PMPRB Framework Modernization - Proposed Application of PE and Market Size Factors to Category 1 Drugs
Disclaimer
This document is not to be viewed as a definitive interpretation of the current or proposed legislation or of the RIAS for the proposed amendments by the PMPRB, is not the Government’s expression of policy intent or an official part of the consultation, and is not intended to bind the PMRPB or the Government in the application and interpretation of legislation.
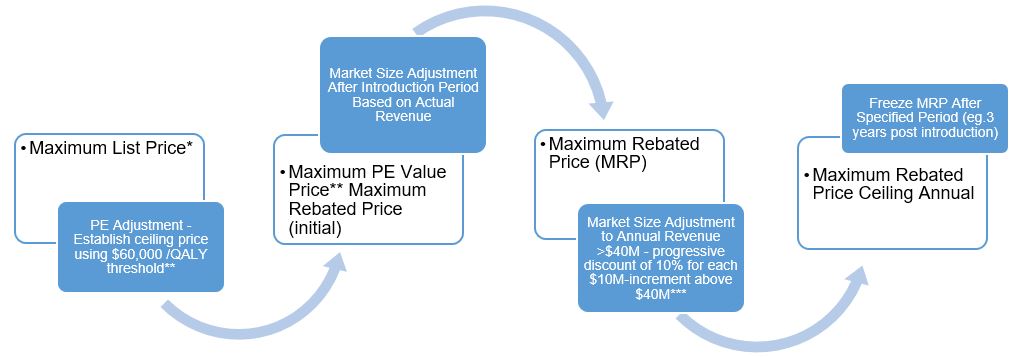
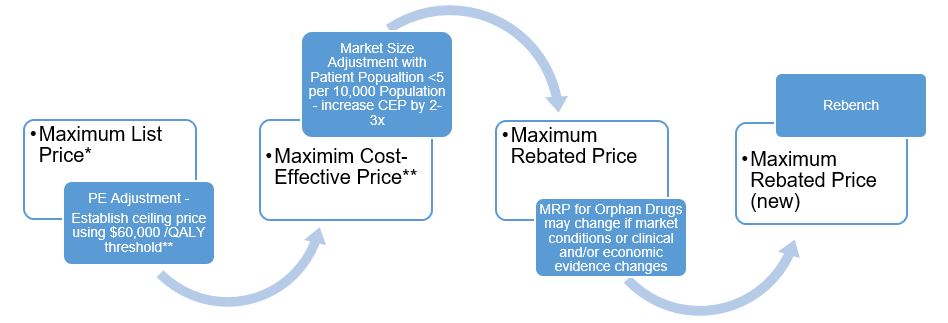
Category 1 drugs are very expensive, new, patented drugs that are an effective treatment for a life threatening and/or debilitating disease. Under the proposed new guidelines, the PMPRB will assign two distinct price ceilings to each Category 1 drugFootnote 1 – the Maximum List Price (MLP) and the Maximum Rebated Price (MRP). The factors related to pharmacoeconomic (PE) value and market size will be applied, in that order, to set the MRPFootnote 2 for a Category 1 drug. The way in which each of the factors will be applied to render the MRP of a Category 1 drug is described in figures 1 and 2. These figures demonstrate approaches to getting to an MRP ceiling that involves a decrease from the PE value price (large market size) or an increase from the maximum PE value price (small market size e.g. orphan drugs).
Figure 1. Getting to MRP for Category 1 Drugs (Non-Orphan)
Long description
-
Maximum List Price*
- PE Adjustment - Establish ceiling price using $60,000 /QALY threshold**
- Maximum PE Value Price** Maximum Rebated Price (initial)
- Market Size Adjustment After Introduction Period Based on Actual Revenue
- Maximum Rebated Price (MRP)
- Market Size Adjustment to Annual Revenue >$40M - progressive discount of 10% for each $10M-increment above $40M***
- Maximum Rebated Price Ceiling Annual
- Freeze MRP After Specified Period (eg.3 years post introduction)
Figure 2. Getting to MRP for Category 1 Orphan Drugs
Long description
-
Maximum List Price*
- PE Adjustment - Establish ceiling price using $60,000 /QALY threshold**
- Maximim Cost-Effective Price**
- Market Size Adjustment with Patient Popualtion <5 per 10,000 Population - increase CEP by 2-3x
- Maximum Rebated Price
- MRP for Orphan Drugs may change if market conditions or clinical and/or economic evidence changes
- Maximum Rebated Price (new)
* The Maximum List Price (MLP) is the lower amount generated by the median international price comparison (MIPC) test or the therapeutic class comparison (TCC) test. This test applies to any new drug (Category 1 and Category 2).
** The Pharmacoeconomic (PE) Value Price is the maximum treatment cost under a set cost per quality-adjusted life year gained ($/QALY) threshold. Best available evidence for Canada suggests that the health care system is currently paying $30,000 to purchase an additional QALY. This implies that a drug whose incremental cost-effectiveness ratio (ICER) exceeds this threshold would generate a net health loss. In order to apply this evidence in the national PMPRB context, setting the baseline $/QALY threshold at two-times that amount ($60,000) is proposed.
*** The Market Size Adjustment for high-impact drugs is based on the annual reported revenues after the initial year. Adjustment to the PE value price ceiling to get to the MRP would reflect annual sales surpassing $40M.Footnote 3 The reduction in MRP involves a progressive discounting methodology. The progressive discount applies to the total annual drug cost where each successive $10M above $40M is discounted by an additional 10%, up to a maximum of 50%. Conversely, market size may be used to increase the ceiling for medicines with small market sizes such as rare diseases. In general, the practice is to adjust the value of the ICER, however, the approach that is being proposed by the PMPRB would use the same baseline ICER threshold, but adjust the final price ceiling up or down taking market size into consideration. The MRP could be adjusted upwards by as much as two to three times depending on the drug and other considerations or modifiers as discussed previously (e.g. drugs for rare disorders).