Patented Medicine Prices Review Board
The Honourable Ginette Petitpas Taylor
Minister of Health
ISSN 2561-0732
Table of Contents
Chairperson’s message
Results at a glance
Raison d’être, mandate and role: who we are and what we do
Operating context and key risks
Results: what we achieved
Analysis of trends in spending and human resources
Supplementary information
Appendix: definitions
Endnotes
Chairperson’s Message
As acting Chairperson, I am pleased to present the Patented Medicine Prices Review Board (PMPRB)’s 2016-17 Departmental Results Report.
The PMPRB is an independent, quasi-judicial administrative agency with a mandate to protect consumers from excessively priced patented medicines and to report to Canadians the latest price trends of all medicines, and on patentees’ research and development spending in Canada.
In December 2015, the PMPRB published its much anticipated 2015-2018 Strategic Plan, an important turning point in the organization’s history as it looks to reform how it carries out its consumer protection mandate in light of recent significant changes in its operating environment. 2016 marked the launch of the Rethinking the Guidelines consultation initiative, which is a key first step in the PMPRB’s efforts to modernize its regulatory framework. The consultation process will resume following publication of the Minister of Health’s recently proposed amendments to the Patented Medicines Regulations in Part I of the Canada Gazette, which is anticipated to take place in the fall of 2017.
The PMPRB’s renewed emphasis on consumer-focused regulation made for another busy year of compliance and enforcement activity in 2016 with the acceptance of eight Voluntary Compliance Undertakings (VCUs) and the paying back of excess revenues totalling $35,856,156.83, in addition to price reductions for the affected drug products.
In terms of its reporting mandate, the PMPRB continued to build strategic partnerships and raise public awareness of its mandate by being more responsive to the specific information needs of pharmaceutical payers while at the same time expanding the scope of its reporting to appeal to a broader stakeholder audience. An example of the latter was the release of the first edition of the new National Prescription Drug Utilization Information System (NPDUIS), Meds Entry Watch publication. An example of the former is the PMPRB’s Market Intelligence Report: Biologic Response Modifying Agents, the first in a series of reports designed to provide greater insight on specific therapeutic market segments of particular importance to public and private payers in Canada.
Budget 2017 proposed a significant increase in funding for the PMPRB, as part of the Government’s commitment to making prescription drugs more accessible and affordable for Canadians. We consider this a vote of confidence in the PMPRB’s potential to play a more meaningful and relevant role in the sustainability of Canada’s health systems. The PMPRB will celebrate its 30 year anniversary in December 2017 and the timing could not be more opportune to celebrate past successes while embracing a very promising future.
Dr. Mitchell Levine
Results at a glance
Actual Spending: $10,133,959
Actual FTEs: 63.7
For more information on the department’s plans, priorities and results achieved, see the “Results: what we achieved” section of this report.
Regulatory Mandate
- In June 2016, the PMPRB launched Phase 1 of the Rethinking the Guidelines consultation initiative, with the release of its Guidelines modernization discussion paper. The PMPRB received 65 submissions from patients, provinces, academics, private insurers, pharmaceutical companies, health professionals, and advocacy groups representing more than 500 Canadian pharmaceutical stakeholders. In May 2017, the Minister announced her intention to move forward with amendments to the Patented Medicines Regulations to equip the PMPRB with more relevant and effective regulatory tools to protect Canadians from excessive prices. This initiative builds on the written submissions received by the PMPRB in response to its June 2016 discussion paper.
- The PMPRB accepted eight Voluntary Compliance Undertakings (VCUs)Footnote i which resulted in price reductions for certain drug products, and the paying back of excess revenues totalling $35,856,156.83 by way of payments to the Government of Canada.
- Closing argument in the PMPRB’s first excessive price hearing in several years, and the first such case in which both public and private insurers sought to participate, took place in April 2017.
Reporting Mandate
- In addition to tabling the 2015 Annual Report, and the PMPRB Guidelines Modernization – Discussion Paper, June 2016, the PMPRB published the following analytical reports:
Market Intelligence Report: Biologic Response Modifier Agents, 2015, October 2016
Meds Entry Watch, 2015, April, 2017;
CompassRx Annual Public Drug Plan Expenditure Report, 3rd Edition, May, 2017;
Raison d’être, mandate and role: who we are and what we do
Raison d’être
The Patented Medicine Prices Review Board (PMPRB) is an independent, quasi-judicial body created by Parliament in 1987. Its mandate is twofold:
- Regulatory – to ensure that prices charged by patentees for patented medicines sold in Canada are not excessive; and
- Reporting – to report on pharmaceutical trends of all medicines and on research and development (R&D) spending by pharmaceutical patentees.
In carrying out its mandate, the PMPRB ensures that Canadians are protected from excessive prices for patented medicines sold in Canada and that stakeholders are informed on pharmaceutical trends.
Mandate and role
The PMPRB was created as a result of amendments to the Patent Act (Act) in 1987 (Bill C-22), and its remedial powers were supplemented by further amendments in 1993 (Bill C-91). These amendments were part of policy reforms intended to balance consumer protection with measures intended to encourage R&D investment by pharmaceutical patentees.
The PMPRB has a dual mandate:
Regulatory
The PMPRB is responsible for ensuring the factory-gate prices that patentees charge for prescription and non-prescription patented medicines sold in Canada to wholesalers, hospitals, pharmacies or others, for human and veterinary use, are not excessive. The PMPRB regulates the price of each patented medicine to which Health Canada has assigned a Drug Identification Number (DIN) as part of its price review process. The PMPRB’s mandate also includes medicines that are available under the Special Access Programme, through a Clinical Trial Application, and Investigational New Drug Products. Over-the-counter (OTC) patented medicines and patented medicines for veterinary use are regulated by the PMPRB on a complaints basis.
Reporting
The PMPRB reports annually to Parliament through the Minister of Health on its price review activities, the prices of patented medicines and price trends of all prescription drugs, and on the R&D expenditures reported by pharmaceutical patentees. In addition, as a result of the establishment of the NPDUISFootnote ii by federal/provincial/territorial (F/P/T) Ministers of Health in September 2001, the PMPRB conducts critical analysis of price, utilization, and cost trends for patented and non-patented prescription drugs to provide Canada’s health system with more comprehensive, accurate information on how all prescription drugs are being used and on the sources of cost increases. This function is aimed at providing F/P/T governments and other interested stakeholders with a centralized credible source of information on pharmaceutical trends. Increasingly, as part of its reporting function, the PMPRB works closely with provincial and territorial (P/T) governments through NPDUIS, and directly with lead jurisdictions through the Council of the Federation, to provide relevant pricing and market analyses aimed at reducing the prices of prescription drugs purchased by public payers in Canada.
For more general information about the department, see the “Supplementary information” section of this report. For more information on the department’s organizational mandate letter commitments, see the Minister’s mandate letter.Footnote iii
Operating context and key risks
Operating context
Prescription drugs are an increasingly important part of the health care system, helping prevent and cure disease as well as save lives. However, Canada’s drug spending, which is high by international standards, is not producing all of the benefits it should.
Drug spending in Canada has increased from less than 10% of total health expenditures, when Medicare was first established, to about 16% today. Drugs are now the second-largest category of spending in health care, ahead of physician services with per capita spending on drugs second only to the United States. Canada is paying higher prices for prescription drugs than most other developed countries which can result in limited access to innovative medicines, place a financial burden on patients, and mean fewer resources for other critical areas of the health care system.
In January 2016, federal, provincial and territorial Ministers agreed to work together to improve the affordability, accessibility and appropriate use of prescription drugs to better meet health care system needs. The federal government is firmly committed to this work and is taking action to significantly lower the cost of prescription drugs; provide faster access to new drugs that are safe and effective and better meet the needs of the health care system; and support the development of tools for more appropriate prescribing. To support these actions, Budget 2017 outlined an investment of $140.3 million over 5 years, starting in 2017, and $18.2 million ongoing. These funds are earmarked for Health Canada, the PMPRB, and CADTH to modernize their therapeutic products review processes to improve access to prescription medications, lower drug prices and support appropriate prescribing.
In May 2017, the Minister outlined a comprehensive plan for improving the pharmaceutical system in Canada. Included in the plan was the launch of a consultation processFootnote iv on potential changes to the Patented Medicines Regulations (Regulations) that would lower the cost of prescription drugs in Canada and better protect Canadians against excessive drug prices. Health Canada’s consultation document outlining these potential regulatory amendments builds on the written submissions received by the PMPRB in response to its recent Rethinking the GuidelinesFootnote v consultation. Given the interdependency of the Regulations and the Guidelines, the PMPRB will await the outcome of the Regulations amendment process before resuming its consultations on Guideline modernization.
Key risks
blank
| Risks |
Mitigating strategy and effectiveness |
Link to the department’s Programs |
Link to mandate letter commitments or to government-wide and departmental priorities |
| There is a risk that Canadian patented drug prices – especially for high cost specialty drugs – will continue to rise to levels that become unsustainable for public and private payers. |
- The PMPRB is modernizing its regulatory framework, through changes to both its Guidelines and Regulations so that it is equipped with more effective and relevant tools to protect Canadians from excessively priced patented drugs in today’s pharmaceutical environment.
- In June 2016, the PMPRB launched consultations on Guideline reform. In May 2017, the Minister launched consultations on proposed amendments to PMPRB regulations.
|
Patented Medicine Prices Regulation Program |
The proposal aligns with Government of Canada priorities, the Minister of Health’s mandate letter, and supports Health Canada’s mandate to help Canadians to maintain and improve their health.
PMPRB priorities:
Framework modernization
|
| There is a risk that the PMPRB will not be able to attract/retain individuals with the specialized skill sets and expertise to operationalize the new excessive pricing factors contemplated under the Minister’s proposed amendments to the regulations. |
- The PMPRB will take a flexible and pragmatic approach to staffing that will enable it to identify and hire individuals in both the public and private sector with the requisite education, background and experience in a timely fashion.
|
Internal Services |
The proposal aligns with the PSC’s New Direction in Staffing.
PMPRB’s priorities:
Employee engagement
|
| There are multiple outstanding court cases that seek to challenge the PMPRB’s jurisdiction and/or the constitutionality of its enabling provisions. There is a risk that these cases may circumscribe the PMPRB’s jurisdiction and make it less able to carry out its consumer protection mandate. |
- The PMPRB will continue to work closely with the Attorney General in defending the remaining cases and any future cases so as to mitigate any risk that consumer protection powers will be curtailed as a result of an adverse decision. In 2016-17, the Federal Court sided with the AG in dismissing two judicial reviews of PMPRB Board decisions.
|
Patented Medicine Prices Regulation Program |
The proposal aligns with the Minister of Health’s mandate letter commitment to affordable prescription drugs
PMPRB’s priorities:
Consumer-focused regulation and reporting
Strategic partnerships and public awareness
|
In the last twenty years, the global environment for pharmaceuticals has significantly changed. Two such changes are of particular relevance in this context: 1) the emergence of higher cost drugs, such as biologics and genetic therapies that are putting increasing pressure on drug spending; and 2) a growing discrepancy between public list prices and lower actual market prices due to the increased use of confidential discounts and rebates.
These changes have led other countries to experiment with new forms of cost containment that are more reliant on assessing the economic value of a new drug to their respective health systems and less on comparing drug prices internationally. The lack of corresponding reform in Canada has contributed to rising Canadian patented drug prices which are among the highest in the world as well as relatively high pharmaceutical spending per capita and as a proportion of GDP.
As a result of its changing operating environment the PMPRB felt it necessary to examine the need for corresponding changes to modernize its regulatory framework. As a first step in this process, the PMPRB launched a public consultation to obtain submissions from stakeholders and the public regarding its Guidelines. The consultation resulted in 65 written submissions from patients, provinces, academics, private insurers, pharmaceutical companies, health professionals, and advocacy groups representing more than 500 Canadian pharmaceutical stakeholders.
Building on the stakeholder feedback, in May 2017, the Minister of Health outlined a comprehensive plan for improving the pharmaceutical system in Canada. Included in the plan was the launch of a consultation process on potential changes to the Patented Medicines Regulations (Regulations) that would equip the PMPRB with enhanced regulatory tools to lower the cost of prescription drugs in Canada and better protect Canadians against excessive drug prices. This online consultation ended June 28, 2017. Given the interdependency of the Regulations and the Guidelines, the PMPRB will await the outcome of Health Canada’s consultation process before resuming its consultations on Guideline modernization.
Results: what we achieved
Programs
Patented Medicine Prices Regulation Program
Description
The PMPRB is an independent quasi-judicial body that is responsible for ensuring that the prices that patentees charge for patented medicines sold in Canada are not excessive based on the price review factors in the Act. To make this determination the Board must consider each of the following factors: prices at which the medicine and other medicines in the same therapeutic class have been sold in Canada and in the seven comparator countries listed in the Patented Medicines Regulations (Regulations); changes in the Consumer Price Index (CPI); and in accordance with the Act, such other factors as may be specified in any regulations made for the purposes of the price review.Footnote vi Under the Act, and as per the Regulations, patentees are required to file price and sales information for each patented medicine sold in Canada, for the duration of the patent(s). Board Staff reviews the introductory and ongoing information filed by patentees, for all patented medicines sold in Canada. When it finds that the price of a patented medicine appears to be excessive, Board Staff will conduct an investigation into the price. An investigation could result in: its closure, where it is concluded that the price was non-excessive; a Voluntary Compliance Undertaking (VCU) by the patentee to reduce the price and offset excess revenues obtained as a result of excessive prices through a payment and/or a price reduction of another patented drug product; or a public hearing to determine if the price is excessive, including any remedial order determined by the Board. In the event that the Board Hearing Panel finds, after a public hearing, that a price is or was excessive, it may order the patentee to reduce the price and take measures to offset any excess revenues. This program, by reviewing the prices charged by patentees for patented medicines sold in Canada, protects Canadians and the health care system from excessive prices.
Results achieved
| Expected results |
Performance indicators |
Target |
Date to achieve target |
2016–17
Actual results |
2015–16
Actual results |
2014–15
Actual results |
| Patentees comply with the Patent Act, the Regulations, and the Excessive Price Guidelines (Guidelines) |
Percentage of patented medicines that are priced within the Guidelines, or at a price which does not trigger the investigation criteria, as a result of voluntary compliance |
95%Footnote vii |
March 31 of each year |
92.3%Footnote viii |
93% |
95.3% |
| Percentage of compliance with Board Orders related to price and/or jurisdiction and with Voluntary Compliance Undertakings (VCUs) |
100% |
March 31 of each year |
100% |
100% |
100% |
| Canadian prices for patented medicines are below the median of international prices |
50% |
March 31 of each year |
58% |
n/aFootnote ix |
n/a |
Budgetary financial resources (dollars)
blank
2016–17
Main Estimates |
2016–17
Planned spending |
2016–17
Total authorities available for use
|
2016–17
Actual spending
(authorities used)
|
2016–17
Difference
(actual minus planned)
|
| 6,646,758 |
6,646,758 |
6,834,705 |
6,098,659 |
(548,099) |
Human resources (full-time equivalents)
blank
2016–17
Planned |
2016–17
Actual |
2016–17
Difference
(actual minus planned) |
| 40.0 |
31.1 |
(8.9) |
The PMPRB’s Compliance Policy was founded on the premise that the most effective and efficient way to protect the public from excessive prices and achieve maximum compliance was through primary reliance on voluntary action by patentees. In spite of the fact the PMPRB has consistently enjoyed a high rate of compliance with its Guidelines, in recent years Canadian patented drug prices have been steadily rising relative to prices in the PMPRB7.
In 2016, Canadian prices were decidedly above prices in the United Kingdom, France, Italy and Sweden. Furthermore, as reported in the PMPRB’s 2016 Annual ReportFootnote x, Canadian prices are on average 20% above median OECD prices. Canadian prices are third highest among the 31 OECD countries, behind only the United States and Switzerland and on par with Germany.
The PMPRB’s existing regulatory framework is not sufficiently equipped to address the current and anticipated future pricing challenges. For instance, in recent years, many pharmaceutical manufacturers have directed their R&D efforts towards more severe diseases afflicting smaller patient populations. The successful drugs that emerge from this type of R&D (often referred to as specialty drugs) tend to be priced at increasingly higher levels to reflect the different market realities associated with the size of the population, the effectiveness of the drug and the lack of therapeutic choice or competition. The current framework, which has not been substantially changed in more than two decades, does not take these factors into consideration. It also has no provisions for considering whether the price of a drug reflects its value to patients or the size of its potential market.
2016 marked the launch of the Rethinking the Guidelines consultation initiative, which is a key first step in the PMPRB’s efforts to modernize its regulatory framework. Phase 1 of this consultation, which ran from June to October 2016, sought stakeholder and public feedback on the Guidelines Modernization Discussion PaperFootnote xi. The written submissions received from interested parties in response to the discussion paper have been made available online and the PMPRB-led consultation process will resume following publication of the Minister of Health’s recently proposed amendments to the Patented Medicines Regulations in Part I of the Canada Gazette, which is anticipated to take place in the fall.
The PMPRB’s renewed emphasis on consumer-focused regulation made for another busy year of compliance and enforcement activity in 2016-17 with the acceptance of eight VCUs and the paying back of excess revenues totalling $35,856,156.83 in addition to reductions in price for the affected drug products. In addition, the PMPRB’s first excessive price hearing in several years, and the first such case in which both public and private insurers sought to participate, continued to wind its way to disposition on the merits, with closing argument taking place in the spring of 2017.
This program has no lower-level programs.
Pharmaceutical Trends Program
Description
The PMPRB reports annually to Parliament through the Minister of Health on its price review activities, the prices of patented medicines and price trends for all drugs, and R&D expenditures as reported by pharmaceutical patentees. In supporting this requirement, the pharmaceutical trends program provides complete and accurate information on trends in manufacturers' prices of patented medicines sold in Canada and on patentees' research-and-development expenditures to interested stakeholders including: industry (i.e., brand-name, biotech, generic); F/P/T governments; consumer and patient advocacy groups; third party payers; and others. This information also provides assurance to Canadians that the prices of patented medicines are not excessive. In addition, as a result of the establishment of the NPDUIS by F/P/T Ministers of Health, the Federal Minister of Health requested that the PMPRB conduct analysis of price, utilization and cost trends for patented and non-patented prescription drugs so that Canada's health system has more comprehensive, accurate information on how all prescription drugs are being used and on the sources of cost increases. This function is aimed at providing F/P/T governments and other interested stakeholders with a centralized credible source of information on all prescription drug prices.
Results achieved
| Expected results |
Performance indicators |
Target |
Date to achieve target |
2016–17
Actual results |
2015–16
Actual results |
2014–15
Actual results |
| Information on pharmaceutical trends and cost drivers is available to stakeholders |
Number of new reports/studies posted on the PMPRB website |
12 reports/ studies |
March 31 of each year |
11 new reports/ studies |
15 new reports/ studies |
15 new reports/ studies |
| Number of presentations made by the PMPRB to an external audience |
10 information sessions |
March 31 of each year |
21 information sessions |
25 information sessions |
25 information sessions |
Budgetary financial resources (dollars)
blank
2016–17
Main Estimates |
2016–17
Planned spending |
2016–17
Total authorities available for use
|
2016–17
Actual spending
(authorities used)
|
2016–17
Difference
(actual minus planned)
|
| 1,704,508 |
1,704,508 |
1,739,258 |
1,616,278 |
(88,230) |
Human resources (full-time equivalents)
blank
2016–17
Planned |
2016–17
Actual |
2016–17
Difference
(actual minus planned) |
| 12.0 |
13.3 |
1.3 |
In 2016-17, the PMPRB continued its efforts to build strategic partnerships and raise public awareness of its mandate by being more responsive to the specific information needs of payers, while at the same time expanding on the scope of its reporting to appeal to a broader stakeholder audience. This included press release distribution, an emphasis on targeted social media campaigns, direct engagement with the public via social media as well as more traditional means (e.g., e-mail and telephone) and engagement with domestic, international and specialized media including the CBC, CTV, Radio-Canada, La Presse, The Globe and Mail, Toronto Star, the Canadian Medical Association Journal, Benefits Canada, CBS, Bloomberg News, Boston Globe, and VICE News.
The PMPRB also continued to support and strengthen its NPDUIS engagement activities by regularly consulting with the NPDUIS Advisory Committee, participating in conferences and stakeholder committees, hosting information exchange sessions with researchers, and organizing information sessions with interested stakeholders to share the results of the analytical studies. In 2016-17 the PMPRB, through the NPDUIS initiative, released two analytical reports and six posters. One of the analytical reports was the first edition of the new, NPDUIS, Meds Entry WatchFootnote xii publication. This will be an annual publication which explores the market entry dynamics of new drugs launched in Canada and other international markets. It is designed to inform decision makers, researchers and patients of the evolving market dynamics associated with emerging drug therapies.
The other publication was the PMPRB’s Market Intelligence Report on Biologic Response Modifying AgentsFootnote xiii; the first in a series of such reports which are designed to provide greater insight on specific therapeutic market segments of particular importance to public and private payers in Canada.
In addition to the aforementioned reports, the PMPRB tabled its 2015 Annual Report, published the PMPRB Guidelines Modernization Discussion Paper, 6 poster presentations and the third edition of CompassRx Annual Public Drug Plan Expenditure ReportFootnote xiv, May 2017.
Furthermore,the NPDUIS conducted a number of ad-hoc studies at the request of the NPDUIS participating jurisdictions.
This program has no lower-level programs.
Internal Services
Description
Internal Services are those groups of related activities and resources that the federal government considers to be services in support of programs and/or required to meet corporate obligations of an organization. Internal Services refers to the activities and resources of the 10 distinct service categories that support Program delivery in the organization, regardless of the Internal Services delivery model in a department. The 10 service categories are: Management and Oversight Services; Communications Services; Legal Services; Human Resources Management Services; Financial Management Services; Information Management Services; Information Technology Services; Real Property Services; Materiel Services; and Acquisition Services.
Results
Budgetary financial resources (dollars)
blank
2016–17
Main Estimates |
2016–17
Planned spending |
2016–17
Total authorities available for use |
2016–17
Actual spending
(authorities used) |
2016–17
Difference
(actual minus planned) |
| 2,613,842 |
2,613,842 |
2,670,837 |
2,419,022 |
(194,820) |
Human resources (full-time equivalents)
blank
2016–17
Planned |
2016–17
Actual |
2016–17
Difference
(actual minus planned) |
| 19.0 |
19.3 |
0.3 |
In its 2015-2018 Strategic Plan, employee engagement was enshrined as one of the PMPRB’s four strategic objectives. In March 2016, an Employee Engagement Strategy was formalized which lays out a plan for creating and maintaining a healthy, respectful and enabling workplace by embracing change, fostering effective communication, and championing recognition.
In 2016-17 the PMPRB continued its Speaker Series, a regularly scheduled forum that allows participating employees the opportunity to learn from and engage with colleagues, industry experts, and stakeholders from across the public, private and non-profit sectors on issues that impact their work and work environment.
An Employee Engagement Survey was conducted in June 2016 in response to a desire from the PMPRB’s Executive Director to ensure employees are given the opportunity to provide their views on the degree to which management is meeting its engagement objectives.
In February 2017, the PMPRB launched its new and improved intranet site, the Atrium, which provides up-to-date tools and mechanisms to facilitate dialogue between branches, management, and employees.
In December 2015, the PMPRB created a Workplace Improvement Team (WIT) comprised of employees, on a voluntary basis, from various groups and levels. The mandate of the WIT is to contribute to a positive change of culture and improvement to the work environment. To that, end, during the year, the WIT continued the implementation of the PMPRB engagement strategy. Initiatives completed include the development of responsibilities’ checklists for managers and employees, ensuring that all employees are aware of, and action, their responsibilities, and onboarding checklists for managers and employees, improving the onboarding experience for employees by ensuring all required tasks were completed for their arrival. The result of these initiatives has been an improvement to the engagement of the PMPRB, a goal to which the WIT continues to contribute.
2016-17 was the first year for the new Public Service Employee Annual Survey (PSEAS). In this survey, the PMPRB showed improvement across all the areas of its 2014 Public Service Employee Survey. The PMPRB also exceeded the results of all other micro-organizations, and the Public Service as a whole, on every indicator, including respect in the workplace, employee engagement, and workplace well-being. These results indicate that the PMPRB engagement strategy has been a success.
The PMPRB will continue to inform and engage employees in the strategic planning process, and provide them clear direction on work objectives and expected behaviours in order to promote a culture of consistent high performance. It will implement a comprehensive internal communications strategy to enable more structured dialogue between branches, management and employees and it will put systems in place to enable employees to rate their managers on the degree to which they are meeting their engagement objectives. The PMPRB will also provide access to a wide range of learning and developmental opportunities, including, but not limited to, mentoring and developmental assignments.
Analysis of trends in spending and human resources
Actual expenditures
Departmental spending trend graph
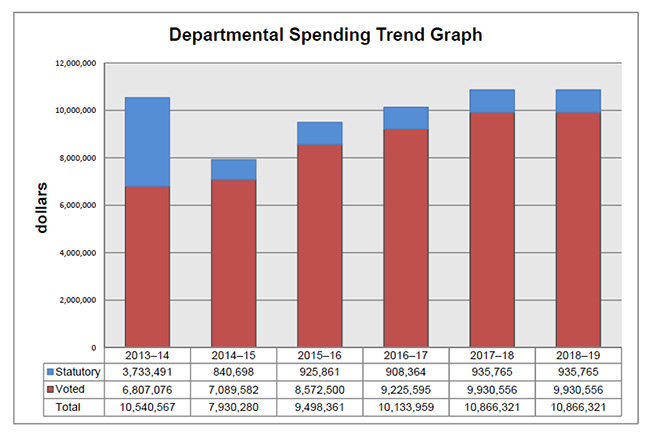
Departmental Spending Trend
The following graph shows the PMPRB's spending trend over time. It illustrates on a bar graph the actual statutory and voted spending for 2013-14 to 2016-17, and planned statutory and voted spending for 2017-18 and 2018-19.
blank
| |
2013-14 |
2014-15 |
2015-16 |
2016-17 |
2017-18 |
2018-19 |
| Statutory |
3,733,491 |
840,698 |
925,861 |
908,364 |
936,765 |
936,765 |
| Voted |
6,807,076 |
7,089,582 |
8,572,500 |
9,255,595 |
9,930,556 |
9,930,556 |
| Total |
10,540,567 |
7,930,280 |
9,498,361 |
10,133,959 |
10,866,321 |
10,866,321 |
Statutory spending in 2013-14 was significantly higher than statutory spending in subsequent years largely due to additional funding received through an adjustment warrant to cover the amount the PMPRB was ordered by the Federal Court to refund a patentee. The Federal Court quashed a Board Order and directed in its judgement that a payment of excess revenues in the sum of $2,801,285 be returned by the PMPRB to the patentee with appropriate interest and specified costs.
Budgetary performance summary for Programs and Internal Services (dollars)
blank
| Programs and Internal Services |
2016–17
Main Estimates |
2016–17
Planned spending |
2017–18
Planned spending |
2018–19
Planned spending |
2016–17
Total authorities available for use |
2016–17
Actual spending (authorities used) |
2015–16
Actual spending (authorities used) |
2014–15
Actual spending (authorities used) |
| Patented Medicine Prices Regulation Program |
6,646,758 |
a6,646,758 |
6,706,989 |
6,706,989 |
6,834,705 |
b6,098,659 |
c5,399,127 |
3,543,891 |
| Pharmaceutical Trends Program |
1,704,508 |
1,704,508 |
1,575,179 |
1,575,179 |
1,739,258 |
1,616,278 |
1,688,584 |
1,301,871 |
| Subtotal |
8,351,266 |
8,351,266 |
8,282,168 |
8,282,168 |
8,573,963 |
7,714,937 |
7,087,711 |
4,845,762 |
| Internal Services |
2,613,842 |
2,613,842 |
2,584,153 |
2,584,153 |
2,670,837 |
2,419,022 |
2,410,650 |
3,084,518 |
| Total |
10,965,108 |
10,965,108 |
10,866,321 |
10,866,321 |
11,244,800 |
10,133,959 |
9,498,361 |
7,930,280 |
a The PMPRB always bases planned spending on the assumption that it will spend the full $2.44 million held in the SPA reserved for conducting public hearings. This is done because these expenditures are dependent on the number of hearings, and the length and complexity of the hearings held, which are difficult to predict.
b Actual spending for 2016-17 was significantly higher than actual spending in 2015-16. This variance is due in large part to expenditures from the SPA of $1,883,121 most of which relate to costs associated with the Soliris Hearing.
c Actual spending for 2015-16 was significantly higher than actual spending in 2014-15. This variance is due in large part to expenditures from the SPA of $1,213,627 most of which relate to costs associated with the Soliris Hearing.
Actual human resources
Human resources summary for Programs and Internal Services
(full-time equivalents)
blank
| Programs and Internal Services |
2014–15
Actual |
2015–16
Actual |
2016–17
Planned |
2016–17
Actual |
2017–18
Planned |
2018–19
Planned
|
| Patented Medicine Prices Regulation Program |
24.7 |
31.1 |
40.0 |
31.1 |
33.0 |
33.0 |
| Pharmaceutical Trends Program |
9.2 |
12.8 |
12.0 |
13.3 |
13.0 |
13.0 |
| Subtotal |
33.9 |
43.9 |
52.0 |
44.4 |
46.0 |
46.0 |
| Internal Services |
22.3 |
18.6 |
19.0 |
19.3 |
20.0 |
20.0 |
| Total |
56.2 |
62.5 |
71.0 |
63.7 |
66.0 |
66.0 |
Expenditures by vote
For information on the PMPRB’s organizational voted and statutory expenditures, consult the Public Accounts of Canada 2017.Footnote xv
Alignment of spending with the whole-of-government framework
Alignment of 2016-17 actual spending with the whole-of-government frameworkFootnote xvi (dollars)
| Program |
Spending area |
Government of Canada activity |
2016–17
Actual spending |
| Patented Medicine Prices Regulation Program |
Social Affairs |
Healthier Canadians |
6,098,659 |
| Pharmaceutical Trends Program |
Social Affairs |
Healthier Canadians |
1,616,278 |
Total spending by spending area (dollars)
| Spending area |
Total planned spending |
Total actual spending |
| Economic affairs |
|
|
| Social affairs |
8,282,168 |
7,714,937 |
| International affairs |
|
|
| Government affairs |
|
|
Financial statements and financial statements highlights
Financial statements
The Patented Medicine Prices Review Board’s financial statementsFootnote xvii [unaudited] for the year ended March 31, 2017, are available on the PMPRB’s website.
Financial statements highlights
Condensed Statement of Operations (unaudited) for the year ended March 31, 2017 (dollars)
| Financial information |
2016–17
Planned
results |
2016–17
Actual |
2015–16
Actual |
Difference
(2016–17
actual
minus
2016–17
planned) |
Difference
(2016–17
actual
minus
2015–16
actual) |
| Total expenses |
12,157,399 |
11,140,340 |
10,716,714 |
(1,017,059) |
423,626 |
| Total revenues |
- |
9,297 |
2,265 |
9,297 |
7,032 |
| Net cost of operations before government funding and transfers |
12,157,399 |
11,131,043 |
10,714,449 |
(1,026,356) |
416,594 |
The PMPRB’s total actual expenses were $11,140,340 in 2016-17, an increase of $423,626 from 2015-16 actual expenses, which is due mainly to the following:
- Salaries and employee benefits decreased by $181,967.
- Professional and special services increased by $586,168.
The PMPRB’s total revenues which are usually made up of fees for Access to information requests and proceeds from disposal of assets, and exchange rate gains were $9,297 in 2016-17, representing an increase of $7,032 over the prior year actual revenues. This year’s increase is primarily a result of an increase in exchange rate gains.
Condensed Statement of Financial Position (unaudited) as at March 31, 2017 (dollars)
| Financial Information |
2016–17 |
2015–16 |
Difference
(2016–17
minus
2015–16) |
| Total net liabilities |
2,294,983 |
1,995,264 |
299,719 |
| Total net financial assets |
1,722,674 |
1,291,599 |
431,075 |
| Departmental net debt |
572,309 |
703,665 |
(131,356) |
| Total non-financial assets |
100,895 |
155,071 |
(54,176) |
| Departmental net financial position |
(471,414) |
(548,594) |
77,180 |
The PMPRB’s total net liabilities were $2,294,983 at the end of 2016-17, an increase of $299,719 from the previous year, which is due mainly to the following:
- Accounts payable and accrued liabilities increased by $431,075.
- Vacation pay and compensatory leave decreased by $41,340.
- Employee future benefits decreased by $90,016.
The PMPRB’s total net financial assets were $1,722,674 at the end 2016-17, an increase of $431,075 from the previous year, which is due mainly to the following:
- Due from the Consolidated Revenue Fund increased by $341,286.
- Accounts receivable and advances increased by $89,789.
Supplementary Information
Corporate information
Organizational profile
Appropriate minister: The Honourable Ginette Petitpas Taylor
Institutional head: Dr. Mitchell Levine, Vice-ChairpersonFootnote xviii
Ministerial portfolio: Health
Enabling instrument(s): Patent Act Footnote xix and Patented Medicines Regulations Footnote xx
Year of incorporation / commencement: 1987
Other: The Minister of Health is responsible for the pharmaceutical provisions of the Patent Act (Act) set out in sections 79 to 103. Although the Patented Medicine Prices Review Board (PMPRB) is part of the Health Portfolio, because of its quasi-judicial responsibilities the PMPRB carries out its mandate at arm’s length from the Minister. It also operates independently of Health Canada, which approves drugs for safety, efficacy and quality; other Health Portfolio members, such as the Public Health Agency of Canada, the Canadian Institutes of Health Research and the Canadian Food Inspection Agency; and federal, provincial and territorial (F/P/T) public drug plans, which approve the listing of drugs for their respective formularies for reimbursement purposes; and the Common Drug Review, administered by the Canadian Agency for Drugs and Technologies in Health (CADTH), which recommends drugs that should qualify for reimbursement purposes by participating public drug plans.
Reporting framework
The Patented Medicine Prices Review Board’s Strategic Outcome and Program Alignment Architecture of record for 2016–17 are shown below.
1. Strategic Outcome: Canadians are protected from excessive prices for patented medicines sold in Canada and stakeholders are informed on pharmaceutical trends.
1.1 Program: Patented Medicine Prices Regulation Program
1.2 Program: Pharmaceutical Trends Program
Internal Services
Supporting information on lower-level programs
The PMPRB does not have any lower-level programs. The PMPRB only has one strategic outcome, two supporting programs and internal services.
Supplementary information tables
The following supplementary information tables are available on the PMPRB’s website:
Federal tax expenditures
The tax system can be used to achieve public policy objectives through the application of special measures such as low tax rates, exemptions, deductions, deferrals and credits. The Department of Finance Canada publishes cost estimates and projections for these measures each year in the Report on Federal Tax Expenditures.Footnote xxii This report also provides detailed background information on tax expenditures, including descriptions, objectives, historical information and references to related federal spending programs. The tax measures presented in this report are the responsibility of the Minister of Finance.
Organizational contact information
The Patented Medicine Prices Review Board
Box L40
Standard Life Centre
333 Laurier Avenue West
Suite 1400
Ottawa, Ontario K1P 1C1
Telephone: (613) 952-7360
Toll-free no.: 1-877-861-2350
Facsimile: (613) 288-9643
TTY: (613) 288-9654
Email: pmprb@pmprb-cepmb.gc.ca
Website: www.pmprb-cepmb.gc.ca
Appendix: definitions
appropriation (crédit)
Any authority of Parliament to pay money out of the Consolidated Revenue Fund.
budgetary expenditures (dépenses budgétaires)
Operating and capital expenditures; transfer payments to other levels of government, organizations or individuals; and payments to Crown corporations.
Core Responsibility (responsabilité essentielle)
An enduring function or role performed by a department. The intentions of the department with respect to a Core Responsibility are reflected in one or more related Departmental Results that the department seeks to contribute to or influence.
Departmental Plan (Plan ministériel)
Provides information on the plans and expected performance of appropriated departments over a three-year period. Departmental Plans are tabled in Parliament each spring.
Departmental Result (résultat ministériel)
A Departmental Result represents the change or changes that the department seeks to influence. A Departmental Result is often outside departments’ immediate control, but it should be influenced by program-level outcomes.
Departmental Result Indicator (indicateur de résultat ministériel)
A factor or variable that provides a valid and reliable means to measure or describe progress on a Departmental Result.
Departmental Results Framework (cadre ministériel des résultats)
Consists of the department’s Core Responsibilities, Departmental Results and Departmental Result Indicators.
Departmental Results Report (Rapport sur les résultats ministériels)
Provides information on the actual accomplishments against the plans, priorities and expected results set out in the corresponding Departmental Plan.
drug product (produit médicamenteux)
A particular presentation of a medicine characterized by its pharmaceutical dosage form and the strength of the active ingredient(s).
Evaluation (évaluation)
In the Government of Canada, the systematic and neutral collection and analysis of evidence to judge merit, worth or value. Evaluation informs decision making, improvements, innovation and accountability. Evaluations typically focus on programs, policies and priorities and examine questions related to relevance, effectiveness and efficiency. Depending on user needs, however, evaluations can also examine other units, themes and issues, including alternatives to existing interventions. Evaluations generally employ social science research methods.
full-time equivalent (équivalent temps plein)
A measure of the extent to which an employee represents a full person-year charge against a departmental budget. Full-time equivalents are calculated as a ratio of assigned hours of work to scheduled hours of work. Scheduled hours of work are set out in collective agreements.
government-wide priorities (priorités pangouvernementales)
For the purpose of the 2016–17 Departmental Results Report, government-wide priorities refers to those high-level themes outlining the government’s agenda in the 2015 Speech from the Throne, namely: Growth for the Middle Class; Open and Transparent Government; A Clean Environment and a Strong Economy; Diversity is Canada's Strength; and Security and Opportunity.
horizontal initiatives (initiative horizontale)
An initiative where two or more federal organizations, through an approved funding agreement, work toward achieving clearly defined shared outcomes, and which has been designated (for example, by Cabinet or a central agency) as a horizontal initiative for managing and reporting purposes.
Management, Resources and Results Structure (Structure de la gestion, des ressources et des résultats)
A comprehensive framework that consists of an organization’s inventory of programs, resources, results, performance indicators and governance information. Programs and results are depicted in their hierarchical relationship to each other and to the Strategic Outcome(s) to which they contribute. The Management, Resources and Results Structure is developed from the Program Alignment Architecture.
medicine (médicament)
Any substance or mixture of substances made by any means, whether produced biologically, chemically, or otherwise, that is applied or administered in vivo in humans or animals to aid in the diagnosis, treatment, mitigation or prevention of disease, symptoms, disorders, abnormal physical states, or modifying organic functions in humans or animals, however administered. For greater certainty, this definition includes vaccines, topical preparations, anaesthetics and diagnostic products used in vivo, regardless of delivery mechanism (e.g., transdermal, capsule form, injectable, inhaler, etc.). This definition excludes medical devices, in vivo diagnostic products and disinfectants that are not used in vivo.
non-budgetary expenditures (dépenses non budgétaires)
Net outlays and receipts related to loans, investments and advances, which change the composition of the financial assets of the Government of Canada.
patent (brevet)
An instrument issued by the Commissioner of Patents in the form of letters patent for an invention that provides its holder with a monopoly limited in time, for the claims made within the patent. A patent gives its holder and its legal representatives, the exclusive right of making, constructing and using the invention and selling it to others to be used.
patentee (breveté)
As defined by subsection 79(1) of the Patent Act, “the person for the time being entitled to the benefit of the patent for the invention and includes, where any other person is entitled to exercise any rights in relation to that patent other than under a license continued by subsection 11(1) of the Patent Act Amendment Act, 1992, that other person in respect of those rights.”
performance (rendement)
What an organization did with its resources to achieve its results, how well those results compare to what the organization intended to achieve, and how well lessons learned have been identified.
performance indicator (indicateur de rendement)
A qualitative or quantitative means of measuring an output or outcome, with the intention of gauging the performance of an organization, program, policy or initiative respecting expected results.
performance reporting (production de rapports sur le rendement)
The process of communicating evidence-based performance information. Performance reporting supports decision making, accountability and transparency.
planned spending (dépenses prévues)
For Departmental Plans and Departmental Results Reports, planned spending refers to those amounts that receive Treasury Board approval by February 1. Therefore, planned spending may include amounts incremental to planned expenditures presented in the Main Estimates.
A department is expected to be aware of the authorities that it has sought and received. The determination of planned spending is a departmental responsibility, and departments must be able to defend the expenditure and accrual numbers presented in their Departmental Plans and Departmental Results Reports.
plans (plans)
The articulation of strategic choices, which provides information on how an organization intends to achieve its priorities and associated results. Generally a plan will explain the logic behind the strategies chosen and tend to focus on actions that lead up to the expected result.
PMPRB7 (CEPMB7)
The seven foreign comparator countries for which patentees must report publicly available prices of patented drug products for price review purposes: France, Germany, Italy, Sweden, Switzerland, the United Kingdom and the United States.
priorities (priorité)
Plans or projects that an organization has chosen to focus and report on during the planning period. Priorities represent the things that are most important or what must be done first to support the achievement of the desired Strategic Outcome(s).
program (programme)
A group of related resource inputs and activities that are managed to meet specific needs and to achieve intended results and that are treated as a budgetary unit.
Program Alignment Architecture (architecture d’alignement des programmes)
A structured inventory of an organization’s programs depicting the hierarchical relationship between programs and the Strategic Outcome(s) to which they contribute.
results (résultat)
An external consequence attributed, in part, to an organization, policy, program or initiative. Results are not within the control of a single organization, policy, program or initiative; instead they are within the area of the organization’s influence.
statutory expenditures (dépenses législatives)
Expenditures that Parliament has approved through legislation other than appropriation acts. The legislation sets out the purpose of the expenditures and the terms and conditions under which they may be made.
Strategic Outcome (résultat stratégique)
A long-term and enduring benefit to Canadians that is linked to the organization’s mandate, vision and core functions.
sunset program (programme temporisé)
A time-limited program that does not have an ongoing funding and policy authority. When the program is set to expire, a decision must be made whether to continue the program. In the case of a renewal, the decision specifies the scope, funding level and duration.
target (cible)
A measurable performance or success level that an organization, program or initiative plans to achieve within a specified time period. Targets can be either quantitative or qualitative.
voted expenditures (dépenses votées)
Expenditures that Parliament approves annually through an Appropriation Act. The Vote wording becomes the governing conditions under which these expenditures may be made.