced consumer surplus from medicines with small market size
A higher ceiling price for medicines with small market size results in greater producer surplus (as illustrated by the combined area of regions 53, 54 and 55), but a correspondingly lower consumer surplus.
Since (in this example) consumer surplus was zero prior to the market size adjustment, it follows that consumer surplus is now negative for medicines with small market size.
Implication 3: Increased profitability for medicines with small market size
For a medicine with a market size of Q5, the producer surplus following the market size adjustment is zero (as illustrated by the area of regions 48, 53 and 54, minus the area of region 52), where previously it was negative.
For a medicine with a market size of Q6, the producer surplus is now positive (as illustrated by the combined area of regions 48, 49, 53, 54 and 55, minus the area of region 52), where previously it was zero.
It follows that the minimum market size at which a medicine is profitable has fallen from Q6 (prior to the market size adjustment) to Q5. Medicines with a market size between Q5 and Q6, which were unprofitable prior to the market size adjustment, now have positive producer surplus. This might, in turn, result in greater access to medicines with small market size.
Appendix 2: Materials Presented at Meetings of the Working Group
Appendix 2.1: Slides from 26 July 2018 (Dr Mike Paulden)
Working Group to Inform the Patented Medicine Prices Review Board (PMPRB) Steering Committee on Modernization of Price Review Process Guidelines
Alt Hotel, Ottawa, ON
26 July 2018
Chair: Dr Mike Paulden
Background
The Patented Medicine Prices Review Board (PMPRB) recently established a ‘Steering Committee on Modernization of Price Review Process Guidelines’.
The mandate of this Steering Committee is to assist the PMPRB in synthesizing stakeholder views on key technical and operational modalities of the PMPRB’s new draft Guidelines.
The Steering Committee’s work will be based in part on the analysis and recommendations of a technical Working Group, which will examine certain issues that the Steering Committee believes would benefit from the review of experts in health technology assessment and other economic and scientific matters.
The Working Group will comprise leading experts in pharmacoeconomics and the clinical evaluation of pharmaceuticals.
The Working Group will meet four times between July and October 2018: twice in-person in Ottawa, and twice via video-conference.
A report of the Working Group’s deliberations and recommendations will be produced by the chair and submitted to the Steering Committee for consideration in October 2018.
Members
- Dr Chris Cameron (Dalhousie University and Cornerstone Research Group);
- Dr Tammy Clifford (University of Ottawa and CADTH);
- Dr Doug Coyle (University of Ottawa);
- Patrick Duford (INESSS);
- Don Husereau (Institute of Health Economics);
- Dr Peter Jamieson (University of Calgary);
- Dr Frédérick Lavoie (Pfizer Canada);
- Dr Karen Lee (University of Ottawa and CADTH);
- Dr Christopher McCabe (University of Alberta and Institute of Health Economics);
- Dr Stuart Peacock (Simon Fraser University and BC Cancer Agency);
- Maureen Smith (Patient – Health Quality Ontario);
- Geoff Sprang (Agmen);
- Dr Tania Stafinski (University of Alberta).
Observers and reviewers
Observers
- Edward Burrows (Innovation, Science and Economic Development);
- Nelson Millar (Health Canada).
External reviewer
- Dr Mark Sculpher (University of York).
Confidentiality
Working Group members may consult with non-members on an ongoing basis but are expected to maintain the confidentiality of any materials provided to them during the course of their work.
The names of the members of the Working Group will be published on the PMPRB’s website, along with a report of its deliberations, analysis and recommendations.
Governance and procedure
It is recognized that members of the Working Group may hold opposing points of view on the above issues and/or disagree with the policy rationale underlying the changes to the PMPRB’s Guidelines.
Members are nonetheless encouraged to work together constructively to assist the Working Group in carrying out its function.
The chair is expected to foster consensus among members, but in order to ensure that Working Group deliberations are as focused and productive as possible, the chair shall have final say on all matters of governance and procedure.
Members who disagree with a decision of the chair in this regard can request that their objection be noted on the record.
The chair shall make every effort to ensure that the Working Group’s final report accurately reflects any important points of convergence or contention between members.
Schedule
| Date |
Event |
Purpose |
| 26 July 2018 |
Full day in-person meeting in Ottawa |
Overview of Working Group objectives. Summary of specific areas of focus under consideration. Allocation of tasks among Working Group members. |
| Week of 20 August 2018 (TBC) |
Two hour video-conference |
Update on Working Group status. Opportunity for input from Working Group members. |
| Week of 10 September 2018 (TBC) |
Two hour video-conference |
Update on Working Group status. Opportunity for input from Working Group members. |
| 5 October 2018 |
Draft report submitted to PMPRB |
Opportunity for input from PMPRB and Working Group members. |
| 12 October 2018 |
Full day in-person meeting in Ottawa |
Present draft report. Report draft recommendations.
Final opportunity for input from PMPRB and Working Group members.
|
| 26 October 2018 |
Final report delivered to PMPRB |
Final deliverable to PMPRB. |
Deliverables
A draft report will be circulated to the Steering Committee and Working Group members on 5 October 2018, prior to the final in-person meeting in Ottawa.
Following delivery of the final report, the chair will be willing to present the recommendations of the Working Group to stakeholders and other interested parties, subject to availability.
Areas of focus
- Options for determining what drugs fall into ‘Category 1’
- Application of supply-side cost effectiveness thresholds in setting ceiling prices for Category 1 drugs
- Drugs with multiple indications
- Options for using the CADTH and/or INESS reference case analyses to set a ceiling price
- Perspectives
- Application of the market size factor in setting ceiling prices
1. Options for determining what drugs fall into ‘Category 1’
A Category 1 drug is one for which a preliminary review of the available clinical, pharmacoeconomic, market impact, treatment cost and other relevant data would suggest is at elevated risk of excessive pricing.
The following criteria have been identified as supporting a Category 1 classification:
- The drug is ‘first in class’ or a ‘substantial’ improvement over existing options
- The drug’s opportunity cost exceeds its expected health gain
- The drug is expected to have a high market impact
- The drug has a high average annual treatment cost
Should other criteria be considered?
What are the relevant metrics for selecting drugs that meet the identified criteria and what options exist for using these metrics?
2. Application of supply-side cost effectiveness thresholds in setting ceiling prices for Category 1 drugs
Potential approaches for implementing a price ceiling based
on a drug’s opportunity cost.
Potential approaches for allowing price ceilings above opportunity cost based on a higher willingness to pay for certain types of drugs (e.g. pediatric, rare, oncology, etc)
What are the potential approaches for considering a drug’s opportunity cost and implementing a price ceiling?
Should higher price ceiling(s) be adopted for certain types of drugs? If so, which drugs? How should the higher price ceiling(s) be determined?
3. Drugs with multiple indications
Options for addressing drugs with multiple indications (e.g. multiple price ceilings or a single ceiling reflecting one particular indication).
What are the available options regarding pricing for multiple indications?
Which option should be recommended, and why?
4. Accounting for uncertainty
Options for using the CADTH and/or INESS reference case analyses to set a ceiling price.
Options for accounting for and/or addressing uncertainty in the point estimate for each value-based price ceiling.
Do existing ‘reference case’ analyses provide the most appropriate estimates from which to derive a ceiling price?
If not, what modifications from the ‘reference case’ assumptions are desirable?
How should uncertainty be accounted for, or addressed, when setting price ceilings?
5. Perspectives
Options to account for the consideration of a public health care system vs societal perspective, including the option of applying a higher value-based price ceiling in cases where there is a ‘significant’ difference between price ceilings under each perspective.
How to define a ‘significant’ difference in price ceilings between each perspective.
What are the key differences between a public health care system vs societal perspective?
What are the options to account for these differences?
How should a ‘significant’ difference be defined?
6. Application of the market size factor in setting ceiling prices
Approaches to derive an appropriate affordability adjustment to a drug’s ceiling price based on an application of the market size and GDP factors (e.g. based on the US ‘ICER’ approach).
What approaches are available to consider an ‘affordability adjustment’ to a drug’s ceiling price?
Should other factors be considered (in addition to market size and GDP)?
How should each of these factors be considered?
Appendix 2.2: Note on Uncertainty (Dr Christopher McCabe)
Note on Uncertainty (Dr Christopher McCabe) (PDF version - 125 kb)
Appendix 2.3: Slides from 12 October 2018 (Dr Mike Paulden)
Slides from 12 October 2018 (Dr Mike Paulden) (PDF version - 1 MB)
Appendix 2.4: Slides from 12 October 2018 (Dr Christopher McCabe)
Slides from 12 October 2018 (Dr Christopher McCabe) (PDF version - 431 kb)
Appendix 2.5: Slides from 5 February 2019 (Dr Mike Paulden)
Slides from 5 February 2019 (Dr Mike Paulden) (PDF version - 622 kb)
Appendix 3: ‘On The Record’ Comments
Appendix 3.1: Email from Frédéric Lavoie and Geoff Sprang (1/4)
Subject: Working Group meeting of July 26, 2018 Date: 9 August 2018 at 15:21 MST
From: Frédéric Lavoie
To: Mike Paulden
Cc: Geoff Sprang
Dear Mike,
On behalf of BIOTECanada and Innovative Medicines Canada, we would like to thank you for chairing the first face to face meeting of the PMPRB Working Group held on July 26, 2018. Although the industry associations we represent do not support the use of economic factors such as cost-effectiveness analyses as part of the proposed amendments to the Patented Medicines Regulations, and are also concerned about the initiation of Guidelines consultations before the finalization of regulatory changes, we felt that you were open to our points of view and invited us with the upmost respect to contribute throughout the meeting.
As the Working Group terms of reference stipulate that points of contention will be recorded by the Chair and reflected in the Working Group final report, we felt it would be appropriate to summarize our perspectives in writing and to provide you with our views on the discussions during the meeting.
Observations on the discussions:
We perceived during the meeting with the academic experts and other stakeholders represented at the Working Group that consensus cannot be achieved for the implementation of economic factors for the purpose of setting price ceilings for patented medicines in Canada. The debates that we observed around the table reinforced the apprehension our industry has communicated regarding the use of pharmacoeconomic factors, and made it clear that it is imperative for the Working Group to communicate to the Steering Committee and to the Federal Government the challenges presented by the proposed use of these factors, so that the scope of discussions with our industry and other stakeholders can be extended to include the consideration of alternative regulatory approaches as quickly as possible.
Determination of a willingness to pay threshold and the use of pharmacoeconomic factors at the PMPRB level:
When it comes to economic factors such as pharmacoeconomics, the proposed utilization of a threshold and of a cost-utility point estimate in the process involving Category One products (as first disclosed by the PMPRB to stakeholders on June 25th, 2018) would produce tremendous uncertainty and is therefore unacceptable.
The different schools of thought and academic debates around the establishment of a willingness to pay threshold through supply side or demand side methods are diverse and evolving. Even if an academic consensus were achievable, the implantation of a single method would always lead to a point estimate around which a distribution of possible results would reflect the high degree of uncertainty that exists regarding the establishment of a willingness to pay threshold and its variability across the diversity of Canadian perspectives it needs to reflect. Citing the work of Neumann et al. on this topic reflects this point: ”Searching for a single benchmark is at best a quixotic exercise because there is no threshold that is appropriate in all decision contexts.” (N ENGL J MED 371;9, August 28, 2014).
The same issue arises from the assessment of cost utility where substantial variability exists around the numerator and the denominator of the cost utility ratio compounded by the variability observed as a function of the analyst that produces the assessment and the peer reviewers that challenge the analyses (i.e. industry, CADTH, INESSS, the private sector, etc.). A review of recent CADTH CDR and pCODR recommendations conducted by Innovative Medicines Canada and EY shows that the degree of divergence between the cost-utility thresholds produced by CADTH versus those submitted by industry is significant: ICURs based on CADTH reassessment are significantly higher than those submitted by the manufacturers in the majority of cases; with the difference being as high as two to three times in many cases. The distribution of possible results around these point estimates is invariably wide and it is therefore inappropriate as a metric for setting the price ceilings of patented medicines. In addition, the perspective employed in CADTH CDR or pCODR submissions is a public drug plan perspective in accordance with the guidance provided by CADTH, and it is inappropriate to apply these pharmacoeconomic analyses to the entire Canadian population.
Given these significant limitations, it is inadvisable to use such an imprecise test of cost utility, compared against an equally controversial willingness to pay threshold, to determine a price ceiling for an innovative medicine. Its usage will lead to frequent and potentially litigious disputes requiring human and financial resources that are best deployed elsewhere by both the regulator and the regulated.
Furthermore, as many of our member companies operate on a global scale and have limited resources to allocate to meet the significant tasks required to bring a product to any individual market around the world, the regulatory signals sent by individual countries need to be as clear as possible to incentivize companies to launch innovative medicines. Contrary to the stated
objective of PMPRB’s proposed new framework, the proposed set of economic factors will provide no “bright line” that will “yield ceiling prices that are foreseeable to patentees”. Under such uncertain circumstances, it is foreseeable that many companies will delay or even forgo the launching of new innovative medicines in Canada.
Risk categorization:
The categorization exercise proposed by the PMPRB is only notionally consistent with the industry’s proposal for a risk-based approach to pricing regulatory scrutiny. As was evident from the Working Group discussions, the identification application of specific criteria must be the subject of careful consideration to avoid unintended consequences. If the categorization is too broadly defined, as was the case with the initial information disclosed by PMPRB to stakeholders on June 25th, 2018, the number of patented medicines that will be subject to an elevated level of regulatory scrutiny will be too large. This in turn will impose a significant operational burden on both the regulator and the regulated, while failing to achieve the stated policy objective of focussing regulatory resources where they add the most value. Furthermore, this categorization needs to be correlated with the magnitude of the risks that concern policy makers. The PMPRB has not offered a compelling policy rationale for each of the proposed screening criteria. From the discussion at the Working Group, we believe that the potential impact of including these criteria requires further evaluation.
Once again, we thank you for listening to our perspective on behalf our industry associations, and for ensuring that the content of this communication is reflected in the proceedings of the Working Group and also communicated back to the Steering Committee.
We look forward to a continued constructive dialogue with you and the Working Group.
Frederic and Geoff
Appendix 3.2: Email from Frédéric Lavoie and Geoff Sprang (2/4)
Subject: Draft summary of 26 July Working Group meeting Date: 17 August 2018 at 11:17 MST
From: Frédéric Lavoie
To: Mike Paulden, Chris Cameron, Christopher McCabe, Donald Husereau, Doug Coyle, Karen Lee, Maureen Smith, Patrick Dufort, Peter Jamieson, Stuart Peacock, Tammy Clifford, Tania Stafinski
Cc: Edward Burrows, Douglas Clark, Guillaume Couillard, Isabel Jaen Raasch, Matthew Kellison, Nelson Millar, Richard Lemay, Tanya Potashnik, Theresa Morrison, Geoff Sprang
Dear Mike,
We wanted to draw your immediate attention to some issues regarding the minutes.
After we sent you by email on August 9, 2018 (attached for reference) a summary of our industry perspectives and our views on the discussions during the first meeting of the technical working group (TWG), we have become aware that meeting minutes from the first meeting of the TWG have been shared with the PMPRB Steering Committee in advance of those minutes being shared and validated with the working group members themselves. As the terms of reference of the TWG stipulates that “the chair shall have final say on all matters of governance and procedures” we feel important to request that certain governance processes be improved. One such usual and customary process is that meeting minutes be reviewed and approved by committee members before they become more broadly circulated. We also recommend the minutes include more detail including time, date, duration of meeting, who was in attendance, who was unable to attend, provide a record of what was said, what was agreed to, and list action items and their status.
Furthermore, in this case, it is particularly problematic because the minutes, in our view, and as confirmed by the observations we shared with you by email on August 9, 2018, do not accurately or completely reflect the discussions of the working group, which could mislead the reader regarding the degree of expert consensus on fundamental issues under consideration. This gap in the minutes limits the ability of external stakeholders to the TWG (i.e. PMPRB steering committee members) to understand the origin and rationale of the points of contention that the chair is required to record in the final report of the TWG (as per terms of reference).
As examples of the issues of concern to us, we would draw your attention to the following passages:
- “Several members expressed the view that the opportunity cost of a drug may not be an appropriate tool for screening purposes. It was suggested further study may be needed to inform the discussion. Members generally agreed that application of supply-side cost effectiveness thresholds were an appropriate approach to consider opportunity cost when setting ceiling prices for Category 1 drugs”
- In our view, there was no general agreement on cost effectiveness thresholds as an appropriate approach to consider opportunity cost and the TWG never resolved the issue of how such a threshold could be determined. There was in fact considerable debate and disagreement on this, leading to PMPRB Chair Mitch Levine to question potential alternatives to the use of pharmacoeconomics. This lack of consensus was evident from your proposal (and the PMPRB staff’s agreement) to schedule additional conference calls beyond what was planned in the terms of reference to allow for further discussion and to arrive at more aligned views.
- “Members discussed using the CADTH and/or INESSS reference case analysis to set a price ceiling, as well as potential approaches to take in situations where the existing reference case was not relevant.”
- We would note that the meeting minutes should reflect that there was fairly widespread agreement that INESSS and CADTH assessments are NOT appropriate as reference cases, that the processes in place do not represent a peer-reviewed approach nor are they conducted from a perspective that is appropriate for price setting. Further, as representatives of our industry, we clearly communicated that the HTA cost-utility point estimates will never provide the level of certainty necessary and appropriate for the purposes of price setting within a quasi-judicial context.
We wanted to bring our concerns to your immediate attention and would welcome further discussion and validation of detailed meeting minutes with the working group. To ensure full transparency, we also want this email as well as our email of August 9 to be posted on the BrightShare site so that the Steering Committee members are able to appreciate our views.
We are happy to discuss either of these points if you have any questions, and looking forward to hearing from you to get your perspective on these issues. Thanks.
Geoff and Frederic
Appendix 3.3: Email from Frédéric Lavoie and Geoff Sprang (3/4)
Subject: Next steps for the PMPRB Technical Working Group Date: 17 August 2018 at 11:17 MST
From: Frédéric Lavoie
To: Mike Paulden
Cc: Edward Burrows, Douglas Clark, Guillaume Couillard, Isabel Jaen Raasch, Matthew Kellison, Nelson Millar, Richard Lemay, Tanya Potashnik, Theresa Morrison, Geoff Sprang, Marie-Claude Aubin, Sylvie Bouchard, Chris Cameron, Christopher McCabe, Donald Husereau Doug Coyle, Karen Lee, Maureen Smith, Patrick Dufort, Peter Jamieson, Stuart Peacock, Tammy Clifford, Tania Stafinski
Dear Mike,
Firstly, we would like to acknowledge and thank you Mike for the manner in which you have conducted and chaired this working group, maintaining a constructive and professional tone throughout the meetings and calls, despite the widely divergent views of the various group members.
As you and the other working group members know from our repeated reminders, the industry has a fundamental disagreement with the premise of using of the proposed economic factors to establish ceiling prices in the context of the PMPRB’s mandate. Chief among those concerns are the difficulties of establishing the so-called “bright lines” which PMPRB itself has identified as an important element of the new regulatory framework, given the inherently subjective nature of point estimates, as well as the technical and operational challenges associated with implementation. These concerns make it very challenging for us to confine our commentary within the very narrow boundaries established by the terms of reference of the Working Group.
Although we have been repeatedly reminded by the PMPRB staff that the mandate of this WG is limited to finding solutions to implement the economic factors proposed in the draft regulations published through the Canada Gazette I process on the assumption that the final regulations published in the Canada Gazette II will be unchanged, we strongly believe it is our responsibility to call attention not only to the issues related to uncertainty and lack of clarity, but also to the significant and, in many cases, insurmountable technical and operational issues associated with the application of these economic factors. We appreciate that many of these issues have also been acknowledged in the perspectives and comments offered by other WG members.
Much if not all of the effort expended by the Working Group in arriving at recommendations will be of limited utility if technical or operational issues render them impossible or impractical to implement. For this reason, we feel strongly that to be informative, the group’s recommendations need to be accompanied by comprehensive commentary on the known and potential technical and operational complexities of implementation.
In addition to participating in the initial Working Group meeting on July 26th, we have now attended all of the 8 hours of conference calls scheduled on August 22 and 24, 2018. It would have been helpful to hear from key stakeholders, such as CADTH staff, who were unfortunately not present during these calls. Through all of these discussions what is consistently apparent to us is that there is little if any consensus around the use of economic factors beyond using a set of international pricing reference tests in the regulatory ceiling price-setting exercise.
Despite the many hours of discussion, it appears that the application of the economic factors proposed by PMPRB to the working group remains associated with a lack of clarity. We have heard that this lack of clarity can be accommodated and may in fact provide a desired level of flexibility where economic factors are applied at the level of budget holders to guide decision making. However, in the context of their application in a prescriptive manner to establish an explicit ceiling price, given PMPRB’s role as a price ceiling regulator, such a lack of clarity constitutes a critical limitation. Our working group discussions to date have only served to heighten our concerns that the uncertainty associated with their use and interpretation is significant and will not provide a bright line conducive to innovative companies understanding the implications of engaging within the Canadian market the significant resources required to commercialize innovations.
While we are cognizant of the limited terms of reference for this working group prescribed by the PMPRB, we feel it is our responsibility to reiterate to policy makers our strong recommendation that the working assumptions of the WG be revisited and that the Government of Canada urgently establish discussions with our industry to consider alternative regulatory approaches excluding the use of economic factors.
Below are our observations from the working group discussions about each of the six topics in scope that support the above industry perspective:
Drugs in Category 1:
- The industry is favourable to a risk-based approach to PMPRB’s regulations;.one that is commensurate to the risk of abuse of a patentee’s monopoly power. However, this risk categorization cannot be the gate towards the implementation of economic factor adjustments as currently intended in the current draft regulations (use of pharmacoeconomic price tests).
- The initial intent published by PMPRB that categorization of risks is framed on the basis of products having a cost-utility point estimate greater than $30,000/QALY (corresponds to a supply side estimate of UK willingness to pay threshold) would capture >90% of current patented medicines in Canada.
- The technical difficulty in establishing a cost-utility estimate for a newly launched medicine led the WG to discourage PMPRB for using this as a criterion to define risk.
- The WG thought that this exercise should exclusively include treatment cost per year, market size and degree of innovative value (breakthrough product).
- Preliminary data on risk-based categorization were only verbally shared with the WG by PMPRB staff. Further details and discussion is required before any conclusions could be made.
- The sensitivity of these criteria also needs to be evaluated post application of the first price test of international price referencing. This was not accounted for by PMPRB during its preliminary analyses.
Supply-side Threshold:
- Industry representatives have repeatedly pointed out that the lack of precision (high levels of uncertainty) associated with cost-effectiveness estimates and thresholds of willingness to pay makes the use of these tools inappropriate for price ceiling determinations. This concern has been echoed by patient and HTA representatives. There does appear to be consensus that cost-effectiveness estimates and willingness to pay thresholds are (and should continue to be) used by payers to guide the allocation of limited resources within the preview of budget holders (public and private payers).
- The debates of the WG highlighted that there are various quantitative methods
- (supply-side and demand-side) that would yield differing estimates of willingness to pay of Canadians all susceptible to uncertainty and therefore open to be debated by stakeholders. Such a subjective estimate is not an appropriate tool to use in a
quasi-judicial price ceiling setting exercise.
- There was general agreement within the WG that PMPRB’s initial position on UK supply-side estimate ($30,000/QALY) was not appropriate and some academic members of the WG suggested more Canadian specific research would need to be conducted before application in this setting and that status quo be observed until conclusion of Canadian research in this area (pause on the application of the economic factors).
- Another area of contention was raised in the WG deliberations as there is misalignment between the suggestions of PMPRB staff to use a supply side estimate of the Canadian willingness to pay threshold while the mandate of PMPRB is to protect the interests of Canadian consumers, aligning with a demand-side willingness to pay threshold quantitative method. Beside this unresolved issue, the use of demand-side thresholds could necessitate that the PMPRB run as many studies to establish thresholds as there are budget holders within the fragmented Canadian pharmaceutical system. Variability across multiple thresholds will also likely raise questions amongst patient stakeholders as to why certain areas and/or diseases are confronted to a lower threshold than other areas and/or diseases. There are many such ethical questions that have not been studied as part of Health Canada and the PMPRB’s proposals.
- The uncertain nature of any cost-effectiveness threshold would represent an unrealistic reference for an innovative patented pharmaceutical tested against its equally uncertain cost-utility value.
Market Size:
- Mitigating the risk of budget impact is an objective of public and private payers in Canada. These stakeholders have effective tools to address the perceived risk pertaining to the anticipated market size a medicine would detain.
- It was acknowledged that use of a gross (or even net) sales number to make ceiling price adjustments ignores the actual budget impact which is more important to payers and which is also a more appropriate consideration in terms of rewarding innovation and influencing the allocation of resources. However, there is no practical or effective way to actually prospectively define this factor and any methodology used to forecast this factor would be accompanied by enormous uncertainty. It is also important to note that such factors are already routinely addressed at the level of budget holders through product listing agreements.
- Establishing a price ceiling threshold based on GDP factors is also problematic given economic variability and more importantly differences across jurisdictions and payer segments in definitions of affordability as well as local or regional healthcare priorities. Affordability and healthcare priorities are ultimately policy decisions best left with individual jurisdictions. Such considerations are already addressed via existing government mechanisms (e.g. pCPA)
- Notwithstanding the industry opposing position, if pharmacoeconomic factors were implemented, why would patentees need to have their prices adjusted further for market size if they are delivering more value for money as use increases? Operationally, when does this adjustment happen?
Multiple Indications:
- The uncertainty associated with potential in-market price adjustments resulting from the introduction of new indications or changes in the mix of business resulting from changes in medical practice or competitive dynamics would discourage manufacturers from launching new indications and make it more difficult to make launch decisions for Canada, thereby resulting in delays or potentially loss of access to innovative medicines.
- The practical limitations of tracking and reporting by indication make implementation effectively impossible in the context of the current Canadian prescription drug setting.
- Even in a hypothetical context when a subsequent indication of an already approved medicine would be associated with a higher cost-utility, there is no mechanism in place to implement differential pricing on a per indication basis. Furthermore, the behaviour of payers in reimbursement negotiation appears to follow a price-volume rationale over medicines’ life cycle.
Perspective:
- The societal perspective is the broadest perspective theoretically speaking but it is associated with important technical measurement hurdles. In a societal perspective, the evaluation of indirect costs has been the subject of important equity issues due to their discriminatory nature. The valuation of productivity through indirect costs often yields to the prioritization of treatments predominantly destined to working age Canadians at the expense of those targeting an older population more likely retired from the work force.
- Again, the expression of a bright line for price ceiling setting of pharmaceuticals would be blurred as a result of the lack of clear consensus in the academic community on which perspective is best, how to measure it adequately and how to shelter it against the accusation of it leading to discriminatory practices. These issues will make it difficult for the WG to come up with a meaningful recommendation.
Accounting for Uncertainty:
- Regulating ceiling prices on the basis of factors that would be measured through payer processes not intended for price setting are a cause for concern. This was raised by the WG during the discussion on uncertainty.
- CADTH and/or INESSS that would produce cost-utility point estimates for medicines in Canada often exhibit differences in their estimates pertaining to heterogeneous assumptions and expert opinions. Their processes do not incorporate state of the art validation steps and levels of peer-reviews.
- The WG discussed the option of creating a new health economics committee to provide enhanced rigour in the evaluation. However, it was noted that the important shortage of trained health economist experts in Canada would make the composition of such group difficult and duplicative. This would also add another layer of complexity and delays on the already difficult Canadian journey of a pharmaceutical innovation.
The compounded uncertainty across multiple proposed economic factors is contrary to the PMPRB’s stated objective of providing innovators with a bright line in forecasting ceiling prices of innovative entrants in the Canadian market.
As the working group moves to the next steps, it will be helpful to get clarity on the process for developing recommendations and the role of the PMPRB Steering Committee (SC) in this regard. We have been informed that the PMPRB staff clarified at the last SC meeting that the role of the SC is not to steer the work of the working group. This raises a serious governance and procedural question regarding the next steps in the process of development of any recommendations through the working group and the role of the SC in approving the recommendations.
Thanks in advance for the work you will do to fully integrate are above considerations into the WG’s outputs.
Sincerely, Frederic & Geoff
Appendix 3.4: Email from Frédéric Lavoie and Geoff Sprang (4/4)
Subject: Feedback related to September 25 meeting Date: 3 October 2018 at 13:55 MST
From: Frédéric Lavoie
To: Mike Paulden
Cc: Geoff Sprang, Marie-Claude Aubin, Sylvie Bouchard, Christopher McCabe, Donald Husereau, Doug Coyle, Karen Lee, Maureen Smith, Patrick Dufort, Chris Cameron, Peter Jamieson, Stuart Peacock, Tammy Clifford, Tania Stafinski, Edward Burrows, Douglas Clark, Guillaume Couillard, Isabel Jaen Raasch, Matthew Kellison, Nelson Millar, Richard Lemay, Tanya Potashnik, Theresa Morrison
Dear Mike,
In follow up to our Technical Working Group call on September 25th, and as the representatives of the industry subject to the PMPRB’s guidelines, we wanted to capture and convey to you our key takeaways from the discussion as well as our understanding of next steps.
Once again, we want to commend you for your thoughtful and inclusive approach to a complex and challenging process given the limiting terms of reference set by PMPRB for the Working Group and diversity of views represented in the group. While we have provided some additional commentary specific to the six pre-specified areas below, it was apparent to us that we are still struggling to arrive at a consensus in any of these six areas and we appreciate your candor in acknowledging this at the close of the meeting. As we have stated repeatedly, the heterogeneity of opinions within the working group and the inability of the group to forge a consensus when it comes to the application of economic factors to price regulation is illustrative of the issues that form the basis of the regulated industry’s concerns; specifically the degree of uncertainty, the lack of “bright lines” and the complexity of implementation which in our view represent critical limitations of the proposed regulatory framework.
We understand that the proposed next steps are the circulation of a draft report by October 5th for review by members prior to a final meeting of the Working Group on October 12th at which voting on the final recommendations will take place. Materials provided to you in advance of October 5th may be incorporated into the draft report. However, those provided after issuance of the draft report may still be considered at the October 12th meeting. The Final Recommendations of the Working Group will be issued at some point shortly thereafter to the Steering Committee for consideration in late October. However, the Steering Committee will not see any draft materials or commentary from the Working Group. Given the complexity of the issues, we believe that the Working Group does not have sufficient time to complete its work in the timeframe defined by PMPRB.
Given that the Working Group’s Terms of Reference state that recommendations will be determined by a simple majority vote, and in view of our comments above, we anticipate that arriving at a single set of coherent recommendations that “do justice” to the complexity of the issues will be extremely challenging and that what is ultimately presented to the Steering Committee may fail to reflect the underlying heterogeneity of opinion. Under these circumstances, we believe it is critical that the questions that will be subject to a vote, the process by which all of the results will be captured and reported, as well as the content and format of what will be shared with the Steering Committee and other relevant stakeholders be well defined in advance. We would therefore ask that these considerations be drafted and shared with the Working Group as soon as possible and before the voting process is launched. The Working Group members should be allowed to comment on the proposed process prior to undertaking any voting. We take comfort with your commitment of filing in the appendix of the final report the written comments of the Working Group members who wish them to be “on record”. As such, please consider this email “on record”.
In addition, and for reasons outlined previously, we believe that it will be important for stakeholders reviewing the output of the Working Group to be provided with information relating to the technical feasibility and other implementation issues and challenges associated with recommendations. It was our understanding from discussions at the September 12th meeting that PMPRB staff were to provide case studies to inform the Working Group’s deliberations and we are disappointed that they have not done so to date. The suggestion from PMPRB staff that Working Group members with expertise and examples may bring these forward has come in the final weeks of the group’s deliberations and provides an insufficient opportunity for their development and consideration. As industry representatives, and although we believe that it should be the responsibility of PMPRB staff as opposed to Working Group members to provide case studies, we will attempt to compile some case studies to share with the group in advance of our next meeting.
With respect to the six specific areas for consideration, as noted previously we do not support the inclusion of economic factors in a quasi-judicial price ceiling regulatory methodology given the uncertainty these would introduce, the practical challenges and complexity of implementation and the fact that the government’s regulatory objectives can be achieved by much simpler, more transparent and predictable mechanisms. Our observations of the group’s discussion are provided below.
- Perspective – while some members expressed the view that a health system perspective would be preferable to a societal perspective in order to minimize discriminatory bias (e.g. productivity considerations), other members raised the concern that the health system perspective fails to account for the private for-profit segment of the market. The concern that private payers are “profit-maximizers” and that potential price reductions may not be passed on to consumers has also been raised.
- Threshold – there were differing points of view on whether the thresholds should be determined by supply side or demand side considerations. There was also a view that PMPRB cannot “enforce” or regulate efficiency and WTP varies, so it may be better to set an upper limit on all but let payers negotiate. There was some support for additional empirical work as general agreement that thresholds used in existing HTA assessments would not appropriately reflect collective WTP. The need for further research in this area in which current empirical work is insufficiently mature and not Canada specific has also been raised.
- Uncertainty – Uncertainty is reflected in HTAs and resulting decision making by considering the range of possible ICERs rather than a point estimate. HTAs are not performed with the objective of determining a point estimate for price setting. New drugs are introduced and priced at the point of maximum uncertainty which typically declines over time.
- Market Size – recognition of the fact that net budget impact is more important than gross sales; challenges in defining this ex ante given uncertainty in forecasting.
- Multiple Indications – general agreement that pricing by indication is theoretically appealing, it is not possible given current limitations in data capture and reporting. Practically it seems necessary to regulate one price across indications, however there was no agreement on how a single price across indications would be established.
- Category 1 Criteria – general agreement that CE would not be appropriate screening criteria, support for risk based approach, some support for use of level of therapeutic improvement and new MOA as a consideration, concerns expressed about market size vs net budget impact as this could distort screening, also concerns expressed about impact of a specific threshold on orphan drugs.
We look forward to your response. If you have any questions, please do not hesitate to contact us.
Regards,
Geoff and Frederic
Appendix 3.5: Summary comments from Frédéric Lavoie and Geoff Sprang
Date: 1 March 2019 at 15:00 MST
As members of the Technical Working Group (TWG) representing BIOTECanada and Innovative Medicines Canada, we wish to enter the following summary of observations and issues into the record on behalf of our respective memberships, who represent most of the patentees subject to the PMPRB’s jurisdiction.
As both organizations have previously communicated, we believe the use of the proposed economic factors in the context of quasi judicial price regulation is inappropriate. Our concerns in this regard and the underlying rationale have been captured elsewhere and for that reason are not restated here but can be reviewed under on the record comments in the appendix of the TWG report. However, our participation in the TWG and the opportunity to further explore the complex issues associated with the use of economic factors in this way has only served to reinforce our concerns that these reforms will, at best, delay access to new therapeutic options for Canadian patients, and potentially impede access altogether to the extent that manufacturers elect not to launch new therapeutic products in Canada.
Overall, a key concern was lack of clarity around the overarching policy objectives. In a number of cases the TWG was unable to arrive at clear recommendations and ultimately determined that the questions posed could only be answered with further clarification of PMPRB’s policy objectives. The fact that such objectives were not sufficiently clear to the TWG is in and of itself problematic and limited the value of the TWG. We also note that the deferral within the proposed recommendations to “policy intent” should not be construed as support for the proposed new economic factors.
Another important and challenging topic for TWG consideration was Topic 5 – Perspective which, under the Terms of Reference, required the TWG to discuss options to account for the consideration of a public health care system versus a societal perspective. Given the heterogeneous nature of the Canadian payor landscape, which includes public payors, employer-sponsored privately funded plans as well as cash paying customers, discussions of this topic reflected very divergent views. It is disappointing and, in our view, inappropriate that, having asked the TWG to provide advice, the PMPRB intervened and imposed the decision to adopt a public health care system perspective without regard to the diverse views of the expert members of the TWG.
In addition, we believe that the Terms of Reference for the TWG greatly limited the value of the exercise in leveraging both the practical and academic expertise of the members. For example, we feel it is important to register our concern and disappointment that important feasibility issues related to implementation were considered out of scope; particularly as the TWG is the only forum specifically charged with consideration of technical questions related to implementation. We find it inconceivable that the proposed regulatory reform process has reached this stage without having given due consideration to technical feasibility.
Our efforts to call out significant feasibility challenges were essentially dismissed by PMPRB staff. In some cases the feasibility issues that we attempted to raise are substantive enough that patentees subject to the proposed regulation changes do not currently have the ability to comply with the new reporting requirements. In other cases, our compliance with the proposed reforms would have major implications for resourcing and enterprise system reconfiguration adding enormously to the existing cost and regulatory burden of reporting by patentees. Significantly adding to the regulatory burden without due consideration to alternative regulatory options makes no sense and runs counter to the federal government’s efforts to reduce so-called “red-tape”.
We also want to register our concern that despite numerous requests and emphasis on the need for case studies to be developed to explore how the proposed reforms would be applied, case studies were only made available in the final stage of the TWG deliberations and a review and discussion of all 6 individual case studies was allocated only 35 minutes on the agenda of the one meeting where they were discussed. A robust discussion of these case studies would have added greatly to the TWG’s deliberations. The case studies themselves, which were developed by the PMPRB, raise numerous issues that are illustrative of the kinds of challenges that will arise if the current regulatory revisions are implemented as proposed. It is noteworthy that despite significant efforts within our respective trade associations as well as the use of external pricing and analytical expertise, we were unable to reverse engineer or replicate the PMPRB’s results. This is concerning in and of itself and underscores the need for additional consultation. The magnitude of price reductions illustrated by the case studies also raises concerns since it is clearly not aligned with the Regulatory Impact Analysis Statement and Cost-Benefit Analysis released by Health Canada with the draft regulatory amendments or with the objective of aligning Canadian prescription drug prices with those of a broader subset of reference markets. When these issues were raised at the TWG they were not adequately addressed by PMPRB staff.
Overall, while we appreciate the efforts of the Chair (Mike Paulden) to execute the mandate he was given as impartially as possible, the mandate itself (Terms of Reference), the limitations placed on the scope of the TWG’s considerations (notably the exclusion of considerations of technical feasibility), the lack of clarity early in the process surrounding the PMPRB’s policy intent that limited the TWG’s ability to provide meaningful recommendations in many areas, the late availability and insufficient time allocated to the consideration of case studies and the decision of the PMPRB to disregard the TWG’s deliberations of Topic 5 (Perspective), combined to render the TWG exercise inadequate as a consultation process.
As representatives of the innovative industry, we have clearly acknowledged the challenges facing governments in meeting the expanding healthcare demands and we reiterate our willingness to work with governments and other stakeholders to find appropriate solutions. These solutions must reflect a comprehensive and balanced policy framework that extends beyond pharmaceutical price ceiling controls to include the objective of ensuring Canadians have timely access to the best treatment options and to preserving Canada’s attractiveness as a destination for life sciences research and investment. Therefore, as it relates to price ceiling regulatory reforms, we continue to advocate for more robust consultations with representatives of industry, patient associations, other federal government ministries as well as provincial governments, all of whom share the objective of improving the health and well-being of Canadians.
Appendix 3.6: Summary comments from Maureen Smith
Date: 1 March 2019 at 15:59 MST
As a member of the Technical Working Group, I would like the following comments to be included in the appendices of the Technical Working Group (WG) Final Report. When I accepted the invitation to join the PMPRB’s Technical Working Group in July 2018, I knew that it would be challenging to provide my own patient perspective in a Working Group whose purpose was to inform the PMPRB Steering Committee on the modernization of price review process guidelines. After all, not many patients know about this quasi-judicial body that sets price ceilings for patented drugs in Canada, yet these ceiling prices are important to patients as they can have consequences on the sustainability of our health care system and access to medications. I have spent the past five years as a patient member of a provincial health technology assessment body, therefore, I felt that I had enough understanding of health economics to participate in the discussions and hopefully bring my lived experience as a Canadian with a rare disease who relies on drugs and has dealt with access issues.
Unfortunately, I believe that the WG was not able to engage in a discussion that would have allowed us to deliver on our terms of reference. Simply put, the terms of reference were not reflective of the scope of the Technical WG. Much of what we were tasked to discuss in the six areas of focus was pre-determined by the Regulatory Impact Analysis Statement (RIAS) that were published in the Canada Gazette, Part 1. For example, after two months of discussion on the options to account for the consideration of a public health care system versus societal perspective, the WG was informed by the PMPRB that, as stated in the RIAS, they were adopting a public health care system perspective. Why then was the WG ever asked to discuss perspective? Given that we do not have a national pharmacare program in Canada and that Canadian consumers use public plans, private insurance, or pay out of pocket for their drugs, it was disappointing that the perspective had already been determined.
The WG was told that other topics were out of scope as well, despite a terms of reference that suggested otherwise. While I appreciate that we were not there to debate the RIAS, the terms of reference should have been more aligned with the RIAS and its constraints. Another barrier to fulfilling our mandate was the lack of a proper review of empirical evidence on each topic. This should have been undertaken, rather than relying on WG members’ own knowledge of what was available and personal biases. Finally, as early as the first meeting and then repeatedly several WG members requested that the PMPRB develop case studies that would allow us to work through the technical details and have a better understanding of the impact. Case studies that were developed for the Steering Committee were finally made available to us and we were granted 30 minutes to discuss this during our final meeting.
The recommendations you see will most likely have a high degree of agreement because, except for a few, they cannot truly be considered recommendations if one looks at the specific questions in our six areas of focus. They are a record of whether the members of the WG agree on our conclusions. There really isn’t much to disagree on, since no resources were invested in synthesizing the existing empirical evidence, resulting in little space for a thoughtful technical discussion. As I see it, the WG’s recommendations fall into five categories: (1) advising the PMPRB to adopt measures that will be consistent with their policy intent; (2) recommendations that simply state that this is the only option because of the policy intent; (3) those that deal with the enormous challenges of applying health technology assessment to a country with 17 jurisdictions who each have their own drug budgets and priorities; (4) recommendations that state the WG’s conclusions such as 2.3 “The WG regards the direction and magnitude of any bias in the $30,000 per QALY estimate by Ochalek et al. (2018) to be unknown”; and (5) recommendations that call on further empirical research. For me, this is the result of 31 hours of discussion and, unfortunately, the impact is minimal due to the failure in the process.
As a patient, my goal was to contribute to the discussion of achieving the fine balance that doesn’t discourage market access while charging prices that payers feel will protect the public health system. Patients are concerned about the prices of drugs but they are also concerned about having access to innovative therapies in Canada. There is some evidence that countries such as Australia and New Zealand who have some of the toughest drug prices have less access. Another concern is whether the application of health technology assessment tools by the PMPRB will result in further inequity in access to drugs for Canadians, especially for those relying on drugs for rare diseases whose coverage is often determined by their postal code. Will they acknowledge the challenges of HTA for rare disease drugs, especially the inappropriateness of thresholds? Finally, if the PMPRB expands its mandate to integrate HTA into setting ceiling prices, they should have a process for patient input into their work similar to the patient submission processes that our Canadian HTA agencies have adopted.
In conclusion, it is my opinion that the PMPRB missed an opportunity to truly consult the WG members as much of the outcome was pre-determined by the key guideline document (the RIAS) and there was a lack of clarity on the policy intent from the outset. It is worrisome that the Technical WG was not able to debate the important considerations and reduce some of the uncertainty in what the consequences will be for Canadian patients by making recommendations that would have reflected our best thinking.
Appendix 4: Terms of Reference
Terms of Reference for Working Group to Inform the Patented Medicine Prices Review Board (PMPRB) Steering Committee on Modernization of Price Review Process Guidelines
Background
The Patented Medicine Prices Review Board (PMPRB) recently established a ‘Steering Committee on Modernization of Price Review Process Guidelines’. The mandate of this Steering Committee is to assist the PMPRB in synthesizing stakeholder views on key technical and operational modalities of the PMPRB’s new draft Guidelines.
The Steering Committee’s work will be based in part on the analysis and recommendations of a technical Working Group, which will examine certain issues that the Steering Committee believes would benefit from the review of experts in health technology assessment and other economic and scientific matters.
The Working Group will comprise leading experts in pharmacoeconomics and the clinical evaluation of pharmaceuticals. The Working Group will meet twice in-person and multiple times via teleconference between July and October 2018. A report of the Working Group’s deliberations and recommendations will be produced by the chair and submitted to the Steering Committee for consideration in October 2018.
Membership
The chair of the Working Group will be Dr. Mike Paulden (University of Alberta).
Thirteen individuals will sit as members of the Working Group (listed alphabetically):
- Dr. Chris Cameron (Dalhousie University and Cornerstone Research Group);
- Dr. Tammy Clifford (University of Ottawa and CADTH);
- Dr. Doug Coyle (University of Ottawa);
- Patrick Dufort (INESSS);
- Don Husereau (University of Ottawa);
- Dr. Peter Jamieson (University of Calgary);
- Dr. Frédérick Lavoie (Pfizer Canada);
- Dr. Karen Lee (University of Ottawa and CADTH);
- Dr. Christopher McCabe (University of Alberta and Institute of Health Economics);
- Dr. Stuart Peacock (Simon Fraser University and BC Cancer Agency);
- Maureen Smith (Patient);
- Geoff Sprang (Agmen);
- Dr. Tania Stafinski (University of Alberta).
Two individuals will sit as observers of the Working Group:
- Edward Burrows (Innovation, Science and Economic Development);
- Nelson Millar (Health Canada).
One individual will act as an external reviewer of the Working Group’s draft report:
- Dr. Mark Sculpher (University of York).
Recommendations of the Working Group will be determined by a vote of the members. In the event of a tie, the chair will have the casting vote.
Areas of focus
The Working Group will examine and make recommendations with respect to the following considerations and questions:
1. Options for determining what medicines fall into ‘Category 1’
- A Category 1 medicine is one for which a preliminary review of the available clinical, pharmacoeconomic, market impact, treatment cost and other relevant data would suggest is at elevated risk of excessive pricing.
- The following criteria have been identified as supporting a Category 1 classification:
- The medicine is ‘first in class’ or a ‘substantial’ improvement over existing options
- The medicine’s opportunity cost exceeds its expected health gain
- The medicine is expected to have a high market impact
- The medicine has a high average annual treatment cost<
- Should other criteria be considered? What are the relevant metrics for selecting medicines that meet the identified criteria and what options exist for using these metrics?
2. Application of supply-side cost effectiveness thresholds in setting ceiling prices for Category 1 medicines
- Potential approaches for implementing a price ceiling based on a medicine’s opportunity cost.
- Potential approaches for allowing price ceilings above opportunity cost for certain types of medicines (e.g. pediatric, rare, oncology, etc.)
3. Medicines with multiple indications
- Options for addressing mediciness with multiple indications (e.g. multiple price ceilings or a single ceiling reflecting one particular indication).
4. Accounting for uncertainty
- Options for using the CADTH and/or INESS reference case analyses to set a ceiling price.
- Options for accounting for and/or addressing uncertainty in the point estimate for each value-based price ceiling.
5. Perspectives
- Options to account for the consideration of a public health care system vs societal perspective, including the option of applying a higher value-based price ceiling in cases where there is a ‘significant’ difference between price ceilings under each perspective.
- How to define a ‘significant’ difference in price ceilings between each perspective.
6. Application of the market size factor in setting ceiling prices
- Approaches to derive an appropriate affordability adjustment to a medicine’s ceiling price based on an application of the market size and GDP factors (e.g. based on the US ‘ICER’ approach).
Additional areas of focus may be identified by the Steering Committee prior to the first meeting of the Working Group in July 2018.
It is anticipated that the approaches or methods recommended by the Working Group may not be identical to approaches or methods currently employed by CADTH or INESSS. Where such departures present potential hurdles for operationalization of its recommendations, the Working Group will identify potential technical or other solutions to these hurdles.
Confidentiality
Working Group members may consult with non-members on an ongoing basis but are expected to maintain the confidentiality of any materials provided to them during the course of their work.
The names of the members of the Working Group will be published on the PMPRB’s website, along with a report of its deliberations, analysis and recommendations.
Governance and procedure
It is recognized that members of the Working Group may hold opposing points of view on the above issues and/or disagree with the policy rationale underlying the changes to the PMPRB’s Guidelines. Members are nonetheless encouraged to work together constructively to assist the Working Group in carrying out its function.
The chair is expected to foster consensus among members, but in order to ensure that Working Group deliberations are as focused and productive as possible, the chair shall have final say on all matters of governance and procedure. Members who disagree with a decision of the chair in this regard can request that their objection be noted on the record. The chair shall make every effort to ensure that the Working Group’s final report accurately reflects any important points of convergence or contention between members.
Schedule
The Working Group will meet for the first time in-person in Ottawa in July, followed by numerous tele-conferences in August and September. Following submission of a draft report, a second in- person meeting will be held in October.
All dates are subject to the availability of the chair and members of the Working Group.
| Date |
Event |
Purpose |
| 26 July 2018 |
Full day in-person meeting in Ottawa |
Overview of Working Group objectives. Summary of specific areas of focus under consideration. Allocation of tasks among Working Group members. |
| 22-24 August 2018 |
One-hour teleconference on each area of focus |
Opportunity for input from Working Group members. |
| 24 August 2018 |
Two-hour teleconference |
Update on Working Group status. Opportunity for input from Working Group members. |
| Week of 10 September 2018 (TBC) |
Two-hour teleconference |
Update on Working Group status. Opportunity for input from Working Group members. |
| 5 October 2018 |
Draft report submitted to PMPRB |
Opportunity for input from PMPRB and Working Group members. |
| 12 October 2018 |
Full day in-person meeting in Ottawa |
Present draft report. Report draft recommendations.
Final opportunity for input from PMPRB and Working Group members. |
| 26 October 2018 |
Final report delivered to PMPRB |
Final deliverable to PMPRB. |
Deliverables
A draft report will be circulated to the Steering Committee and Working Group members on 5 October 2018, prior to the final in-person meeting in Ottawa.
Following delivery of the final report, the chair will be willing to present the recommendations of the Working Group to stakeholders and other interested parties, subject to availability.
Budget
The PMPRB may cover reasonable travel and accommodation costs of members where such funding is requested and approved in advance. Where possible, the chair of the Working Group will arrange meetings to attempt to minimize expenditures for participants.
Appendix 5: Policy Intent
Appendix 5.1: Regulations Amending the Patented Medicines Regulations
Appendix 5.2: PMPRB Guidelines Scoping Paper
Appendix 5.3: PMPRB Framework Modernization Presentation
Appendix 5.4: PMPRB Short Primer
Patented Medicine Prices Review Board -
Prepared for the Working Groups on Guideline Reforms
July 2018
The following short description is intended to address questions raised during the technical working group meeting about the PMPRB’s mandate and role.
Prior to 1987, the Canadian Patent Act (“Act”) allowed generic drug manufacturers to obtain compulsory licences to produce generic versions of patented brand name drugs at any time during the patent term. In addition, the Act only allowed for the patenting of processes to make medicines, but not the medicines themselves.
In 1987, the Act was substantially amended to reduce the availability of compulsory licences to generic manufacturers and to allow patents for the medicine themselves. These changes gave rise to a concern that patentees would abuse their newfound patent rights by charging prices above “reasonable” levels. To address this concern, the Act was further amended to create the PMPRB. All of these amendments were made to the Act through Bill C-22.
In introducing Bill C-22 in Parliament, the responsible Minister, the Hon. Harvie Andre, had the following to say regarding the dual intentions underlying the legislation:
In essence, the amendments I propose in Bill C-22 will create a climate favourable to new investment in research and development in Canada by giving patent holding firms in Canada a guaranteed period of protection. These changes will also ensure consumer protection by creating a new prices review board to monitor drug prices. 1
There is the question of consumer protection. What good would come of it if we had all kinds of new drugs and no one could afford them? If the sick and elderly could not get access to the drugs, what good would come of it? 2
I hereby submit that anybody who takes an objective view of what we are proposing will see that we have in place enormous checks and balances to ensure that consumer prices of drugs remain reasonable. 3
Thus, while the purpose of stronger patent rights for pharmaceutical manufacturers is to incent innovation in Canada, the purpose of the PMPRB is to act as an effective check on these rights by ensuring that patentees do not charge excessive prices during the statutory monopoly period. The consumer protection the PMPRB provides extends to all Canadian purchasers of medicines, be they government, insurers, wholesalers or private individuals.
In a statutory monopoly situation, a seller has the ability to limit competition and thus can set a higher price than would otherwise exist, possibly to excessive levels. This risk of excessive pricing is exacerbated where demand for the product is high and there are few, if any, substitutes. In the pharmaceutical realm, this situation is most likely for patented medicines that are the first effective treatment of their kind for life threatening ailments. The PMPRB’s existence as the only sector-specific regulator under the Act is attributable to this fact and a recognition by policy makers that the unfettered monopoly pricing of patented medicines is not in the public interest.
In 1993, the Act was amended again to eliminate the special compulsory licencing regime that had applied only to patented medicines and, as an offsetting measure, to provide the PMPRB with additional remedial powers in dealing with cases of excessively priced patented medicines. In speaking to the latter set of amendments, the sponsoring Minister, the Hon. Pierre Blais, explained to Parliament that their purpose was “to strengthen consumer protection, so that consumers can continue to obtain patented medicines at reasonable prices” and to “assure Canadian consumers, of reasonable prices, like those they have had since 1987.”
The scope of the PMPRB’s powers reside in sections 83 and 85 of the Act. Section 83 enables the Board to order a patentee to lower its maximum price where it is found to be “excessive”.
Where the Board finds that a patentee of an invention pertaining to a medicine is selling the medicine in any market in Canada at a price that, in the Board’s opinion, is excessive, the Board may, by order, direct the patentee to cause the maximum price at which the patentee sells the medicine in that market to be reduced to such level as the Board considers not to be excessive.
The Act does not define what an “excessive” price is, and instead directs the PMPRB to consider the following factors at section 85 in making that determination:
- the prices at which the medicine has been sold in the relevant market;
- the prices at which other medicines in the same therapeutic class have been sold in the relevant market;
- the prices at which the medicine and other medicines in the same therapeutic class have been sold in countries other than Canada;
- changes in the Consumer Price Index;
- [proposed factor] the size of the market for the medicine in Canada and in countries other than Canada;
- [proposed factor] the gross domestic product in Canada and the gross domestic product per capita in Canada;
- [proposed factor] the pharmacoeconomic value in Canada of the medicine and that of other medicines in the same therapeutic class
While the PMPRB can order price reductions following a hearing, it also issues Guidelines that outline how it monitors the prices of patented medicines to identify whether the price of any particular medicine should be considered potentially excessive and the subject of a hearing. The Guidelines are not binding, but they provide guidance on patentee pricing behaviour and adherence with the Guidelines reduces the likelihood that a patentee may find itself in a hearing before the Board. Although it is part of the Health Portfolio, the PMPRB as a whole maintains an arm’s length relationship with other entities including the Minister of Health and stakeholders. In other words, the PMPRB conducts its price monitoring and decides hearings independently from those entities. For example, while complaints from third parties may initiate an investigation under the Guidelines, the complainant has no part or role in the actual investigation or its resolution.
The PMPRB has no mandate or policy tools to incent innovation in Canada, cannot bar a patented medicine from being marketed in Canada; makes no decisions or recommendations regarding the approval of medicines for safety, efficacy and quality; and makes no decisions or recommendations regarding the listing or reimbursement of medicines in drug plans.
The Government believes that the PMPRB’s current regulatory framework does not provide it with adequate tools to effectively protect Canadians from excessive prices, or for optimal identification of maximum prices in today’s pharmaceutical environment. That is why Health Canada is advancing the proposed regulatory amendments, including new s.85 factors in the form of pharmacoeconomic value, market size and GDP.
- 1 House of Commons Debates, 33rd Parliament, 2nd Session, Vol. 1, page 1369, Hon. Harvie Andre (Minister of Consumer and Corporate Affairs).
- 2 House of Commons Debates, 33rd Parliament, 2nd Session, Vol. 1, page 1371, Hon. Harvie Andre (Minister of Consumer and Corporate Affairs).
- 3 House of Commons Debates, 33rd Parliament, 2nd Session, Vol. 1, page 1373, Hon. Harvie Andre (Minister of Consumer and Corporate Affairs).
Appendix 6: Case Studies
Guideline Modernization: Case Studies
Appendix 7: Disclaimers
Appendix 7.1: Disclaimer from the PMPRB
The PMPRB provided the chair with the following disclaimer:
“The views expressed herein are those of the author and of the parties to whom certain views are attributed and should not be understood to constitute or reflect the views of the PMPRB or the Government of Canada unless specifically stated.”
Appendix 7.2: Disclaimer from Innovative Medicines Canada
Frédéric Lavoie provided the chair with the following disclaimer on behalf of Innovative Medicines Canada (IMC):
“IMC understands that the PMPRB intends to take steps to modernize its Guidelines within the framework of the proposed amendments to the Regulations. While IMC is committed to constructive engagement with the PMPRB on Modernization of Price Review Process Guidelines, our participation on the Steering Committee and the Working Group should not be interpreted as supporting the proposed amendments to the Regulations. IMC continues to have serious policy and process concerns about the proposed amendments and reserves its right to oppose the proposed amendments and the work of the Steering Committee and Working Group to the extent it is intended to implement or reflect the proposed amendments. IMC also has many concerns with the June 25, 2018 Guideline Proposals and will provide more detailed commentary once we have had an opportunity to fully assess their potential impacts on patentees. With respect to the Working Group’s governance, IMC intends to participate constructively but is concerned that minority and/or dissenting opinions should be fully and accurately placed on the record throughout the process including the draft and final report from the working group and the publication, following a request from one or more Working Group members.”
Appendix 8: External Review of Draft Report
The following is an external review of the draft report conducted by Dr Mark Sculpher from the Centre for Health Economics at the University of York.
This review was emailed to the chair on 4 March 2019.
General comments
Overall, the report reads well, and the guidance and advice offered to the PMPRB seems appropriate and well balanced.
Chair’s response: I would like to thank Dr Sculpher for reviewing the draft report and providing a number of thoughtful comments. I have responded to each of these below.
I have struggled to understand the role of PMPRB in relation to the CDR and provincial HTA arrangements. Presumably these different levels of policy review of drug prices will work synergistically and coherently. It seems to me that the most obvious version of such arrangements would be for PMPRB to set a ceiling price which CDR/provincial HTA to take as a maximum which may not be considered cost-effective from the perspective of a given province, indication or patient sub-group. In other words, PMPRB’s ceiling price becomes a starting point for further evidence review, analysis and price negotiation that may very well bring prices down further. As such, some of the challenges considered in the report may well simplify (see below). Although this is not the remit of the report, there does seem to be a need to consider how PMPRB will work with CDR and provincial HTA, avoiding duplication and contradiction.
Chair’s response: There are many possible approaches for setting a single ceiling price across multiple provinces, indications and/or patient subgroups. The Working Group recognized that the choice of which approach to adopt is a matter for policy makers. As a result, the Working Group did not advocate for any specific approach. Instead, we considered the technical implications of several possible approaches, in order to support policy makers in coming to an informed decision regarding which approach to adopt.
Dr Sculpher proposes a specific arrangement under which the PMPRB first sets a ceiling price informed by the maximum price at which a medicine is ‘just’ cost-effective within a single province, indication or patient subgroup, and then the price is negotiated down further using other mechanisms at the provincial level. The Working Group discussed some of the technical implications of such an arrangement. It was noted that provinces with lower supply-side thresholds might not have the negotiating power to bargain down the price to a level at which consumer surplus is positive for that province. As a result, such an approach might result in diminished population health in these provinces, which might in turn result in diminished population health across Canada as a whole.
The report covers the key areas of evidence and analysis that I would have expected given the policy context, with three exceptions. The first is the importance of patient-level heterogeneity. There is considerable discussion about pricing by indication, but the same issues exist in relation to patient sub-groups within an indication. There is a trade-off between the product’s ceiling price and the number of sub-groups for which it would be cost-effective. This is particularly obvious for products where cost-effectiveness is a function of the underlying risk of a clinical event (e.g. heart disease, osteoporosis etc), but it also applies to a large proportion of pharmaceuticals in other disease areas. I will come back to this below.
The report covers the key areas of evidence and analysis that I would have expected given the policy context, with three exceptions. The first is the importance of patient-level heterogeneity. There is considerable discussion about pricing by indication, but the same issues exist in relation to patient sub-groups within an indication. There is a trade-off between the product’s ceiling price and the number of sub-groups for which it would be cost-effective. This is particularly obvious for products where cost-effectiveness is a function of the underlying risk of a clinical event (e.g. heart disease, osteoporosis etc), but it also applies to a large proportion of pharmaceuticals in other disease areas. I will come back to this below.
Chair’s response: I agree with Dr Sculpher that patient heterogeneity within an indication is an important consideration. As a result of this heterogeneity, there might be specific patient subgroups within an indication that are more cost-effective to treat than others.
In principle, the implications for consumer and producer surplus of setting a single ceiling price across patient subgroups within an indication are similar to those associated with pricing across multiple indications (as considered in the Conceptual Framework). Among many possible approaches, the ceiling price might be informed by the price at which:
- The most cost-effective patient subgroup is ‘just’ cost-effective to treat (resulting in negative overall consumer surplus within the indication in question);
- The least cost-effective patient subgroup is ‘just’ cost-effective to treat (resulting in positive overall consumer surplus within the indication in question);
- The ‘average’ patient within the subgroup is ‘just’ cost-effective to treat (resulting in zero overall consumer surplus within the indication in question).
The second area where I would have expected more to be said relates to why there should be interest in producer surplus. A good deal of the report (most notably the first appendix on the conceptual framework) focuses on the balance between producer and consumer surplus, but the interest in the former is surely only because of its anticipated link with enhanced consumer surplus in the future. The challenge is that there is little evidence on how much producer surplus is necessary to generate future consumer surplus, particularly in an individual and relatively small market. So much of what is in the report hinges on how much producer surplus (or probability of that surplus) should the system ‘give away’ now to incentivize research and development to generate future consumer surplus, but there is no discussion about how this might be determined given existing evidence.
Chair’s response: It is for policy makers to decide upon the appropriate balance between consumer and producer surplus. The Working Group therefore did not take a position on whether greater producer surplus is inherently desirable or, as Dr Sculpher suggests, is desirable only if it results in greater consumer surplus in the future. Instead, the Working Group considered some of the potential implications for producer surplus associated with various possible approaches for informing a ceiling price.
A related issue here is that the report often talks about consumer and producer surplus during a product’s patent period as if it exists in perpetuity. For example, on page 26, in looking at the implications of a different supply-side thresholds across provinces. The implications of a patent ending for prices and consumer and producer surpluses under different policies seems relevant to consider.
Chair’s response: The Working Group discussed the potential for prices to fall following patent expiry, with implications for the allocation of consumer and producer surplus over the long term. However, in a July 2018 document prepared for the Working Group (Appendix 5.4), the PMPRB clarified that the purpose of the PMPRB is to ensure that patentees “do not charge excessive prices during the statutory monopoly period”. As a result, the Working Group focused only on the price during the statutory monopoly period.
The final element of evidence and analysis on which I would have expected to see more relates to uncertainty. There is good coverage of the underlying challenges of uncertainty in the evidence and modelling and its implications for decision uncertainty, but I was surprised there was not more on policy responses to this and implications for ceiling prices. I am thinking here about frameworks that consider the value of additional evidence, whether evidence can be generated alongside reimbursement, the implications for irreversible costs and the importance of a product’s price and its flexibility (e.g. Claxton et al). A reasonable response to this critique is that PMPRB only have one ‘policy decision’ in the domain of value and resources, namely setting a ceiling price. But more could perhaps have been said about what this means for provincial HTA bodies which could, in principle, have other policy levers at their disposal such as funding only in research, funding alongside research and further price reductions.
Chair’s response: I agree with Dr Sculpher that uncertainty has important implications for provincial decision makers, who may have a variety of policy levers at their disposal. However, the purpose of the Working Group was to provide specific technical recommendations to the Steering Committee regarding how the PMPRB might inform a ceiling price for a new medicine, so these implications were considered out of scope.
Specific comments
Page 22: The implication here is that supply side thresholds are only relevant to systems with a constrained budget. This is not the case: all systems have many other opportunities to enhance patient benefits, so incur opportunity costs when they make investment decisions (see Sculpher et al).
Chair’s response: I agree with Dr Sculpher. The text on p.22 has been revised to remove reference to a “constrained budget”.
Page 28: It may be worth emphasising that any equity weights used as part of analysis supporting pricing and reimbursement decisions should also be applied to the empirical supply-side threshold.
Chair’s response: I agree with Dr Sculpher that equity weights, if adopted, should also be applied to patients who bear the opportunity cost. Approaches for doing this include weighting the QALYs forgone directly, or adjusting the cost-effectiveness threshold. However, the latter approach has limitations that do not apply to direct QALY weighting.19 The existing text notes that “there is also an ongoing and unresolved debate regarding whether weights should be applied directly to QALYs or to the cost-effectiveness threshold”. I have not made any revisions to the text in response to this comment.
Page 29: I was unclear how the PMPRB process would give information about the location of the demand curve if its focus is the maximum price a product should command in Canada. There would presumably also need to be information about the relationship between lower prices that might emerge from the provincial HTA process and volumes.
Chair’s response: The Working Group recommended that “any estimate of the supply-side threshold adopted by the PMPRB for the purposes of informing a price ceiling be clearly specified, so as to reduce uncertainty for stakeholders” (Recommendation 2.7).
This would provide information to stakeholders on the location of the demand curve, given the incremental cost and effectiveness of the medicine in question.
Regarding the supply curve for new medicines, are there examples of any health system being able to estimate this credibly? I am not aware of any and, if that’s the case, it would be helpful to reflect on its implications for the PMPRB process.
Chair’s response: Difficulties associated with estimating supply curves, and some potential implications for the PMPRB, are noted throughout the Conceptual Framework. These implications include the potential that ceiling prices might be lowered to the extent that new medicines are not launched, potentially resulting in a loss in economic surplus and negating any positive consumer surplus that might otherwise have arisen.
Page 32: There may be a case to mention in Section 2.3.9 the distinction between a policy threshold (i.e. the cost per QALYs (or its range) which generally leads to a positive funding/pricing decision) and an empirical estimate of the supply-side threshold. These are often confused in my experience and the examples of ‘thresholds’ quoted in this page are instances of the former rather than the latter.
Chair’s response: I agree with Dr Sculpher. The text on p.32 has been revised to change all references to a non-supply-side “threshold” to “policy threshold”.
Page 34-35: This section could be repeated in the context of patient sub-groups by indication, but I saw no mention of this.
Chair’s response: I agree with Dr Sculpher that individual level heterogeneity is an important consideration. However, since the Working Group did not explicitly consider approaches for setting a ceiling price across heterogeneous patient subgroups within a single indication, I have not modified the text in this section.
I return to the point mentioned under ‘general comments’, that if PMPRB is defining a maximum price, then surely option 2 is appropriate. This would allow provinces to make decisions and undertake negotiations that involve bringing the price down so that other indications are also cost-effective.
This point could be generalised to cover deliberations regarding the choice of supply-side threshold (given variation across jurisdictions), patient sub-groups and reflecting uncertainty. That is, the PMPRB defines a maximum price, and the provinces may come down from that to reflect lower supply-side thresholds, agreement to include more sub-groups as well as indications, and the implications of uncertainty.
Chair’s response: As noted earlier, the choice of which approach to adopt is ultimately a matter for policy makers. As a result, the Working Group did not advocate for any specific approach but instead explored the technical implications of several possible approaches.
Page 38: I wonder whether invoking the concept of ‘risk neutrality’ and ‘risk aversion’ is helpful here. The underlying normative starting point for the report is a set of objectives relating to population health (perhaps augmented with equity considerations), rather than an unspecified utility function. What role is there, therefore, is considering risk preferences?
Chair’s response: As noted in the Conceptual Framework, uncertainty raises the potential that the actual impact of a new medicine on population health at a given ceiling price is negative, even if the expected impact on population health is zero.
If the PMPRB is ‘risk neutral’ then this is offset by the possibility that the actual impact on population health is positive, such that no adjustment is needed to the ceiling price.
However, if the PMPRB is adverse to the risk that the actual impact on population health is negative, then it may wish to lower the ceiling price. This would increase the expected impact on population health and reduce the risk that the actual impact is negative.
The latter position represents a departure from the default assumption of risk neutrality. However, the implied objective is still “related to population health (perhaps augmented with equity considerations)”. Specifically, the implied objective is related not only to the expected population health but also the distribution of uncertainty around the expected population health, in both cases potentially augmented with equity considerations.
The Working Group was unaware of the PMPRB’s precise risk attitude, and did not attempt to specify a “utility function” to account for any potential risk aversion. Rather, the Working Group acknowledged that the PMPRB might adopt an approach to risk that departs from the default assumption of risk neutrality, and noted that this would have implications for the specification of a ceiling price.
Page 45: The term ‘societal perspective’ is used quite loosely here. It may be helpful to be more specific about what this means and how it aligns with a general normative starting point of objectives relating to population health and opportunity costs relating to health care resources.
Chair’s response: On the previous page, reference is made to the CADTH guidelines which explicitly specify the differences between a ‘public health care system’ and ‘societal’ perspective (see “Differences between perspectives” on p.44).
I agree with Dr Sculpher that the use of a societal perspective raises important questions regarding the normative position with respect to population health and opportunity costs. As noted in the text, one Working Group member argued that “adopting a societal perspective implies that policy makers are willing to trade health benefits for other societal benefits, which may not be the case”. Other members noted that a societal perspective raises “ethical concerns, including the potential for productivity to be valued less for those with lower earning power”, which may not align with the preferred normative position. I have not made any modifications to the text in this section in response to this comment.
References
- Ochalek, J., Lomas, J. & Claxton, K. Assessing health opportunity costs for the Canadian health care systems. (University of York, 2018).
- Pandey, H., Paulden, M. & McCabe, C. Theoretical models of the cost-effectiveness threshold, value assessment, and health care system sustainability. (Institute of Health Economics, 2018).
- International statistics: Compare countries on just about anything! NationMaster.com. Available at: https://www.nationmaster.com/. (Accessed: 17th February 2019)
- Bound, J., Jaeger, D. A. & Baker, R. The Cure Can Be Worse than the Disease: A Cautionary Tale Regarding Instrumental Variables. (1993). doi:10.3386/t0137
- Bound, J., Jaeger, D. A. & Baker, R. M. Problems with Instrumental Variables Estimation when the Correlation between the Instruments and the Endogenous Explanatory Variable is Weak. J. Am. Stat. Assoc. 90, 443–450 (1995).
- Chao, J. C. & Swanson, N. R. Consistent Estimation with a Large Number of Weak Instruments. Econometrica 73, 1673–1692 (2005).
- Estimating Cost Effectiveness Thresholds for Canadian Provinces (NOAHE Seminar) (Question on IVs at 48:20). (NOAHE, 2018).
- Paulden, M., O’Mahony, J. & McCabe, C. Determinants of Change in the Cost-effectiveness Threshold. Med. Decis. Making 37, 264–276 (2017).
- Lomas, J., Claxton, K., Martin, S. & Soares, M. Resolving the ‘Cost-Effective but Unaffordable’ Paradox: Estimating the Health Opportunity Costs of Nonmarginal Budget Impacts. Value Health (2018). doi:10.1016/j.jval.2017.10.006
- Claxton, K. et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol. Assess. 19, (2015).
- Canadian Agency for Drugs and Technologies in Health (CADTH). Guidelines for the Economic Evaluation of Health Technologies: Canada (4th Edition). (CADTH, 2017).
- Hampson, G., Mott, D., Shah, K. & Others. Public Preferences for Health Gains and Cures: A Discrete Choice Experiment. (Office of Health Economics, 2019).
- Bentley, C. et al. Trade-offs, fairness, and funding for cancer drugs: key findings from a deliberative public engagement event in British Columbia, Canada. BMC Health Serv. Res. 18, 339 (2018).
- Stafinski, T. & Menon, D. Explicating social values for resource allocation decisions on new cancer technologies: We, the jury, find·. Journal of Cancer Policy 14, 5–10 (2017).
- Carman, K. L. et al. Effectiveness of public deliberation methods for gathering input on issues in healthcare: Results from a randomized trial. Soc. Sci. Med. 133, 11–20 (2015).
- van Exel, J., Baker, R., Mason, H., Donaldson, C. & Brouwer, W. Public views on principles for health care priority setting: Findings of a European cross-country study using Q methodology. Soc. Sci. Med. 126, 128–137 (2015).
- Ratcliffe, J., Lancsar, E., Walker, R. & Gu, Y. Understanding what matters: An exploratory study to investigate the views of the general public for priority setting criteria in health care. Health Policy 121, 653–662 (2017).
- Menon, D. & Stafinski, T. Engaging the public in priority-setting for health technology assessment: findings from a citizens’ jury. Health Expect. 11, 282–293 (2008).
- Mike Paulden, James F O’Mahony, Anthony J Culyer & Christopher McCabe. Some inconsistencies in NICE’s consideration of social values. Pharmacoeconomics 32, 1043–1053 (2014).
- Buist, S. New cancer drugs cost more than $10,000 each month. The Hamilton Spectator (2016).
- Marin, A. A Vast Injustice. (Ombudsman of Ontario, 2009).
- Vallejo-Torres, L., García-Lorenzo, B. & Serrano-Aguilar, P. Estimating a cost-effectiveness threshold for the Spanish NHS. Health Econ. (2017). doi:10.1002/hec.3633
- Edney, L. C., Haji Ali Afzali, H., Cheng, T. C. & Karnon, J. Estimating the Reference Incremental Cost-Effectiveness Ratio for the Australian Health System. Pharmacoeconomics (2017). doi:10.1007/s40273-017-0585-2
- Towse, A., Cole, A., Zamora, B. & Others. The Debate on Indication-Based Pricing in the US and Five Major European Countries. London: OEH Consulting (2018).
- Pearson, S. D., Dreitlein, W. B., Henshall, C. & Towse, A. Indication-specific pricing of pharmaceuticals in the US healthcare system. Journal of Comparative Effectiveness Research 6, 397–404 (2017).
- Institut national d’excellence en santé et en services sociaux (INESSS). Guidance Document for Submitting a Request to INESSS. (INESSS, 2018).
- Arrow, K. J. & Lind, R. C. Uncertainty and the Evaluation of Public Investment Decisions. Am. Econ. Rev. 60, 364–378 (1970).
- Pekarsky, B. Trust, constraints and the counterfactual: Reframing the political economy of new drugs. University of Adelaide (2012). doi:10.1007/978-3-319-08903-4_3
- Eckermann, S. & Pekarsky, B. Can the real opportunity cost stand up: displaced services, the straw man outside the room. Pharmacoeconomics 32, 319–325 (2014).
- Jayasundara, K. et al. Estimating the clinical cost of drug development for orphan versus non-orphan drugs. Orphanet J. Rare Dis. 14, 12 (2019).
- Berdud, M., Drummond, M. F. & Towse, A. Establishing a reasonable price for an orphan drug. OHE Research Paper. London, Office of Health Economics (2018).