Annual Public Drug Plan Expenditure Report, 2013/14
Executive Summary
The CompassRx annual report explores the underlying forces driving prescription drug expenditures in Canadian public drug plans. It analyzes trends in demographics, pricing and the use of drugs, and measures their impact on expenditure levels. The report also monitors major developments in drug approval, review, pricing and reimbursement in Canada. This edition of the report focuses on the 2013/14 fiscal year and provides a retrospective review of trends since 2009/10.
The change in prescription drug expenditures is driven by a number of opposing “push” and “pull” effects. Increases in the beneficiary population, the increased use of drugs, and/or the use of more expensive drugs put an upward pressure on expenditures, resulting in a push effect; while generic substitutions and price reductions exert a downward pull effect. In any given year, the weight of each of these effects may vary, and as a result, the rates of change in prescription drug expenditures evolve over time and may differ across public drug plans.
The main data source for this report is the National Prescription Drug Utilization Information System (NPDUIS) Database managed by the Canadian Institute for Health Information (CIHI). Results are presented for the following public drug plans participating in NPDUIS: British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, New Brunswick, Nova Scotia, Prince Edward Island, Newfoundland and Labrador, and Health Canada’s Non-Insured Health Benefits drug plan. The data for British Columbia and Newfoundland and Labrador have been added to this edition of the report, giving a broader overview of the public plans.
Identifying the major drivers of change and the effect they have on prescription drug expenditures supports policy makers and researchers in better understanding the current trends and anticipating future cost pressures and expenditure levels.
Key findings
Overview of Prescription Drug Expenditures for 2013/14
Prescription drug expenditures for the NPDUIS public drug plans totaled $9.8 billion in 2013/14 and included $7.3 billion in drug costs (74.2%), $2.2 billion in pharmacy dispensing costs (22.3%), and $0.3 billion in markups (3.5%).
The NPDUIS public drug plans paid 78.7% of the overall prescription drug expenditures. The remaining share was paid by drug plan beneficiaries either out-of-pocket or through a third-party private insurer.
Drug Cost Component of Prescription Drug Expenditures
The average rate of change in the cost of drugs for all NPDUIS public plans steadily declined from 2010/11 to 2012/13, reaching a low of -1.5%. This trend reversed in 2013/14, with the average drug costs increasing by 2.0%.
In 2013/14, generic drug policies markedly reduced drug costs by 6.0%; however, this decrease was almost completely offset by an increase in the use of higher-cost drugs, which put a 5.4% upward pressure on costs. The impact of higher-cost drugs was more pronounced in 2013/14 than in 2012/13, when it was 4.1%Footnote I.
The tipping point towards positive growth rates in 2013/14 was the modest cost-saving effect of generic substitution (1.5%), which only partially compensated for the increases in the beneficiary population and their use of drugs (2.1% and 2.2%, respectively). The low generic substitution effect signals the end of the patent cliff, as no major blockbuster drugs lost patent protection in 2013/14. In contrast, 2012/13 was marked by a sizable savings due to generic substitution, which pulled drug costs downward by 7.2%Footnote I.
Highlights for 2013/14
- The demographic, volume, and drug-mix effects had an important “push” effect in 2013/14. Without the impact of generic savings, increases in the size and age of the active beneficiary population, the volume of drugs and the use of higher-cost drugs would have driven up drug costs by 9.7%.
- Generic substitution and especially price changes had an important “pull” effect in 2013/14. In the absence of other cost pressures, lower generic drug prices and the shift from brand-name to generic drugs would have decreased drug cost levels by 7.5%.
Drug cost drivers 2012/13* versus 2013/14
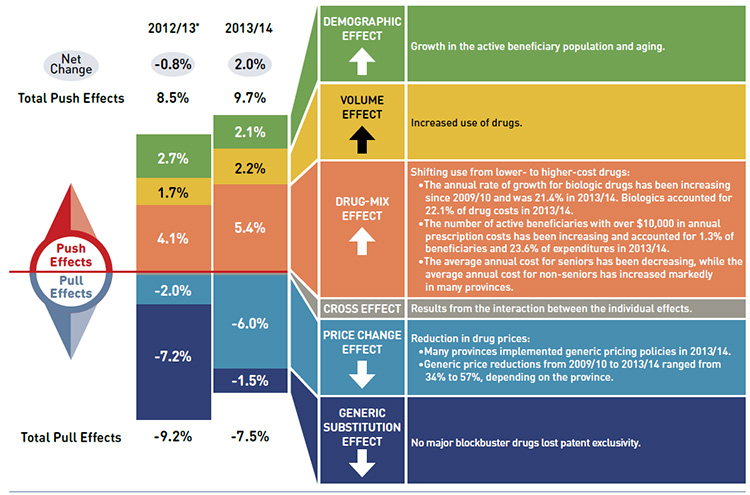
* Results for 2012/13 do not capture the data for the British Columbia and Newfoundland and Labrador provincial public drug plans.
Note: Values may not add to totals due to rounding and the cross effect.
Data source: National Prescription Drug Utilization Information System Database, Canadian Institute for Health Information.
Figure description
This diagram illustrates the drug cost drivers for 2013/14 and compares them to the overall results for 2012/13 for the NPDUIS public drug plans in Canada. Cost drives are categorized as push (increasing) or pull (decreasing) effects.
The overall net change in drug costs from 2012/13 to 2013/14 was 2.0% compared to a -0.8% change from 2011/12 to 2012/13.
The total push effect in 2013/14 was 9.7% (compared to 8.5% in 2012/13) and included the following key effects:
- The demographic effect was 2.1% due to a growth in the active beneficiary population and aging. In 2012/13 it was 2.7%.
- The volume effect was 2.2% due to the increased use of drugs. In 2012/13 it was 1.7%.
- The drug-mix effect was 5.4% (up from 4.1% in 2012/13) due to the shifting use from lower- to higher-cost drugs:
- The annual rate of growth for biologic drugs has been increasing since 2009/10 and was 21.4% in 2013/14. Biologics accounted for 22.1% of drug costs in 2013/14.
- The number of active beneficiaries with over $10,000 in annual prescription costs has been increasing and accounted for 1.3% of beneficiaries and 23.6% of expenditures in 2013/14.
- The average annual costs for seniors have been decreasing, while the average annual costs for non-seniors have increased markedly in many provinces.
The total pull effect was -7.5% (compared to -9.2% in 2012/13) and included the following key effects:
- The price change effect was -6.0% (compared to -2.0% in 2012/13) due to a reduction in drug prices:
- Many provinces implemented generic pricing policies in 2013/14.
- Generic price reductions from 2009/10 to 2013/14 ranged from 34% to 57%, depending on the province.
- The generic substitution effect was -1.5% (compared to -7.2% in 2012/13) as no blockbuster drugs lost patent exclusivity in 2013/14.
A cross effect resulted from the interaction between the individual effects. No value is given for the cross effect.
Dispensing Cost Component of Prescription Drug Expenditures
Dispensing costs have been increasing in recent years in most public plans, growing at a rate of 5.9% from 2012/13 to 2013/14. Dispensing costs accounted for an increased share of prescription drug expenditures: 22.3% in 2013/14, up from 19.0% in 2010/11.
Highlights for 2013/14
- The 5.9% rate of change in dispensing costs was mainly driven by increases in the size and age of the active beneficiary population (2.1%), growth in the use of drugs (1.3%), and increases in dispensing fee levels (1.1%), as well as a trend toward smaller prescription sizes in some provinces (1.4%).
Dispensing cost drivers 2013/14
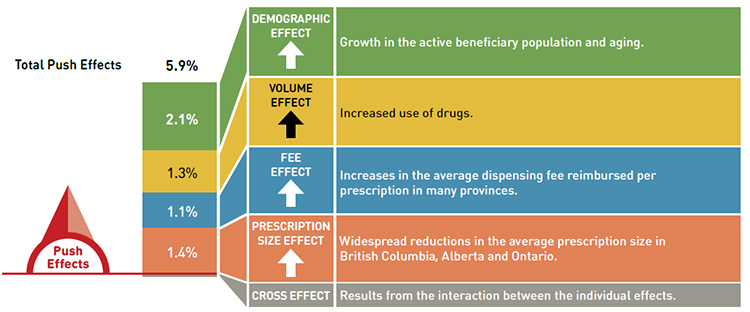
Data source: National Prescription Drug Utilization Information System Database, Canadian Institute for Health Information.
Figure description
This diagram illustrates dispensing cost drivers for 2013/14. It shows the totals for the NPDUIS public drug plans in Canada.
The total push (increasing) effect from 2012/13 to 2013/14 was 5.9% and included the following key effects:
- The demographic effect (2.1%) was driven by the growth in the active beneficiary population and aging.
- The volume effect (1.3%) was driven by the increased use of drugs.
- The fee effect (1.1%) was driven by increases in the average dispensing fee reimbursed per prescription in many provinces.
- The prescription size effect (1.4%) was driven by widespread reductions in the average prescription size in British Columbia, Alberta, and Ontario.
A cross effect resulted from the interaction between the individual effects. No value is given for the cross effect.
Note that overall key findings mask important variations at the jurisdictional level, which are detailed in the report.
Canadian Pricing and Reimbursement Environment, 2013/14
- Five provinces implemented generic pricing policies in 2013/14.
- British Columbia, New Brunswick, Prince Edward Island, and Newfoundland and Labrador lowered the prices of generic drugs to 25% of their brand-name equivalents, while Alberta reduced this ratio to 18%, with a few exceptions for specific drugs.
- The PMPRB reviewed 115 new drug products in 2013.
- Of the new drugs, 7 were either breakthrough drugs or demonstrated a substantial improvement; and 20 were classified as having a moderate improvement; and the remaining 88 drugs were classified as having slight or no improvement.
- In 2013, the Canadian Agency for Drugs and Technologies in Health (CADTH) Common Drug Review made 34 recommendations for 29 drugs; a few drugs received multiple recommendations depending on their indication.
- Recommendations included: list: 0; list with criteria/condition: 18; list with clinical criteria and/or conditions: 4; do not list at the submitted price: 4; and do not list: 8.
Acknowledgements
This report was prepared by the Patented Medicine Prices Review Board (PMPRB) as part of the National Prescription Drug Utilization Information System (NPDUIS).
The PMPRB would like to acknowledge the contributions of:
- The members of the NPDUIS Advisory Committee, for their expert oversight and guidance in the preparation of this report.
- The PMPRB staff for their contribution to the analytical content of the report:
- Tanya Potashnik – Director, Policy and Economic Analysis
- Elena Lungu – Manager, NPDUIS
- Greg McComb – Senior Economic Analyst
- Ai Chau – SAS Analyst
- Carol McKinley – Publications Advisor
- The PMPRB scientific and editing groups
Disclaimer
NPDUIS is a research initiative that operates independently of the regulatory activities of the Board of the PMPRB. The statements and opinions expressed in this report do not represent the position of the PMPRB with respect to any regulatory matter.
Parts of this material are based on data and information provided by the Canadian Institute for Health Information. However, the analyses, conclusions and/or statements expressed herein are not those of the Canadian Institute for Health Information.
- Executive Summary
- Introduction
- Methods
- Limitations
- 1 Canadian Pricing and Reimbursement Environment, 2013/14
- 2 Overview of Prescription Drug Expenditures and Utilization, 2013/14
- 3 Trends in Prescription Drug Expenditures, 2009/10 to 2013/14
- 4 The Drivers of Drug Costs, 2012/13 to 2013/14
- 5 The Drivers of Dispensing Costs, 2012/13 to 2013/14
- References
- Appendix A – Public Drug Plan Designs
- Appendix B – Pricing Policies for Generic Drugs in Provincial Drug Plans
- Appendix C – Markup Policies in Public Drug Plans, 2013/14
- Appendix D – Dispensing Fee Policies in Public Drug Plans, 2013/14
- Appendix E – Common Drug Review Listing Recommendations by Drug and Indication, 2013/14
- Appendix F – Top 100 Patented Drugs, NPDUIS Public Drug Plans, 2013/14
- Appendix G – Top 100 Non-Patented Single Source Drugs, NPDUIS Public Drug Plans, 2013/14
- Appendix H –Top 100 Multi-Source Generic Drugs, NPDUIS Public Drug Plans, 2013/14
- Appendix I –Top 100 Manufacturers by Drug Cost, NPDUIS Public Drug Plans, 2013/14
- Appendix J – Glossary
Introduction
The amount spent on prescription drugs in Canada represents a significant component of the overall health care costs. After sustained double-digit rates of growth in prescription drug expenditures a decade ago, the annual rates have gradually declined in recent years,
reaching 2.3% in 2013, the second lowest point in more than two decades.Footnote 1
To aid in understanding the recent trends in prescription drug expenditures and to anticipate their future direction, the CompassRx report provides a comprehensive cost driver analysis of prescription drug expenditures for all of the Canadian provincial public drug
plans (except Quebec), as well as the federal Non-Insured Health Benefits (NIHB) drug plan. The report highlights the most significant cost pressures, measures their impact on expenditure levels, and delves into the factors determining trends in costs, pricing and utilization in public plans. It also monitors major developments in the drug approval, review, pricing and reimbursement environment in Canada.
The 2013/14 CompassRx is the second edition of this report and identifies developing trends based on the baseline established in the 2012/13 publication. Two new jurisdictions have been added to this edition, providing a more comprehensive view of the public
plan environment.
The recent low rates of growth in prescription drug expenditures are the net result of a number of “push” and “pull” effects. Factors such as an increase in the beneficiary population, the increased use of drugs, and the use of more expensive drugs are putting an upward pressure (“push”) on expenditures. At the same time, expenditure levels are pulled downward by factors such as generic substitution and generic price reductions.
The analysis in this report isolates and quantifies the impact of each of the principal contributing factors. Four broad categories of effects are considered: demographic effects, volume effects, price effects and drug-mix effects. Important sub-effects are also analyzed.
In any given year, the weight of the opposing “push” and “pull” effects may vary due to changes in market trends, reimbursement decisions, changing treatment practices and other factors. These factors evolve over time and may vary across public drug plans.
This report is divided into five sections. Section 1 monitors recent pricing and reimbursement developments. Section 2 provides an overview of the prescription drug expenditure and utilization levels in 2013/14 for the NPDUIS public drug plans. Section 3 reports on five-year trends in prescription drug expenditures (from fiscal year 2009/10 to 2013/14). Sections 4 and 5 provide a cost driver analysis of the factors that drive drug and dispensing costs, respectively.
Methods
The main data source for this report is the National Prescription Drug Utilization Information System (NPDUIS) Database, developed by the Canadian Institute for Health Information (CIHI). This database houses pan-Canadian information on public drug programs, including anonymous claims-level data collected from the plans participating in the NPDUIS initiative.
Results are presented for all NPDUIS provincial plans, which include the public drug plans in British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, New Brunswick, Nova Scotia, Prince Edward Island, and Newfoundland and Labrador, as well as Health Canada’s Non-Insured Health Benefits (NIHB) drug plan. The totals reported include data from all of the NPDUIS plans. A detailed description of the plans contributing to the NPDUIS Database is available in CIHI’s Plan Information Document.Footnote 2
The study analyzes data from 2009/10 to 2013/14, with a focus on the rates of change in prescription drug expenditures from 2012/13 to 2013/14. The drug costs, markups and dispensing costs reported in this study are the amounts accepted toward reimbursement by the public plans. (See the glossary in Appendix J for definitions of the variables in the report).
The results reported for Saskatchewan and Manitoba include the accepted prescription drug expenditures for individuals who are eligible for coverage but have not submitted an application and, therefore, do not have a defined deductible.Footnote 1 For the NIHB, claims that were coordinated with provincial public drug plans are excluded from the analysis to ensure consistency in the annual data reporting. The results reported for New Brunswick include the number of active beneficiaries enrolled in the Medavie Blue Cross Seniors’ Prescription Drug Program and their related drug expenditures, which are offset by monthly premiums.
The analysis of the drivers of drug and dispensing costs follows the methodological approach detailed in the PMPRB report The Drivers of Prescription Drug Expenditures: A Methodological Report.Footnote 3
Analyses of the average prescription size, as well as generic pricing, are limited to oral solid formulations. This is to avoid data reporting inconsistencies that may exist in the day supply and unit reporting of non-oral formulations.
Population data is derived from the Non-Insured Health Benefits Annual Report and Statistics Canada census data for 2007 and 2013.
Limitations
The results presented in this report are intended for individual reviews of each public plan. Comparative analyses across plans are limited by differences in the plan designs, demographics and the disease profiles of the eligible beneficiary populations. For example, British Columbia, Saskatchewan and Manitoba have universal income-based drug programs that provide broad-based coverage for the general population. Other public drug plans offer programs with specific design structures for seniors, income assistance recipients and
various patient groups.
The Non-Insured Health Benefits Program provides universal coverage to First Nations and Inuit people across Canada. This population has specific demographic and health profiles that differ from those reimbursed by other public plans.
The NPDUIS Database includes sub-plan data specific to particular jurisdictions. This further limits the comparability of results across plans. For instance, some sub-plans that are available in most provinces are not captured in the data for Alberta, Nova Scotia and Prince Edward Island. Appendix A provides a comprehensive summary of the sub-plans available in the NPDUIS Database, along with the eligibility criteria.
The totals for the NPDUIS public drug plans are heavily skewed toward Ontario due to its size.
The prescription drug expenditure data for the NPDUIS public drug plans represents only one segment of the overall pharmaceutical market, and hence, the findings in this report should not be extrapolated to the overall Canadian marketplace. The total prescription drug
expenditure reported for the NPDUIS public plans was $9.8 billion in the fiscal year 2013/14. This represents 33.4% of the $29.3 billion in total Canadian prescription drug spending in the 2013 calendar year. In total, 41.6% of prescription drug spending was financed by
public drug plans in calendar year 2013, with the remainder financed by private plans (35.5%) and out-of-pocket by households and individuals (23.9%).Footnote 1
This edition of the CompassRx reports on data up to and including the 2013/14 fiscal year. Important developments that have taken place in the Canadian environment since then are not captured in this report.
Drug costs reported are the amounts accepted toward reimbursement by the public plans and do not reflect off-invoice price rebates or price reductions resulting from confidential product listing agreements.
1 Canadian Pricing and Reimbursement Environment, 2013/14
This section provides an overview of provincial and federal developments related to public drug plan expenditure and utilization in 2013/14.
Public Drug Plans: Initiatives and Policy Updates
The information in this section was obtained from publicly available sources, including CIHI’s NPDUIS Plan Information DocumentFootnote 2 and IMS Brogan’s Provincial Reimbursement Advisor.Footnote 4
Generic and Brand-name Drug Prices
Since 2010 most provincial governments have implemented generic pricing policies that reduced the price of generic drugs in Canada and resulted in important cost savings. In 2013/14, five provinces implemented new generic pricing policies. British Columbia,
New Brunswick, Prince Edward Island, and Newfoundland and Labrador lowered the prices of generic drugs to 25% of the equivalent brand-name prices, while Alberta reduced this ratio to 18%, with some exceptions for specific drugs.
As part of a continuing effort to reduce the cost of drugs, the pan-Canadian Generic Value Price Initiative was established in 2013 (currently referenced as the pan-Canadian Pharmaceutical Alliance or pCPA). As of April 1, 2013, the prices of six of the most commonly used generic drugs were lowered to 18% of the brand-name price: atorvastatin, ramipril, venlafaxine, amlodipine, omeprazole and rabeprazole.
Since 2013/14, subsequent generic pricing policies have been introduced, either individually by the provinces or collectively through the pCPA process. In addition, all provinces and territories (except Quebec) reached an agreement on a Tiered Pricing Framework. This framework sets the prices of generic drugs based on the number of products available in the Canadian market. Tiered pricing is not intended to supersede the existing provincial regulations and policies. Appendix B provides a summary of generic pricing policies implemented since 2010.
The pCPA also conducts joint provincial/territorial negotiations for brand-name drugs to achieve greater value for Canadian publicly funded drug programs. A total of 90 joint negotiations or product listing agreements (PLAs) for brand-name drugs were completed as of January 31, 2016. PLA prices are not reflected in the drug costs captured by the NPDUIS Database.
Dispensing Fees
Several provinces increased their dispensing fees in 2013/14. Saskatchewan increased the maximum dispensing fee from $10.25 to $10.75, while Ontario raised dispensing fees for non-rural pharmacies from $8.40 to $8.62, and set the range for rural pharmacies at $9.69 to $12.92. Nova Scotia increased dispensing fees from $10.90 to $11.05, and Prince Edward Island raised their fees from $11.65 to $12.00.
Public drug plans may also reimburse fees for professional pharmacy services other than the dispensing of medications. These fees are not reflected in the data reported in this study.
Plan Design Changes
Prince Edward Island introduced a Catastrophic Drug Program on October 1, 2013. This program assists individuals or families with high prescription costs relative to their income. Beneficiary co-payments are capped on a sliding scale based on annual income.
Both Alberta and Nova Scotia introduced insulin pump therapy programs in 2013. These programs provide funding for eligible residents with type 1 diabetes.
In British Columbia, Health Canada transferred management and delivery of First Nations health programs – including prescription drugs – to the new First Nations Health Authority on October 1, 2013. British Columbia also made additional vaccines available through pharmacists including vaccines for measles, mumps, hepatitis and tetanus.
In July 2013, New Brunswick introduced a Frequency of Dispensing and Payment Policy. According to this policy, pharmacies are eligible for one dispensing fee every 28 days or more for drugs taken continuously (long-term).
Approval, Review and Assessment of Drugs and Prices in Canada
Three separate federal institutions are responsible for drug approval, price reviews, and health technology assessments: Health Canada, the Patented Medicine Prices Review Board (PMPRB), and the Canadian Agency for Drugs and Technologies in Health (CADTH).
Health Canada
Health Canada grants the authority to market a drug in Canada once it has met the regulatory requirements for safety, efficacy and quality, and issues a Notice of Compliance (NOC). In 2013/14, Health Canada issued 979 NOCsFootnote 5 (Table 1.1).
Table 1.1 Health Canada Notices of Compliance issued in 2013/14
| Pharmaceutical / biologic status |
Number of NOCs |
| Prescription pharmaceutical |
908 |
| Biologic |
71 |
| Total |
979 |
Table 1.1
| Brand name, generic or supplement status |
Number of NOCs |
| Brand name |
204 |
| Generic |
431 |
| Supplements to existing drugs* |
344 |
| Total |
979 |
* After an initial NOC is issued for a drug, a subsequent NOC may be issued for reasons such as a change to the drug’s name, a new indication or strength, a new manufacturing site or a new process to manufacturer the drug.
Patented Medicine Prices Review Board
The PMPRB reviews the factory-gate prices of patented drugs sold in Canada and ensures that they are not excessive. It also reports on pharmaceutical trends for all medicines and research and development spending by patentees. In 2013, the PMPRB reviewed
115 new drug products and classified each based on its level of therapeutic improvement (Table 1.2).
Table 1.2 Patented Medicine Prices Review Board, drugs reviewed in 2013 by level of therapeutic improvement
| Level of therapeutic improvement |
Number of drugs |
| Breakthrough |
4 |
| Substantial improvement |
2 |
| Moderate improvement |
20 |
| Slight / no improvement |
85 |
| Category 2* |
1 |
| Category 1* |
3 |
| Total |
115 |
* Drugs reviewed by the PMPRB prior to the implementation of the 2010 Guidelines. Category 2 drugs are equivalent to breakthrough and substantial improvement levels; Category 1 drugs are line extensions, which are the equivalent of a slight/no improvement under the 2010 Guidelines.
As part of its reporting mandate, the PMPRB uses the Patented Medicines Price Index (PMPI) to monitor trends in prices of patented drug products. The PMPI measures the average year-over-year change in the factory-gate (manufacturer) prices of patented drug
products sold in Canada. These prices are based on publically available information and do not include confidential rebates. In 2013, the PMPI, on average, increased slightly by 0.5%, which was less than the 0.9% increase in inflation measured by the Consumer Price
Index (CPI).Footnote 6
The PMPRB compares the prices of Canadian patented drug products to the median price of a basket of seven comparator countries (PMPRB7): France, Italy, Germany, Sweden, Switzerland, United Kingdom and the United States. While average foreign prices were 6% higher than Canadian prices in 2013, this result was greatly influenced by the high drug prices in the United States. In fact, Canadian prices were decidedly higher than prices in the United Kingdom, France and Italy, and somewhat higher than prices in Sweden and
Switzerland.Footnote 6
Canadian Agency for Drugs and Technologies in Health
The CADTH Common Drug Review (CDR) conducts evaluations of the clinical, economic, and patient evidence on drugs marketed in Canada and uses this information to provide reimbursement recommendations and advice to Canada’s publicly funded drug plans, with
the exception of Quebec. The provinces take these recommendations under advisement when determining what drugs will be listed in their formularies.
In 2013/14, the CDR made 34 recommendations for 29 drugs; a few drugs received multiple recommendations depending on the indication.Footnote 7 See Table 1.3 for a summary of results and Appendix E for a complete list of drugs and their recommendations by indication.
Table 1.3 Common Drug Review listing recommendations,* 2013/14
| Recommendation |
Number of recommendations |
Number of drugs |
| List |
0 |
0 |
| List with criteria/condition |
18 |
14 |
| List with clinical criteria and/or conditions |
4 |
4 |
| Do not list at submitted price |
4 |
4 |
| Do not list |
8 |
8 |
| Total |
34 |
29† |
* CADTH implemented revised Canadian Drug Expert Committee (CDEC) recommendation options on November 21, 2012, which included the creation of the “do not list at the submitted price” category and greater usage of conditions related to price in
the “list with clinical criteria and/or conditions” category.
† The value does not add to the sum of the number of drugs by listing recommendation, as several drugs had separate recommendations for two indications.
2 Overview of Prescription Drug Expenditures and Utilization, 2013/14
This section provides an overview of prescription drug expenditures and utilization for the NPDUIS public drug plans in fiscal year 2013/14. The expenditures reported here include the drug costs, dispensing costs, and markups, where applicable. These expenditures reflect both the plan-paid and beneficiary-paid portions of the costs, such as co-payments and deductibles. They represent the total amount accepted for reimbursement by the public drug plans (including the amount eligible toward deductibles). See Appendix A for a
summary of the individual plan designs and the glossary in Appendix J for a definition of the expenditure components.
Figure 2.1 presents the total prescription drug expenditures levels for the NPDUIS public drug plans in 2013/14 broken down into the three major components: drug costs, dispensing costs and markups.
Figure 2.1 Prescription drug expenditures in NPDUIS public drug plans, 2013/14 ($million, % share)
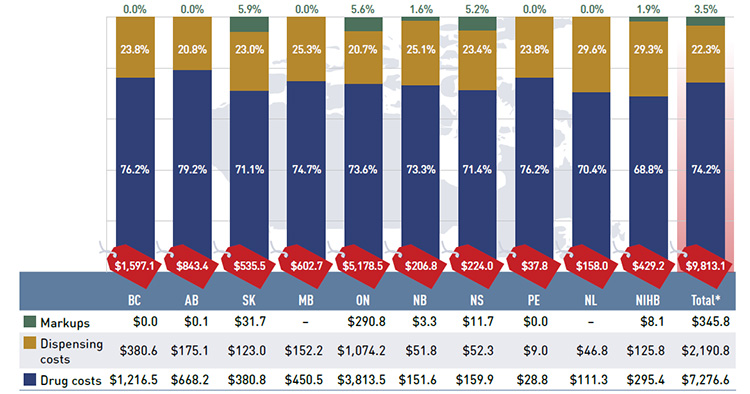
*Total results for the public drug plans reported in this figure.
Note: Values may not add to totals due to rounding.
A wholesale upcharge amount may be captured either in the drug cost or the markup component, depending on the reimbursement policies specific to each drug plan (see Appendix C). Thus, the comparison of the relative size of these two components across plans is limited.
Data source: National Prescription Drug Utilization Information System Database, Canadian Institute for Health Information.
Figure description
This bar graph describes prescription drug expenditures for the NPDUIS public drug plans in 2013/14 broken out into the three components: drug costs, dispensing costs and markups.
|
British Columbia |
Alberta |
Saskatchewan |
Manitoba |
Ontario |
New Brunswick |
Nova Scotia |
Prince Edward Island |
Newfoundland and Labrador |
Non-Insured Health Benefits |
Total |
| Total prescription drug expenditures (millions of dollars) |
$1,597.1 |
$843.4 |
$535.5 |
$602.7 |
$5,178.5 |
$206.8 |
$224.0 |
$37.8 |
$158.0 |
$429.2 |
$9,813.1 |
| Markups (millions of dollars, percent share) |
$0.0 (0.0%) |
$0.1 (0.0%) |
$31.7 (5.9%) |
‒ |
$290.8 (5.6%) |
$3.3 (1.6%) |
$11.7 (5.2%) |
$0.0 (0.0%) |
‒ |
$8.1 (1.9%) |
$345.8 (3.5%) |
| Dispensing costs (millions of dollars, percent share) |
$380.6 (23.8%) |
$175.1(20.8%) |
$123.0 (23.0%) |
$152.2 (25.3%) |
$1,074.2 (20.7%) |
$51.8 (25.1%) |
$52.3 (23.4%) |
$9.0 (23.8%) |
$46.8 (29.6%) |
$125.8 (29.3%) |
$2,190.8 (22.3%) |
| Drug costs (millions of dollars, percent share) |
$1,216.5 (76.2%) |
$668.2 (79.2%) |
$380.8 (71.1%) |
$450.5 (74.7%) |
$3,813.5 (73.6%) |
$151.6 (73.3%) |
$159.9 (71.4%) |
$28.8 (76.2%) |
$111.3 (70.4%) |
$295.4 (68.8%) |
$7,276.6 (74.2%) |
Of the $9.8 billion in total prescription expenditures, nearly three quarters (74.2%) was represented by the drug cost component. Dispensing costs made up 22.3% of the total, and markups represented 3.5%.
Prescription drug expenditure levels differ widely among the plans. This is mainly due to variations in the size of the beneficiary populations, but also reflects the demographic and disease profiles of the beneficiaries, as well as differences in plan
designs. The relative size of the these components also varies across the plans, reflecting differences in the reimbursement of markups and dispensing costs, as well as the quantity of drugs dispensed per prescription and the choice of drugs.
Appendices C and D summarize the policies governing markups and dispensing fees for the NPDUIS public drug plans in 2013/14.
A portion of the prescription drug expenditures reported in Figure 2.1 is reimbursed by the public plans, while the rest is paid by the beneficiaries either out-of-pocket or through a third-party private insurer. Figure 2.2 reports the share paid by the public plans.
Figure 2.2 Plan-paid share of prescription drug expenditures for NPDUIS public drug plans, 2013/14 ($million, % share)
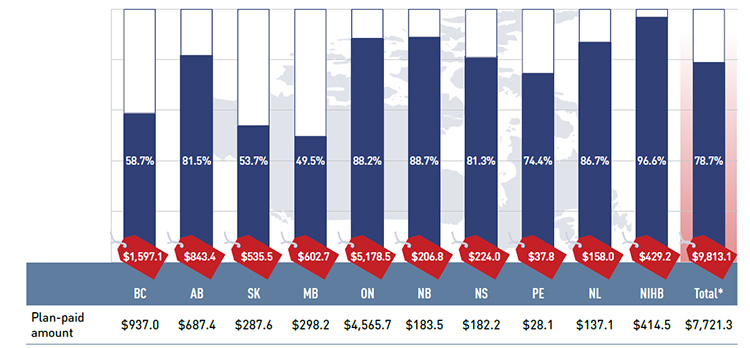
*Total results for the public drug plans reported in this figure.
Data source: National Prescription Drug Utilization Information System Database, Canadian Institute for Health Information.
Figure description
This bar graph describes the plan-paid share of prescription drug expenditures for the NPDUIS public drug plans in 2013/14. Absolute values are given in millions of dollars. British Columbia: $937.0 (58.7%); Alberta: $687.4 (81.5%); Saskatchewan: $287.6 (53.7%); Manitoba: $298.2 (49.5%); Ontario: $4,565.7 (88.2%); New Brunswick: $183.5 (88.7%); Nova Scotia: $182.2 (81.3%); Prince Edward Island: $28.1 (74.4%); Newfoundland and Labrador: $137.1 (86.7%); Non-Insured Health Benefits: $414.5 (96.6%); Total plans: $7,721.3 (78.7%).
The graph also shows total prescription drug expenditures for the plans in millions of dollars: British Columbia: $1,597.1; Alberta: $843.4; Saskatchewan: $535.5; Manitoba: $602.7; Ontario: $5,178.5; New Brunswick: $206.8; Nova Scotia: $224.0; Prince Edward Island: $37.8; Newfoundland and Labrador: $158.0; Non-Insured Health Benefits: $429.2; Total plans: $9,813.1.
The results suggest that the public drug plans paid 78.7% of the overall prescription drug expenditure level for their beneficiaries, including drug costs, dispensing costs and markups.
Variations among the plans are mainly due to differences in plan designs and the specific government–patient cost-sharing structures (Appendix A). These differences limit the comparability of results among the jurisdictions. For instance, public drug plans in British Columbia, Saskatchewan and Manitoba provide income-based coverage to the general population, and the expenditure levels include accepted amounts for individuals who are eligible for coverage but have not submitted an application and, therefore, do not have a
defined deductible.Footnote 1
Figure 2.3 gives the number of active beneficiaries as an absolute number and as a share of the total population for each jurisdiction for 2013/14.Footnote 8 Footnote 9 It also reports the number of prescriptions that were accepted for reimbursement.
Figure 2.3 Number of active beneficiaries and associated number of prescriptions in NPDUIS public drug plans, 2013/14
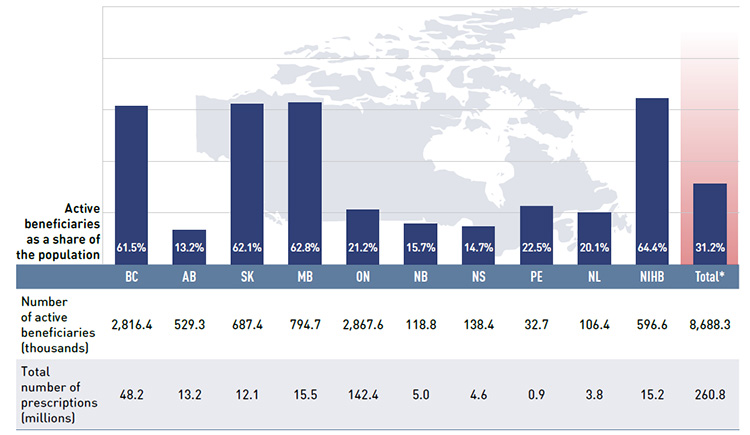
*Total results for the public drug plans reported in this figure.
Data sources: National Prescription Drug Utilization Information System Database, Canadian Institute for Health Information;
Statistics Canada, CANSIM Table 051-0001; Non-Insured Health Benefits Program Annual Report, 2013/14.
Figure description
This bar graph describes the number of active beneficiaries, their share of the population, and the associated number of prescriptions for the NPDUIS public drug plans in 2013/14.
|
British Columbia |
Alberta |
Saskatchewan |
Manitoba |
Ontario |
New Brunswick |
Nova Scotia |
Prince Edward Island |
Newfoundland and Labrador |
Non-Insured Health Benefits |
Total |
| Number of active beneficiaries (thousands); percent share of population |
2,816.4 (61.5%) |
529.3 (13.2%) |
687.4 (62.1%) |
794.7 (62.8%) |
2,867.6 (21.2%) |
118.8 (15.7%) |
138.4 (14.7%) |
32.7 (22.5%) |
106.4 (20.1%) |
596.6 (64.4%) |
8,688.3 (31.2%) |
| Total number of prescriptions (millions) |
48.2 |
13.2 |
12.1 |
15.5 |
142.4 |
5.0 |
4.6 |
0.9 |
3.8 |
15.2 |
260.8 |
Nearly 8.7 million active beneficiaries had 260.8 million prescriptions accepted towards a deductible or paid for (in full or in part) by the NPDUIS public drug plans. These beneficiaries accounted for almost a third (31.2%) of the total provincial and NIHB client populations.
The variations in the active beneficiary share of the population are related to the plan designs, with income-based plans in British Columbia (61.5%), Saskatchewan (62.1%) and Manitoba (62.8%) providing drug coverage for the general population. Other plans that focused their coverage on seniors, income assistance recipients and various patient groups had a smaller representation of active beneficiaries in the population, ranging from 13.2% to 22.5%. Nevertheless, these provinces also paid a higher share of the prescription cost for their active beneficiaries (Figure 2.2). The NIHB had the highest participation rate (64.4%), as it provided universal coverage to its clients.
Figure 2.4 reports the shares of non-senior and senior beneficiaries in 2013/14. Overall there was a greater proportion of non-seniors (58.9%) than seniors (41.1%).
There were wide variations in distribution at the jurisdictional level, mainly related to the specific plan designs. As discussed, British Columbia, Saskatchewan and Manitoba have income-based plans, and hence, a relatively high non-senior representation (76.4%, 78.5%
and 78.7%, respectively). In other plans, the share of non-senior beneficiaries ranged from 19.0% to 53.9%. In the NIHB, non-seniors accounted for 92.6% of the active beneficiaries, reflecting its unique demographic profile.
Alberta, Nova Scotia and Prince Edward Island do not submit data to NPDUIS for all their sub-plans, so their non-senior shares may be under-represented.
Figure 2.4 Shares of non-senior and senior active beneficiaries in NPDUIS public drug plans, 2013/14
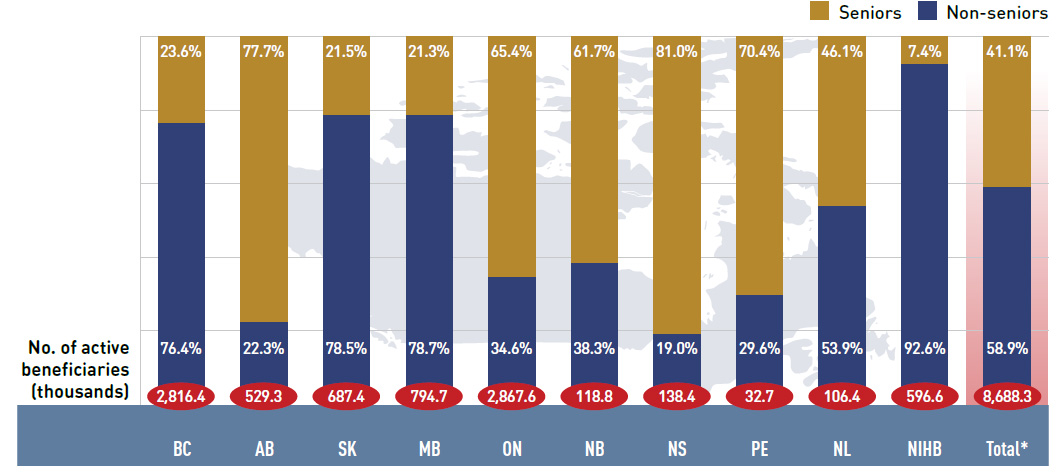
*Total results for the public drug plans reported in this figure.
Data source: National Prescription Drug Utilization Information System Database, Canadian Institute for Health Information.
Figure description
This bar graph describes the shares of senior and non-senior active beneficiaries for the NPDUIS public drug plans in 2013/14.
|
British Columbia |
Alberta |
Saskatchewan |
Manitoba |
Ontario |
New Brunswick |
Nova Scotia |
Prince Edward Island |
Newfoundland and Labrador |
Non-Insured Health Benefits |
Total |
| Number of active beneficiaries (thousands) |
2,816.4 |
529.3 |
687.4 |
794.7 |
2,867.6 |
118.8 |
138.4 |
32.7 |
106.4 |
596.6 |
8,688.3 |
| Seniors |
23.6% |
77.7% |
21.5% |
21.3% |
65.4% |
61.7% |
81.0% |
70.4% |
46.1% |
7.4% |
41.1% |
| Non-seniors |
76.4% |
22.3% |
78.5% |
78.7% |
34.6% |
38.3% |
19.0% |
29.6% |
53.9% |
92.6% |
58.9% |
Figure 2.5 reports the average annual prescription drug cost per senior beneficiary in 2013/14, stratified by five-year age bands. Limiting the data to seniors allows for a greater comparability across plans.
With a few exceptions, the results show that the annual drug cost for seniors was higher in the older age groups. The average drug cost for all plans ranged from $1,240 for beneficiaries between 65 and 69 years old to $1,976 for those over 85, as comorbidity and chronic
conditions generally increase with age.
There is some jurisdictional variation in the annual drug costs for these age groups. This may be due to the differences in plan designs, the disease profiles of the population, drug coverage or prescribing patterns. Annual drug costs for seniors have declined in recent years due to generic entry and pricing policies for drugs that are generally used by older beneficiaries. This trend is explored further in Figure 3.5.
Figure 2.5 Average annual prescription drug cost per senior beneficiary, by five-year age bands, NPDUIS public drug plans, 2013/14
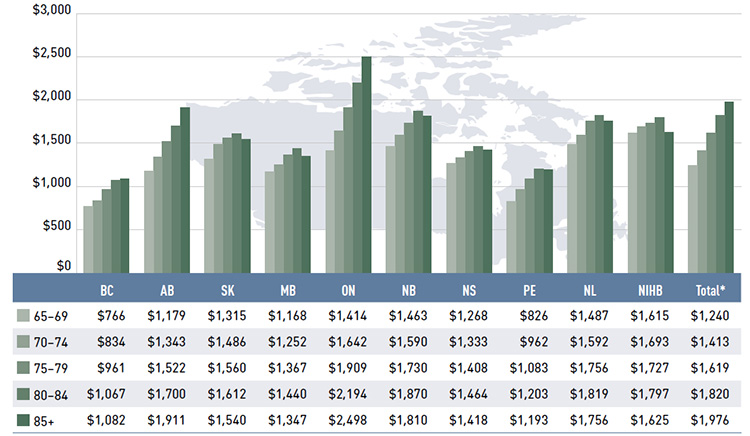
*Total results for the public drug plans reported in this figure.
Data source: National Prescription Drug Utilization Information System Database, Canadian Institute for Health Information.
Figure description
This bar graph describes the average annual prescription drug cost per senior beneficiary by five-year age bands for the NPDUIS drug plans in 2013/14.
|
British Columbia |
Alberta |
Saskatchewan |
Manitoba |
Ontario |
New Brunswick |
Nova Scotia |
Prince Edward Island |
Newfoundland and Labrador |
Non-Insured Health Benefits |
Total |
| 65-69 |
$766 |
$1,179 |
$1,315 |
$1,168 |
$1,414 |
$1,463 |
$1,268 |
$826 |
$1,487 |
$1,615 |
$1,240 |
| 70-74 |
$834 |
$1,343 |
$1,486 |
$1,252 |
$1,642 |
$1,590 |
$1,333 |
$962 |
$1,592 |
$1,693 |
$1,413 |
| 75-79 |
$961 |
$1,522 |
$1,560 |
$1,367 |
$1,909 |
$1,730 |
$1,408 |
$1,083 |
$1,756 |
$1,727 |
$1,619 |
| 80-84 |
$1,067 |
$1,700 |
$1,612 |
$1,440 |
$2,194 |
$1,870 |
$1,464 |
$1,203 |
$1,819 |
$1,797 |
$1,820 |
| 85+ |
$1,082 |
$1,911 |
$1,540 |
$1,347 |
$2,498 |
$1,810 |
$1,418 |
$1,193 |
$1,756 |
$1,625 |
$1,976 |
Figure 2.6 shows the distribution of active beneficiaries in 2013/14 based on their annual prescription cost levels: ‹$500, $500–$1,000, $1,000–$10,000 and $10,000+. The share of active beneficiaries in each of these groups is presented in Figure 2.6a, with the
corresponding share of prescription drug expenditures provided in Figure 2.6b.
The results show that high-cost beneficiaries with $10,000 or more in annual prescription costs represented a small proportion of the active beneficiaries, ranging from 0.6% to 2.3% depending on the plan. However, they accounted for a disproportionate share of expenditures, ranging from 16.4% to 29.2% across the public drug plans. These high-cost beneficiaries are more likely to have chronic conditions, comorbiditiesFootnote 10 or require treatment with expensive therapies such as biologics.
Conversely, those with annual treatment costs under $1,000 represented the majority of active beneficiaries, ranging from 54.5% to 88.0% depending on the plan. These beneficiaries accounted for a relatively low share of prescription drug expenditures, ranging from
11.5% to 31.3% of the total for 2013/14.
Figure 2.6 Share of active beneficiaries and prescription drug expenditures, by annual individual prescription drug cost levels, NPDUIS public drug plans, 2013/14
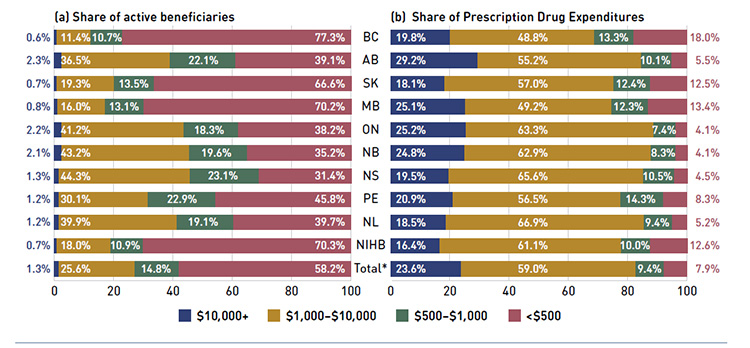
*Total results for the public drug plans reported in this figure.
Note: Values may not add to totals due to rounding.
Data source: National Prescription Drug Utilization Information System Database, Canadian Institute for Health Information.
Figure description
This figure shows two complementary bar graphs side-by-side.
The left-hand graph shows the shares of active beneficiaries in 2013/14 by annual prescription drug cost levels in the NPDUIS public drug plans:
|
$10,000 or greater |
$1,000 to $10,000 |
$500 to $1,000 |
Less than $500 |
| Total |
1.3% |
25.6% |
14.8% |
58.2% |
| Non-Insured Health Benefits |
0.7% |
18.0% |
10.9% |
70.3% |
| Newfoundland and Labrador |
1.2% |
39.9% |
19.1% |
39.7% |
| Prince Edward Island |
1.2% |
30.1% |
22.9% |
45.8% |
| Nova Scotia |
1.3% |
44.3% |
23.1% |
31.4% |
| New Brunswick |
2.1% |
43.2% |
19.6% |
35.2% |
| Ontario |
2.2% |
41.2% |
18.3% |
38.2% |
| Manitoba |
0.8% |
16.0% |
13.1% |
70.2% |
| Saskatchewan |
0.7% |
19.3% |
13.5% |
66.6% |
| Alberta |
2.3% |
36.5% |
22.1% |
39.1% |
| British Columbia |
0.6% |
11.4% |
10.7% |
77.3% |
The right-hand graph depicts the corresponding shares of prescription drug expenditures:
|
$10,000 or greater |
$1,000 to $10,000 |
$500 to $1,000 |
Less than $500 |
| Total |
23.6% |
59.0% |
9.4% |
7.9% |
| Non-Insured Health Benefits |
16.4% |
61.1% |
10.0% |
12.6% |
| Newfoundland and Labrador |
18.5% |
66.9% |
9.4% |
5.2% |
| Prince Edward Island |
20.9% |
56.5% |
14.3% |
8.3% |
| Nova Scotia |
19.5% |
65.6% |
10.5% |
4.5% |
| New Brunswick |
24.8% |
62.9% |
8.3% |
4.1% |
| Ontario |
25.2% |
63.3% |
7.4% |
4.1% |
| Manitoba |
25.1% |
49.2% |
12.3% |
13.4% |
| Saskatchewan |
18.1% |
57.0% |
12.4% |
12.5% |
| Alberta |
29.2% |
55.2% |
10.1% |
5.5% |
| British Columbia |
19.8% |
48.8% |
13.3% |
18.0% |
3 Trends in Prescription Drug Expenditures, 2009/10 to 2013/14
A review of the recent trends in prescription drug expenditures suggests that the rate of growth in the dispensing cost component exceeded that in the drug cost component, with dispensing costs accounting for an increased share of prescription costs. In 2013/14, drug cost levels in some plans continued to decline, following the trend observed in 2012/13; however, other plans saw a reversal of this trend, having positive rates of growth in drug cost.
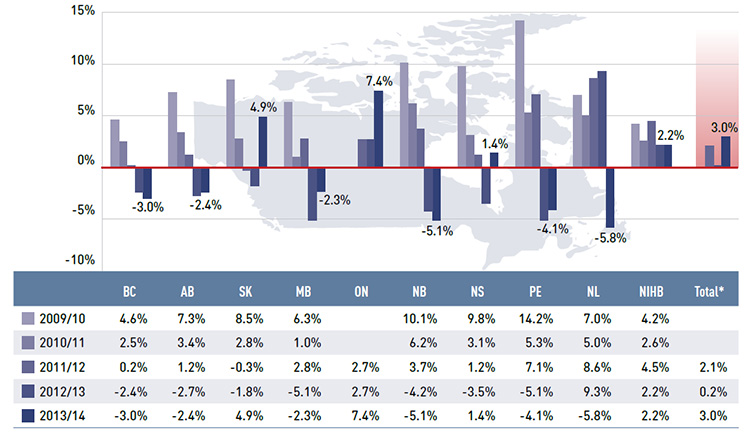
Figure 3.1 reports the annual rates of change in prescription drug expenditures from fiscal year 2009/10 to 2013/14. Growth has slowed considerably in recent years, with low positive or negative rates of change in most public plans.
In 2013/14, the rates of change in prescription costs averaged 3.0% for the public drug plans, which exceeded the overall growth in the previous two years (0.2% in 2012/13 and 2.1% in 2011/12). For just over half of plans, these rates were negative, ranging from –5.8% in Newfoundland and Labrador to –2.3% in Manitoba. Ontario and Saskatchewan had the highest rate of growth at 7.4% and 4.9%, respectively, while Nova Scotia and the NIHB had low positive rates of growth of 1.4% and 2.2%, respectively. The prescription
drug expenditures reported include drug costs, dispensing costs and markups, where applicable.
A number of factors drive the year-over-year change in prescription drug expenditures, such as demographic, volume, price and drug-mix effects. These are discussed in detail in Sections 4 and 5, with a focus on the rates of change from 2012/13 to 2013/14.
Figure 3.1 Annual rates of change in prescription drug expenditures, NPDUIS public drug plans, 2009/10 to 2013/14
*Total results for the public drug plans reported in this figure.
Data source: National Prescription Drug Utilization Information System Database, Canadian Institute for Health Information.
Figure description
This bar graph shows the trend in annual rates of change in prescription drug expenditures from 2009/10 to 2013/14 for the NPDUIS public drug plans.
|
British Columbia |
Alberta |
Saskatchewan |
Manitoba |
Ontario |
New Brunswick |
Nova Scotia |
Prince Edward Island |
Newfoundland and Labrador |
Non-Insured Health Benefits |
Total |
| 2009/10 |
4.6% |
7.3% |
8.5% |
6.3% |
‒ |
10.1% |
9.8% |
14.2% |
7.0% |
4.2% |
‒ |
| 2010/11 |
2.5% |
3.4% |
2.8% |
1.0% |
‒ |
6.2% |
3.1% |
5.3% |
5.0% |
2.6% |
‒ |
| 2011/12 |
0.2% |
1.2% |
-0.3% |
2.8% |
2.7% |
3.7% |
1.2% |
7.1% |
8.6% |
4.5% |
2.1% |
| 2012/13 |
-2.4% |
-2.7% |
-1.8% |
-5.1% |
2.7% |
-4.2% |
-3.5% |
-5.1% |
9.3% |
2.2% |
0.2% |
| 2013/14 |
-3.0% |
-2.4% |
4.9% |
-2.3% |
7.4% |
-5.1% |
1.4% |
-4.1% |
-5.8% |
2.2% |
3.0% |
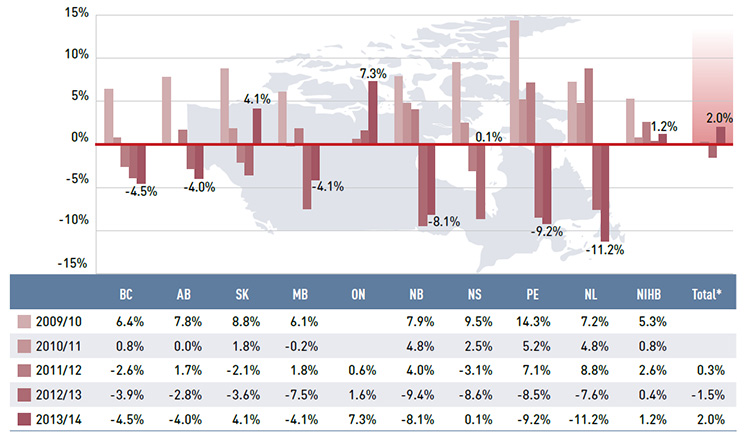
Figure 3.2 reports the annual rates of change in drug costs, which is the largest component of prescription expenditure (74.2% in 2013/14, see Figure 2.1).
While the overall drug cost levels increased by 2.0% in 2013/14, this reflects very diverse rates of growth across the public plans. Many plans continued the trend of declining drug costs observed in previous years. A few provinces saw positive rates of growth in 2013/14, a reversal of what was observed the year before. Ontario had the highest rate of growth at 7.3%, followed by Saskatchewan at 4.1%. The NIHB and Nova Scotia had small or virtually no growth in drug costs, with rates of 1.2% and 0.1%, respectively. All the other drug plans
had negative rates of change in drug costs ranging from -4.0% to -11.2%.
The changes in drug costs are driven by several opposing “push” (positive) effects and “pull” (negative) effects which nearly off-set each other in recent years. Section 4 provides a detailed analysis of the factors behind these trends.
Figure 3.2 Annual rates of change in drug costs, NPDUIS public drug plans, 2009/10 to 2013/14
*Total results for the public drug plans reported in this figure.
Data source: National Prescription Drug Utilization Information System Database, Canadian Institute for Health Information.
Figure description
This bar graph shows the trend in annual rates of change in drug costs from 2009/10 to 2013/14 for the NPDUIS public drug plans.
|
British Columbia |
Alberta |
Saskatchewan |
Manitoba |
Ontario |
New Brunswick |
Nova Scotia |
Prince Edward Island |
Newfoundland and Labrador |
Non-Insured Health Benefits |
Total |
| 2009/10 |
6.4% |
7.8% |
8.8% |
6.1% |
‒ |
7.9% |
9.5% |
14.3% |
7.2% |
5.3% |
‒ |
| 2010/11 |
0.8% |
0.0% |
1.8% |
-0.2% |
‒ |
4.8% |
2.5% |
5.2% |
4.8% |
0.8% |
‒ |
| 2011/12 |
-2.6% |
1.7% |
-2.1% |
1.8% |
0.6% |
4.0% |
-3.1% |
7.1% |
8.8% |
2.6% |
0.3% |
| 2012/13 |
-3.9% |
-2.8% |
-3.6% |
-7.5% |
1.6% |
-9.4% |
-8.6% |
-8.5% |
-7.6% |
0.4% |
-1.5% |
| 2013/14 |
-4.5% |
-4.0% |
4.1% |
-4.1% |
7.3% |
-8.1% |
0.1% |
-9.2% |
-11.2% |
1.2% |
2.0% |
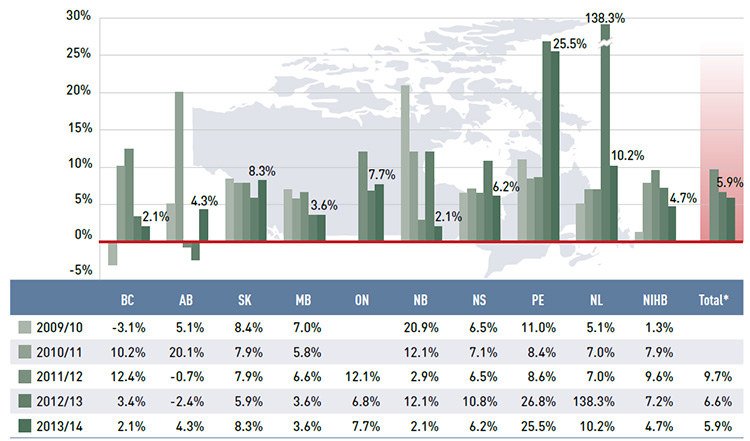
Figure 3.3 reports the annual rates of change in the dispensing component of the prescription cost. Unlike drug costs, dispensing costs grew in all public drug plans. In 2013/14, the rate of change averaged 5.9%, following considerable increases in previous years (6.6% in 2012/13 and 9.7% in 2011/12). Prince Edward Island had a particularly high rate of growth of 25.5%, mainly due to an increase in the dispensing fees per prescription reimbursed (see Section 5). Newfoundland and LabradorFootnote II, Saskatchewan and Ontario had substantial rates of growth in dispensing costs: 10.2%, 8.3% and 7.7%, respectively. The rates of growth in dispensing costs in other plans ranged from 2.1% in New Brunswick and British Columbia to 6.2% in Nova Scotia.
Figure 3.3 Annual rates of change in dispensing costs, NPDUIS public drug plans, 2009/10 to 2013/14
*Total results for the public drug plans reported in this figure.
Note: Due to the lack of available data, a limited number of years are reported for Ontario.
Data source: National Prescription Drug Utilization Information System Database, Canadian Institute for Health Information.
Figure description
This bar graph shows the trend in annual rates of change in dispensing costs from 2009/10 to 2013/14 for the NPDUIS public drug plans.
|
British Columbia |
Alberta |
Saskatchewan |
Manitoba |
Ontario |
New Brunswick |
Nova Scotia |
Prince Edward Island |
Newfoundland and Labrador |
Non-Insured Health Benefits |
Total |
| 2009/10 |
-3.1% |
5.1% |
8.4% |
7.0% |
‒ |
20.9% |
6.5% |
11.0% |
5.1% |
1.3% |
‒ |
| 2010/11 |
10.2% |
20.1% |
7.9% |
5.8% |
‒ |
12.1% |
7.1% |
8.4% |
7.0% |
7.9% |
‒ |
| 2011/12 |
12.4% |
-0.7% |
7.9% |
6.6% |
12.1% |
2.9% |
6.5% |
8.6% |
7.0% |
9.6% |
9.7% |
| 2012/13 |
3.4% |
-2.4% |
5.9% |
3.6% |
6.8% |
12.1% |
10.8% |
26.8% |
138.3% |
7.2% |
6.6% |
| 2013/14 |
2.1% |
4.3% |
8.3% |
3.6% |
7.7% |
2.1% |
6.2% |
25.5% |
10.2% |
4.7% |
5.9% |
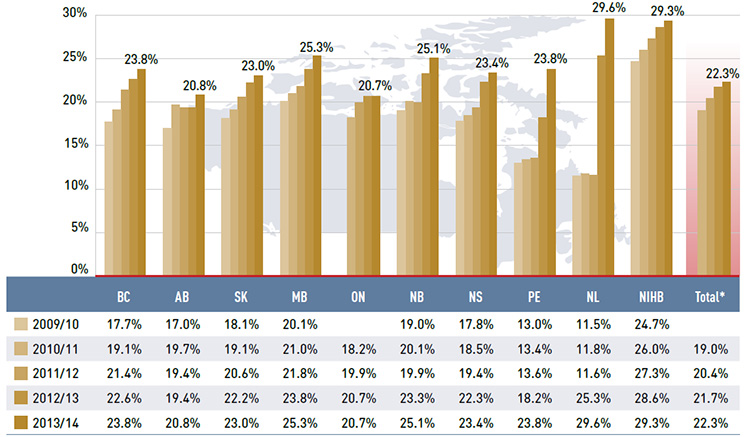
Figure 3.4 reports the dispensing costs as a share of total prescription costs by fiscal year. Without exception, dispensing cost shares increased markedly in recent years. In 2010/11, the average share was 19.0% and by 2013/14 it rose to 22.3%, an increase of 3.3%. The increase from 2009/10 to 2013/14 was most notable in two jurisdictions: Newfoundland and Labrador, where the dispensing cost share increased from 11.5% to 29.6%; and Prince Edward Island, with a 10.8% increase in the dispensing cost share over the study period.
These increases were mainly due to changes in plan designs and the reimbursed dispensing fees (see Table 5.1). Other jurisdictions with notable increases included Nova Scotia, British Columbia and New Brunswick, with the dispensing cost share increasing in the range
of 5.6% to 6.1% over the five-year period.
Jurisdictional variations are driven by differences in the average dispensing fee per prescription, prescription size and the market share of brand-name and generic drugs in each plan. The results only reflect fees for dispensing medications; other professional pharmacy services are excluded.
Section 5 provides a detailed analysis of the factors impacting dispensing costs from 2012/13 to 2013/14.
Figure 3.4 Annual dispensing costs as a share of prescription costs, NPDUIS public drug plans, 2009/10 to 2013/14
* Total results for the public drug plans reported in this figure.
Note: Due to the lack of available data, a limited number of years are reported for Ontario.
Data source: National Prescription Drug Utilization Information System Database, Canadian Institute for Health Information.
Figure description
This bar graph shows the trend in annual dispensing costs as a share of prescription costs from 2009/10 to 2013/14 for the NPDUIS public drug plans.
|
British Columbia |
Alberta |
Saskatchewan |
Manitoba |
Ontario |
New Brunswick |
Nova Scotia |
Prince Edward Island |
Newfoundland and Labrador |
Non-Insured Health Benefits |
Total |
| 2009/10 |
17.7% |
17.0% |
18.1% |
20.1% |
‒ |
19.0% |
17.8% |
13.0% |
11.5% |
24.7% |
‒ |
| 2010/11 |
19.1% |
19.7% |
19.1% |
21.0% |
18.2% |
20.1% |
18.5% |
13.4% |
11.8% |
26.0% |
19.0% |
| 2011/12 |
21.4% |
19.4% |
20.6% |
21.8% |
19.9% |
19.9% |
19.4% |
13.6% |
11.6% |
27.3% |
20.4% |
| 2012/13 |
22.6% |
19.4% |
22.2% |
23.8% |
20.7% |
23.3% |
22.3% |
18.2% |
25.3% |
28.6% |
21.7% |
| 2013/14 |
23.8% |
20.8% |
23.0% |
25.3% |
20.7% |
25.1% |
23.4% |
23.8% |
29.6% |
29.3% |
22.3% |
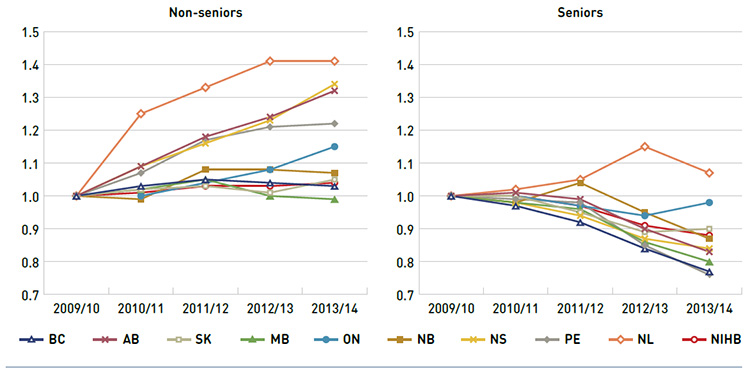
Public drug plan expenditures can vary depending on the plan designs and the demographic and disease profiles of the beneficiary populations. Some plans mainly cover the senior population and catastrophic drug costs, while others are more broadly income-based. To provide for greater comparability across plans, Figure 3.5 reports separately on trends in the average annual prescription cost per active beneficiary for non-seniors and seniors from 2009/10 to 2013/14. An index was used to equate the average annual cost in each plan and for each patient group to the value of 1 for the base year 2009/10. The values for subsequent years were then calculated relative to the base year.
The results indicate that, in general, the annual cost of drug treatment for senior beneficiaries has been declining. This is mainly due to their relatively high use of drugs that benefited from generic launches and generic pricing policies. Newfoundland and Labrador is the only plan with increases in the average annual prescription cost per active beneficiary in the senior population.
In contrast, the cost to treat non-senior patients rose rapidly in several provinces: Alberta, Nova Scotia, Prince Edward Island, Newfoundland and Labrador, and Ontario. This may be due to the increased use of high-cost drugs, such as biologics, and the introduction of new sub-plans that expanded drug coverage to non-seniors (e.g., Nova Scotia’s Family Pharmacare Program, launched in March 2008). The plans in British Columbia, Saskatchewan, Manitoba and the NIHB, which provide coverage to a general population, had a more stable average annual prescription cost for non-seniors.
The variability in results for Newfoundland and Labrador are due to changes in the dispensing fee portion of total prescription cost, which includes fees, drug costs and markup. There was a large increase in dispensing fee costs in 2012/13 for two reasons: (i) fees became part of the co-payment structure for seniors; and (ii) there were increases in the fees paid to pharmacies in light of generic price reductions.
Figure 3.5 Index of the average annual prescription cost per beneficiary, non-seniors and seniors, NPDUIS public drug plans, 2009/10 to 2013/14
Note: Due to a lack of available data, the index for Ontario starts with 2010/11.
Data source: National Prescription Drug Utilization Information System Database, Canadian Institute for Health Information.
Figure description
This figure shows two complementary line graphs side-by-side. Using an index, the left graph describes the change in the annual prescription cost per beneficiary for non-seniors in the NPDUIS public drug plans over a five-year period:
|
2009/10 |
2010/11 |
2011/12 |
2012/13 |
2013/14 |
| British Columbia |
1.00 |
1.03 |
1.05 |
1.04 |
1.03 |
| Alberta |
1.00 |
1.09 |
1.18 |
1.24 |
1.32 |
| Saskatchewan |
1.00 |
1.02 |
1.03 |
1.01 |
1.05 |
| Manitoba |
1.00 |
1.02 |
1.05 |
1.00 |
0.99 |
| Ontario |
‒ |
1.00 |
1.04 |
1.08 |
1.15 |
| New Brunswick |
1.00 |
0.99 |
1.08 |
1.08 |
1.07 |
| Nova Scotia |
1.00 |
1.09 |
1.16 |
1.23 |
1.34 |
| Prince Edward Island |
1.00 |
1.07 |
1.17 |
1.21 |
1.22 |
| Newfoundland and Labrador |
1.00 |
1.25 |
1.33 |
1.41 |
1.41 |
| Non-Insured Health Benefits |
1.00 |
1.01 |
1.03 |
1.03 |
1.04 |
The right graph shows the same index for seniors:
|
2009/10 |
2010/11 |
2011/12 |
2012/13 |
2013/14 |
| British Columbia |
1.00 |
0.97 |
0.92 |
0.84 |
0.77 |
| Alberta |
1.00 |
1.01 |
0.99 |
0.90 |
0.83 |
| Saskatchewan |
1.00 |
1.00 |
0.95 |
0.89 |
0.90 |
| Manitoba |
1.00 |
0.98 |
0.96 |
0.86 |
0.80 |
| Ontario |
‒ |
1.00 |
0.97 |
0.94 |
0.98 |
| New Brunswick |
1.00 |
0.98 |
1.04 |
0.95 |
0.87 |
| Nova Scotia |
1.00 |
0.98 |
0.94 |
0.87 |
0.84 |
| Prince Edward Island |
1.00 |
0.99 |
0.98 |
0.85 |
0.76 |
| Newfoundland and Labrador |
1.00 |
1.02 |
1.05 |
1.15 |
1.07 |
| Non-Insured Health Benefits |
1.00 |
1.00 |
0.97 |
0.91 |
0.88 |
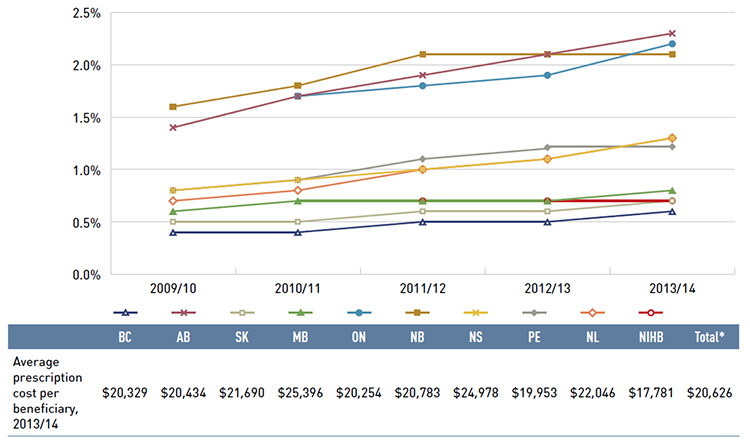
Figure 3.6 reports on trends in the share of high-cost beneficiaries with annual prescription drug costs exceeding $10,000. The results indicate that although the proportion of these patients is relatively small (1.3% across plans in 2013/14, see Figure 2.6), it has been gradually increasing in all public drug plans from 2009/10 to 2013/14.
In 2013/14, the average annual prescription cost per beneficiary for this group ranged from $17,781 in the NIHB to $25,396 in Manitoba.
Figure 3.6 Share of patients with $10,000+ in annual prescription drug costs, NPDUIS public drug plans, 2009/10 to 2013/14
* Total results for the public drug plans reported in this figure.
Note: Due to the lack of available data, a limited number of years are reported for Ontario (2010/11 to 2013/14) and the NIHB (2011/12 to 2013/14).
Data source: National Prescription Drug Utilization Information System Database, Canadian Institute for Health Information.
Figure description
This figure gives the share of patients with more than $10,000 in annual prescription drug costs in the NPDUIS public drug plans from 2009/10 to 2013/14. An associated table lists the average prescription costs per beneficiary in 2012/13 for all of the plans.
The share of patients is as follows:
|
2009/10 |
2010/11 |
2011/12 |
2012/13 |
2013/14 |
| British Columbia |
0.4% |
0.4% |
0.5% |
0.5% |
0.6% |
| Alberta |
1.4% |
1.7% |
1.9% |
2.1% |
2.3% |
| Saskatchewan |
0.5% |
0.5% |
0.6% |
0.6% |
0.7% |
| Manitoba |
0.6% |
0.7% |
0.7% |
0.7% |
0.8% |
| Ontario |
‒ |
1.7% |
1.8% |
1.9% |
2.2% |
| New Brunswick |
1.6% |
1.8% |
2.1% |
2.1% |
2.1% |
| Nova Scotia |
0.8% |
0.9% |
1.0% |
1.1% |
1.3% |
| Prince Edward Island |
0.8% |
0.9% |
1.1% |
1.2% |
1.2% |
| Newfoundland and Labrador |
0.7% |
0.8% |
1.0% |
1.1% |
1.2% |
| Non-Insured Health Benefits |
‒ |
‒ |
0.7% |
0.7% |
0.7% |
The average prescription cost per beneficiary for the public drug plans in 2013/14 was British Columbia: $20,329; Alberta: $20,434; Saskatchewan: $21,690; Manitoba: $25,396; Ontario: $20,254; New Brunswick: $20,783; Nova Scotia: $24,978; Prince Edward Island: $19,953; Newfoundland and Labrador: $22,046; Non-Insured Health Benefits: $17,781; Total NPDUIS plans: $20,626.
4 The Drivers of Drug Costs, 2012/13 to 2013/14
Changes in drug costs are driven by a number of opposing “push” and “pull” effects. An increase in the beneficiary population, the use of drugs, and the use of more expensive drugs will put an upward pressure on costs, resulting in a push effect; while generic substitutions and price reductions will exert a downward pull effect. The net effect of these opposing forces yields the overall rate of change.
In any given year, the weight of each of these effects may vary, and as a result, the rates of change in drug costs evolve over time and vary across public drug plans.
This section of the CompassRx report provides a comprehensive cost driver analysis that reveals the most important cost pressures, measures their impact on drug cost levels, and delves into the factors determining trends in costs, pricing and utilization in public plans.
This edition of the report focuses on the rates of change in drug expenditures from fiscal year 2012/13 to 2013/14 and includes data for two additional public plans – British Columbia and Newfoundland and Labrador – that were not included in the first edition. Thus, the results reported for the total of all drug plans in the two editions are not directly comparable. However, plan-by-plan comparisons are still relevant, and the interpretation of general trends is still appropriate.
Four broad categories of effects are analyzed along with their corresponding sub-effects.
Price Effects
- Price change effect – changes in the prices of both brand-name and generic drugs
- Generic substitution effect – shifts from brand-name to generic drugs
Demographic Effects
- Population effect – changes in the number of active beneficiaries
- Aging effect – shifts in the distribution of the population across age groups
- Gender effect – shifts in the distribution of the population by gender
Volume Effects
- Prescription volume effect – changes in the number of prescriptions dispensed to patients
- Prescription size effect – changes in the average number of units of a drug dispensed per prescription
- Strength-form effect – shifts in the use of various strengths or forms of an ingredient
Drug-Mix EffectsFootnote III
- Shifts in the use of drugs
Each of these effects was determined by assuming that all the other effects remained constant over the periods analyzed. The results provide an answer to the following question:
"How much would public plan drug costs have changed between 2012/13 and 2013/14 if only one factor (e.g., the price of drugs) changed while all the others remained the same?"
In reality, multiple factors change simultaneously, creating a residual or a cross effect, which is also reported to account for the total change.
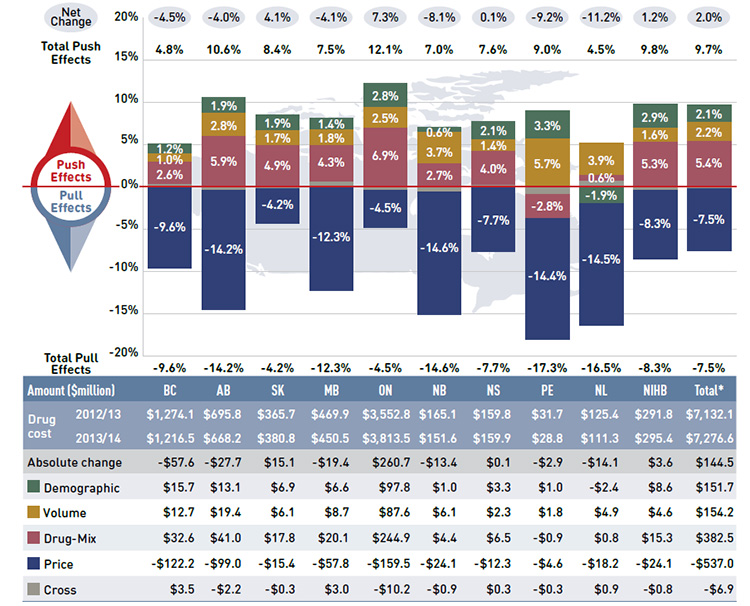
Figure 4.1 reports the rate of change in drug costs for the public drug plans over the fiscal years 2012/13 to 2013/14 separated into the four broad categories of effects. The bar graph and the associated table show the impacts of each effect as a percent and absolute change in drug cost, respectively.
While the overall rate of change in drug costs across all plans was 2.0% or $144.5 million in absolute terms, there are important jurisdictional variations, with some plans such as New Brunswick, Prince Edward Island, and Newfoundland and Labrador experiencing
significant negative rates of change. The net effect conceals the underlying opposing components of change, and the total rate of change is heavily influenced by Ontario because of its size.
Figure 4.1 Rates of change in drug costs by demographic, volume, price and drug-mix effects, NPDUIS public drug plans, 2012/13 to 2013/14
*Total results for the public drug plans reported in this figure.
Note: Values may not add to totals due to rounding.
Data source: National Prescription Drug Utilization Information System Database, Canadian Institute for Health Information.
Figure description
This bar graph and table describe the factors that impacted the rates of change in drug cost in the NPDUIS public drug plans from 2012/13 to 2013/14.
The bar graph shows the percent change from 2012/13 to 2013/14 for each public drug plan. The results are as follows:
|
British Columbia |
Alberta |
Saskatchewan |
Manitoba |
Ontario |
New Brunswick |
Nova Scotia |
Prince Edward Island |
Newfoundland and Labrador |
Non-Insured Health Benefits |
Total |
| Net change |
-4.5% |
-4.0% |
4.1% |
-4.1% |
7.3% |
-8.1% |
0.1% |
-9.2% |
-11.2% |
1.2% |
2.0% |
| Total push effects |
4.8% |
10.6% |
8.4% |
7.5% |
12.1% |
7.0% |
7.6% |
9.0% |
4.5% |
9.8% |
9.7% |
| Demographic Effect |
1.2% |
1.9% |
1.9% |
1.4% |
2.8% |
0.6% |
2.1% |
3.3% |
-1.9% |
2.9% |
2.1% |
| Volume Effect |
1.0% |
2.8% |
1.7% |
1.8% |
2.5% |
3.7% |
1.4% |
5.7% |
3.9% |
1.6% |
2.2% |
| Drug-Mix Effect |
2.6% |
5.9% |
4.9% |
4.3% |
6.9% |
2.7% |
4.0% |
-2.8% |
0.6% |
5.3% |
5.4% |
| Price Effects |
-9.6% |
-14.2% |
-4.2% |
-12.3% |
-4.5% |
-14.6% |
-7.7% |
-14.4% |
-14.5% |
-8.3% |
-7.5% |
| Cross Effect |
0.3% |
-0.3% |
-0.1% |
0.6% |
-0.3% |
-0.5% |
0.2% |
-0.9% |
0.7% |
-0.3% |
-0.1% |
The accompanying table gives the corresponding changes in millions of dollars.
|
British Columbia |
Alberta |
Saskatchewan |
Manitoba |
Ontario |
New Brunswick |
Nova Scotia |
Prince Edward Island |
Newfoundland and Labrador |
Non-Insured Health Benefits |
Total |
| Drug cost 2012/13 |
$1,274.1 |
$695.8 |
$365.7 |
$469.9 |
$3,552.8 |
$165.1 |
$159.8 |
$31.7 |
$125.4 |
$291.8 |
$7,132.1 |
| Drug cost 2013/14 |
$1,216.5 |
$668.2 |
$380.8 |
$450.5 |
$3,813.5 |
$151.6 |
$159.9 |
$28.8 |
$111.3 |
$295.4 |
$7,276.6 |
| Absolute change |
-$57.6 |
-$27.7 |
$15.1 |
-$19.4 |
$260.7 |
-$13.4 |
$0.1 |
-$2.9 |
-$14.1 |
$3.6 |
$144.5 |
| Demographic Effect |
$15.7 |
$13.1 |
$6.9 |
$6.6 |
$97.8 |
$1.0 |
$3.3 |
$1.0 |
-$2.4 |
$8.6 |
$151.7 |
| Volume Effect |
$12.7 |
$19.4 |
$6.1 |
$8.7 |
$87.6 |
$6.1 |
$2.3 |
$1.8 |
$4.9 |
$4.6 |
$154.2 |
| Drug-Mix Effect |
$32.6 |
$41.0 |
$17.8 |
$20.1 |
$244.9 |
$4.4 |
$6.5 |
-$0.9 |
$0.8 |
$15.3 |
$382.5 |
| Price Effects |
-$122.2 |
-$99.0 |
-$15.4 |
-$57.8 |
-$159.5 |
-$24.1 |
-$12.3 |
-$4.6 |
-$18.2 |
-$24.1 |
-$537.0 |
| Cross Effect |
$3.5 |
-$2.2 |
-$0.3 |
$3.0 |
-$10.2 |
-$0.9 |
$0.3 |
-$0.3 |
$0.9 |
-$0.8 |
-$6.9 |
Price effects had the greatest “pull” on drug cost levels, with the implementation of generic price reductions and generic substitutions resulting in significant savings to the public plans. If all other factors had remained unchanged, the reduction in drug prices along with the shift from higher-cost brand-name products to lower-cost generic products would have reduced the drug costs in 2013/14 by an average of 7.5% ($537.0 million). Note that price effects exerted a less pronounced pull force on drug costs in 2013/14 (-7.5%) than
in 2012/13 (-9.2%).Footnote 11
Conversely, demographic, volume and drug-mix effects had a large “push” effect, increasing drug cost levels. For most plans, these push effects offset most or all of the cost savings resulting from generic substitutions and price reductions. In the absence of price effects, the combined impact of increases in the active beneficiary populations, the volume of drugs used and the use of more expensive drugs would have raised the drug cost levels in 2013/14 by an average of 9.7% ($688.4 million).
These effects exerted a more pronounced push force on drug costs in 2013/14 (9.7%) than in 2012/13 (8.5%),Footnote 11 mainly due to a higher drug-mix effect in 2013/14 (5.4% or $382.5 million) compared to the previous year (4.1%). The demographic and volume effects pushed the drug cost levels upwards by 2.1% ($151.7 million) and 2.2% ($154.2 million), respectively, in 2013/14. The combined cross effect was -0.1% (-$6.9 million).
In the following sections, each of the broad categories of effects is examined in more detail.
4.1 Price Effects
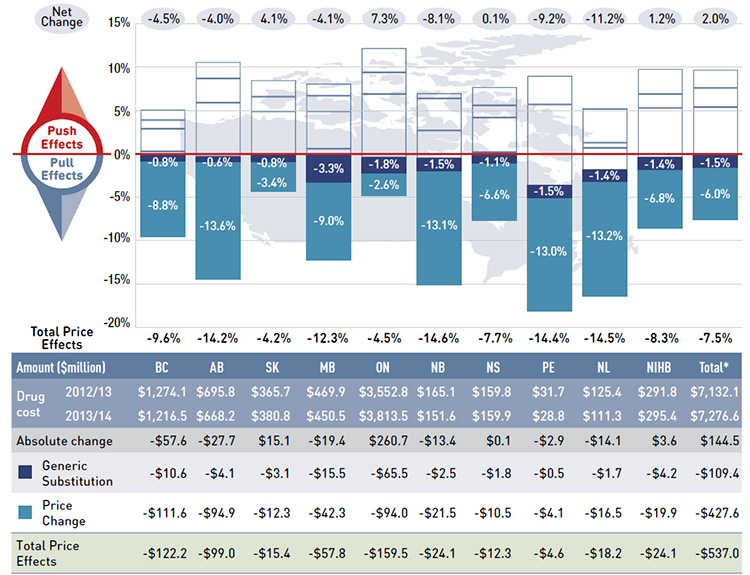
The general category of price effects can be further broken down to capture the precise impact of the price change and generic substitution effects. These effects had a marked pull down effect on drug cost levels in 2013/14, resulting in significant cost savings to the public drug plans.
Price Change Effect
This effect captures the impact of changes in drug prices and is determined at the strength, form and brand-name or generic level. It can have either a positive (increasing) or negative (decreasing) impact on drug costs. For instance, the recent generic price reforms that resulted in lower prices would have a negative price change effect on drug costs. In this analysis, drug prices are measured as the average unit cost accepted for reimbursement.
Generic Substitution Effect
This effect captures the impact of shifts in use from higher-cost brand-name products to lower-cost generic products, and has a negative (decreasing) impact on drug costs.
Figure 4.1.1 reports the rate of change in drug costs from 2012/13 to 2013/14 focusing on the two price effects: price change and generic substitution. The bar graph and accompanying table show the year-over-year impacts of each effect as a relative and absolute change in drug cost.
Figure 4.1.1 Rates of change in drug costs due to price effects, NPDUIS public drug plans, 2012/13 to 2013/14
*Total results for the public drug plans reported in this figure.
Note: Values may not add to totals due to rounding.
Data source: National Prescription Drug Utilization Information System Database, Canadian Institute for Health Information.
Figure description
This figure describes the factors that impacted the rates of change in drug cost due to price effects in the NPDUIS public drug plans from 2012/13 to 2013/14. It is composed of a bar graph with a table underneath.
The bar graph shows the percent change from 2012/13 to 2013/14 for each public drug plan. The results are as follows:
|
British Columbia |
Alberta |
Saskatchewan |
Manitoba |
Ontario |
New Brunswick |
Nova Scotia |
Prince Edward Island |
Newfoundland and Labrador |
Non-Insured Health Benefits |
Total |
| Net change |
-4.5% |
-4.0% |
4.1% |
-4.1% |
7.3% |
-8.1% |
0.1% |
-9.2% |
-11.2% |
1.2% |
2.0% |
| Total Price Effects |
-9.6% |
-14.2% |
-4.2% |
-12.3% |
-4.5% |
-14.6% |
-7.7% |
-14.4% |
-14.5% |
-8.3% |
-7.5% |
| Price Change Effect |
-8.8% |
-13.6% |
-3.4% |
-9.0% |
-2.6% |
-13.1% |
-6.6% |
-13.0% |
-13.2% |
-6.8% |
-6.0% |
| Generic Substitution Effect |
-0.8% |
-0.6% |
-0.8% |
-3.3% |
-1.8% |
-1.5% |
-1.1% |
-1.5% |
-1.4% |
-1.4% |
-1.5% |
The table below the bar graph gives the corresponding changes in millions of dollars.
|
British Columbia |
Alberta |
Saskatchewan |
Manitoba |
Ontario |
New Brunswick |
Nova Scotia |
Prince Edward Island |
Newfoundland and Labrador |
Non-Insured Health Benefits |
Total |
| Drug cost 2012/13 |
$1,274.1 |
$695.8 |
$365.7 |
$469.9 |
$3,552.8 |
$165.1 |
$159.8 |
$31.7 |
$125.4 |
$291.8 |
$7,132.1 |
| Drug cost 2013/14 |
$1,216.5 |
$668.2 |
$380.8 |
$450.5 |
$3,813.5 |
$151.6 |
$159.9 |
$28.8 |
$111.3 |
$295.4 |
$7,276.6 |
| Absolute change |
-$57.6 |
-$27.7 |
$15.1 |
-$19.4 |
$260.7 |
-$13.4 |
$0.1 |
-$2.9 |
-$14.1 |
$3.6 |
$144.5 |
| Price Change Effect |
-$111.6 |
-$94.9 |
-$12.3 |
-$42.3 |
-$94.0 |
-$21.5 |
-$10.5 |
-$4.1 |
-$16.5 |
-$19.9 |
-$427.6 |
| Generic Substitution Effect |
-$10.6 |
-$4.1 |
-$3.1 |
-$15.5 |
-$65.5 |
-$2.5 |
-$1.8 |
-$0.5 |
-$1.7 |
-$4.2 |
-$109.4 |
| Total Price Effect |
-$122.2 |
-$99.0 |
-$15.4 |
-$57.8 |
-$159.5 |
-$24.1 |
-$12.3 |
-$4.6 |
-$18.2 |
-$24.1 |
-$537.0 |
The price effects pulled drug cost levels downward by 7.5% in 2013/14, translating to a savings of $537.0 million in drug costs for the NPDUIS public plans. This was mainly due to reductions in the prices of generic drugs, which pulled costs down by 6.0%, but also by generic substitution, which pulled costs by 1.5%. The price effects were less pronounced in 2013/14 (-7.5%) than in 2012/13 (-9.2%),Footnote 11 mainly due to reduced savings from generic substitution.
The impact of the price change effect varies across plans due to differences in the timing of generic reforms, the magnitude of generic price reductions, and the utilization rates of generic drugs. The price change effect for 2013/14 (-6.0%) was more pronounced
than for 2012/13 (-2.0%),Footnote 11 as several plans implemented important generic pricing policies in 2013/14. In addition, through the pan-Canadian Pharmaceutical Alliance (pCPA),Footnote 12 the provinces and territories collectively reduced the prices of six commonly-used generic drugs to 18% of the brand-name level as of April 1, 2013 (Appendix B).
Public plans in British Columbia, New Brunswick, Prince Edward Island, and Newfoundland and Labrador reduced generic prices from levels ranging from 35% to 45% of the reference brand-name drug to 25%. Alberta lowered generic drug prices even further in 2013/14,
to 18% of the brand-name levels, with some exceptions for specific drugs. These price reductions, along with those achieved by the pCPA, resulted in substantial pull-down effects in 2013/14, ranging from -8.8% to -13.6% across these plans.
Ontario, which was the first province to reduce the prices of generic drugs to 25% of the brand-name levels in 2010, had the lowest savings from generic price reductions in 2013/14. The -2.6% price change effect was most likely driven by the generic price reductions achieved through the pCPA.
The generic substitution effect (the shift in use from brand-name drugs to less expensive generic drugs) had less impact in 2013/14 (-1.5%) than in 2012/13 (-7.2%).Footnote 11 The modest change reflects the residual cost-saving effect of large-selling generics that entered the market in previous years, as only relatively minor generic drugs were launched in 2013/14. This marks the end of the "patent cliff" influence, as most top-selling brand-name drugs have already reached the end of their patent life.
The additional figures in this section provide supporting statistics on price indices, the generic share of prescriptions and drug costs, and generic savings for the public plans.
Figure 4.1.2a, b and c reports on the trends in the average unit cost for multi-source generic drugs, patented drugs and single-source non-patented drugs from 2009/10 to 2013/14, (see the glossary in Appendix J for the definitions of market segments). The results are presented as an index.
The index is constructed by setting the prices in each plan to the value of 1 in 2009/10, and it is calculated using the cost-weighted average of the average unit cost changes at the individual drug level. This approach is similar to the one used by Statistics Canada to calculate the Consumer Price Index. This analysis was restricted to oral solid formulations to ensure unit reporting consistency.
In the cost driver model, the price change effect is mainly the result of reductions in the average unit cost reimbursed for multi-source generics drugs, as the prices of patented drugs have increased slightly over the last five years. Prices of single-source non-patented drugs have increased substantially for some public drug plans (British Columbia, Saskatchewan, Manitoba and Prince Edward Island); however, this market segment is relatively small.
The results for the multi-source generic market (Figure 4.1.2.a) show a rapid decline in generic drug prices for Ontario beginning in 2010/11, with the other plans following the same trend starting in 2011/12. This reflects the timing of the introduction of generic price reforms (see Appendix B). The average generic price reductions from 2009/10 to 2013/14 ranged from 32% to 50%, depending on the plan.
Figure 4.1.2 Average unit cost index, multi-source generic drugs, patented drugs and single-source non-patented drugs, NPDUIS public drug plans, 2009/10 to 2013/14
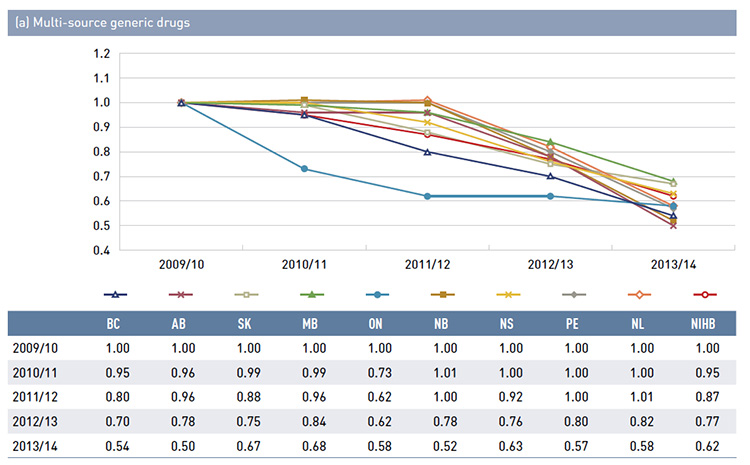
Figure description
These three line graph describe the change in the average unit drug cost index from 2009/10 to 2013/14 for the NPDUIS public drug plans for (a) multi-source generic drugs; (b) patented drugs; and (c) single-source non-patented drugs. The results for each graph are as follows:
(a) Multi-source generic drugs
|
2009/10 |
2010/11 |
2011/12 |
2012/13 |
2013/14 |
| British Columbia |
1.00 |
0.95 |
0.80 |
0.70 |
0.54 |
| Alberta |
1.00 |
0.96 |
0.96 |
0.78 |
0.50 |
| Saskatchewan |
1.00 |
0.99 |
0.88 |
0.75 |
0.67 |
| Manitoba |
1.00 |
0.99 |
0.96 |
0.84 |
0.68 |
| Ontario |
1.00 |
0.73 |
0.62 |
0.62 |
0.58 |
| New Brunswick |
1.00 |
1.01 |
1.00 |
0.78 |
0.52 |
| Nova Scotia |
1.00 |
1.00 |
0.92 |
0.76 |
0.63 |
| Prince Edward Island |
1.00 |
1.00 |
1.00 |
0.80 |
0.57 |
| Newfoundland and Labrador |
1.00 |
1.00 |
1.01 |
0.82 |
0.58 |
| Non-Insured Health Benefits |
1.00 |
0.95 |
0.87 |
0.77 |
0.62 |
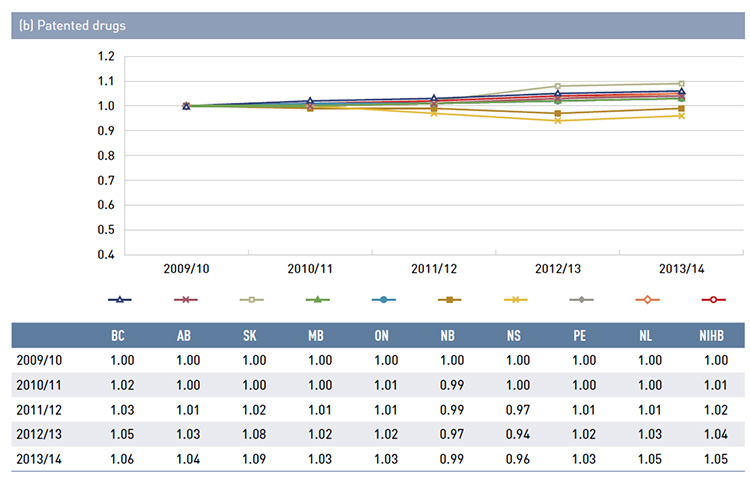 blank
blank
Figure description
|
2009/10 |
2010/11 |
2011/12 |
2012/13 |
2013/14 |
| British Columbia |
1.00 |
1.02 |
1.03 |
1.05 |
1.06 |
| Alberta |
1.00 |
1.00 |
1.01 |
1.03 |
1.04 |
| Saskatchewan |
1.00 |
1.00 |
1.02 |
1.08 |
1.09 |
| Manitoba |
1.00 |
1.00 |
1.01 |
1.02 |
1.03 |
| Ontario |
1.00 |
1.01 |
1.01 |
1.02 |
1.03 |
| New Brunswick |
1.00 |
0.99 |
0.99 |
0.97 |
0.99 |
| Nova Scotia |
1.00 |
1.00 |
0.97 |
0.94 |
0.96 |
| Prince Edward Island |
1.00 |
1.00 |
1.01 |
1.02 |
1.03 |
| Newfoundland and Labrador |
1.00 |
1.00 |
1.01 |
1.03 |
1.05 |
| Non-Insured Health Benefits |
1.00 |
1.01 |
1.02 |
1.04 |
1.05 |
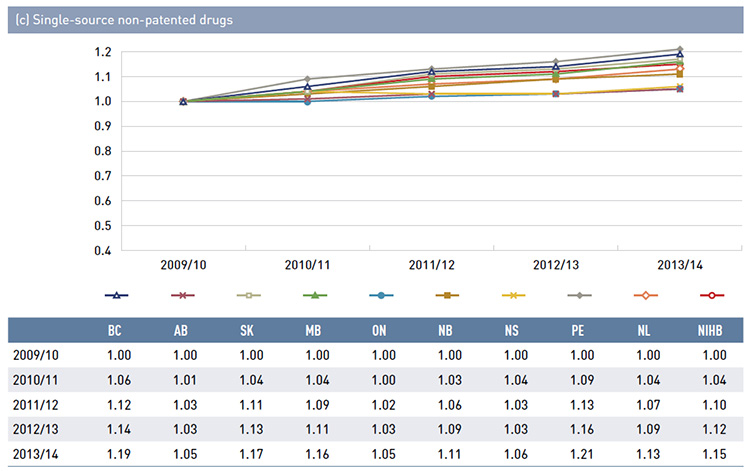 blank
blank
Note: The average unit cost reimbursed was used to calculate the index. The analysis was limited to oral solid formulations.
Data source: National Prescription Drug Utilization Information System Database, Canadian Institute for Health Information;
PMPRB DIN-level database.
Figure description
|
2009/10 |
2010/11 |
2011/12 |
2012/13 |
2013/14 |
| British Columbia |
1.00 |
1.06 |
1.12 |
1.14 |
1.19 |
| Alberta |
1.00 |
1.01 |
1.03 |
1.03 |
1.05 |
| Saskatchewan |
1.00 |
1.04 |
1.11 |
1.13 |
1.17 |
| Manitoba |
1.00 |
1.04 |
1.09 |
1.11 |
1.16 |
| Ontario |
1.00 |
1.00 |
1.02 |
1.03 |
1.05 |
| New Brunswick |
1.00 |
1.03 |
1.06 |
1.09 |
1.11 |
| Nova Scotia |
1.00 |
1.04 |
1.03 |
1.03 |
1.06 |
| Prince Edward Island |
1.00 |
1.09 |
1.13 |
1.16 |
1.21 |
| Newfoundland and Labrador |
1.00 |
1.04 |
1.07 |
1.09 |
1.13 |
| Non-Insured Health Benefits |
1.00 |
1.04 |
1.10 |
1.12 |
1.15 |
Figure 4.1.3 reports on trends in the generic share of total prescriptions and drug costs from 2009/10 to 2013/14.
In the cost driver model, the negative effect of generic substitution on drug costs is the result of the increased market capture of generic drugs.
The results in 4.1.3a show a marked increase in the generic share of prescriptions across the public drug plans: from lows of 52.8% (SK) to 59.9% (BC) in 2009/10, shares rose to between 62.1% (ON) and 70.0% (AB) in 2013/14. The generic market share was the
lowest in the NIHB, increasing from 46.3% in 2009/10 to 51.7% in 2013/14.
By comparison, the generic share of drug costs (4.1.3b) declined markedly for all public drug plans because of policies that reduced generic drug prices (see Appendix B). There was a pronounced decrease from 2012/13 to 2013/14 in several jurisdictions coinciding with the implementation of generic policy reforms: Alberta: -11.4%; New Brunswick: -9.3%; Newfoundland and Labrador: -7.2%; Prince Edward Island: -7.0% and British Columbia: -6.0%.
Figure 4.1.3 Generic drug share of prescriptions and drug cost, NPDUIS public drug plans, 2009/10 to 2013/14
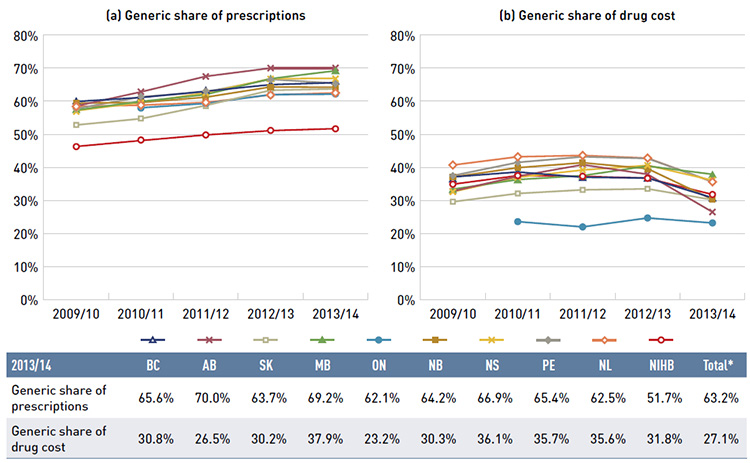
*Total results for the public drug plans reported in this figure.
Note: Due to the lack of available data, 2009/10 results are not reported for Ontario.
Data source: National Prescription Drug Utilization Information System Database, Canadian Institute for Health Information.
Figure description
This figure shows two complementary line graphs side-by-side.
The left-hand graph shows the generic share of prescriptions from 2009/10 to 2013/14 for the NPDUIS public drug plans. The right-hand graph shows the generic share of drug costs for the same period and plans. A table below the figure gives the generic shares of prescriptions and drug costs for 2013/14.
Generic share of prescriptions
| |
2009/10 |
2010/11 |
2011/12 |
2012/13 |
2013/14 |
| British Columbia |
59.9% |
61.1% |
63.0% |
65.0% |
65.6% |
| Alberta |
58.5% |
62.8% |
67.5% |
70.0% |
70.0% |
| Saskatchewan |
52.8% |
54.7% |
58.7% |
63.3% |
63.7% |
| Manitoba |
57.3% |
59.9% |
62.0% |
66.8% |
69.2% |
| Ontario |
‒ |
58.0% |
59.3% |
62.0% |
62.1% |
| New Brunswick |
59.4% |
59.6% |
61.2% |
64.3% |
64.2% |
| Nova Scotia |
57.1% |
59.6% |
62.5% |
66.8% |
66.9% |
| Prince Edward Island |
57.7% |
61.3% |
62.8% |
66.6% |
65.4% |
| Newfoundland and Labrador |
58.4% |
58.8% |
59.6% |
61.9% |
62.5% |
| Non-Insured Health Benefits |
46.3% |
48.1% |
49.8% |
51.1% |
51.7% |
| Total for 2013/14 |
|
|
|
|
63.2% |
Generic share of drug costs
| |
2009/10 |
2010/11 |
2011/12 |
2012/13 |
2013/14 |
| British Columbia |
37.1% |
38.6% |
37.0% |
36.8% |
30.8% |
| Alberta |
32.8% |
37.3% |
40.8% |
37.9% |
26.5% |
| Saskatchewan |
29.6% |
32.1% |
33.2% |
33.5% |
30.2% |
| Manitoba |
33.4% |
36.3% |
37.5% |
40.4% |
37.9% |
| Ontario |
‒ |
23.6% |
22.0% |
24.7% |
23.2% |
| New Brunswick |
37.1% |
39.9% |
41.4% |
39.6% |
30.3% |
| Nova Scotia |
32.6% |
37.0% |
39.2% |
40.6% |
36.1% |
| Prince Edward Island |
37.5% |
41.5% |
43.2% |
42.7% |
35.7% |
| Newfoundland and Labrador |
40.7% |
43.2% |
43.6% |
42.8% |
35.6% |
| Non-Insured Health Benefits |
34.9% |
37.5% |
37.3% |
36.7% |
31.8% |
| Total for 2013/14 |
|
|
|
|
27.1% |
The differences in generic market shares across Canada are driven by many factors, including, but not limited to, the disease profile of the populations, prescribing practices, coverage of brand-name products and generic price levels.
4.2 Demographic Effects
The demographic effects include the following sub-effects: the population effect, the aging effect and the gender effect. In 2013/14, the combined demographic effects had a slight push effect on drug cost levels.
Population Effect
This effect captures the extent to which a change in the active beneficiary population contributes to a change in drug costs. Note that in the public drug plan population this effect may also capture an aging component, as people become eligible for coverage when
they become seniors.
Aging Effect
This effect captures the impact of changes in the distribution of the population by age groups. An older population is generally associated with increased drug use and cost (Figure 2.5). Therefore, population shifts toward an older or a younger population may slightly increase or decrease drug expenditures, respectively.
Gender Effect
This effect captures the impact of changes in the gender split in the population. Unless major changes occur, this effect is minimal.
Figure 4.2.1 reports the rate of change in drug costs for the public drug plans from 2012/13 to 2013/14 focusing on the three demographic effects: population, aging and gender. The bar graph and the associated table below show the year-over-year impacts of each effect as a relative and absolute change in drug cost.
The demographic effects pushed drug cost levels upwards by 2.1% in 2013/14, which translates into an increase of $151.7 million in drug cost expenditures in the NPDUIS public plans. This effect was mainly due to the increase in the size of the active beneficiary
population. The impact of the demographic effects was slightly lower in 2013/14 (2.1%) than in 2012/13 (2.7%)Footnote 11.
Figure 4.2.1 Rates of change in drug costs due to demographic effects, NPDUIS public drug plans, 2012/13 to 2013/14
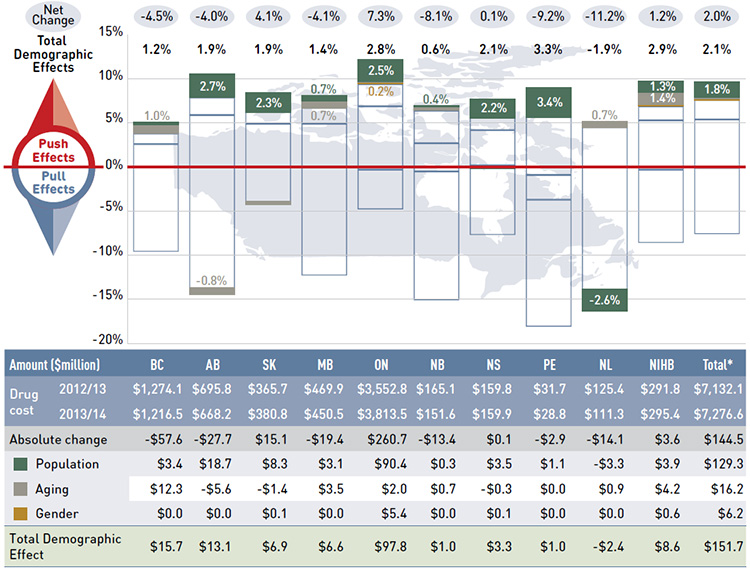
*Total results for the public drug plans reported in this figure.
Note: Values may not add to totals due to rounding.
Data source: National Prescription Drug Utilization Information System Database, Canadian Institute for Health Information.
Figure description
This figure describes the factors that impacted the rates of change in drug cost due to demographic effects in the NPDUIS public drug plans from 2012/13 to 2013/14. It is composed of a bar graph with a table underneath.
The bar graph shows the percent change from 2012/13 to 2013/14 for each public drug plan. The results are as follows:
|
British Columbia |
Alberta |
Saskatchewan |
Manitoba |
Ontario |
New Brunswick |
Nova Scotia |
Prince Edward Island |
Newfoundland and Labrador |
Non-Insured Health Benefits |
Total |
| Net change |
-4.5% |
-4.0% |
4.1% |
-4.1% |
7.3% |
-8.1% |
0.1% |
-9.2% |
-11.2% |
1.2% |
2.0% |
| Total demographic effects |
1.2% |
1.9% |
1.9% |
1.4% |
2.8% |
0.6% |
2.1% |
3.3% |
-1.9% |
2.9% |
2.1% |
| Population effect |
0.3% |
2.7% |
2.3% |
0.7% |
2.5% |
0.2% |
2.2% |
3.4% |
-2.6% |
1.3% |
1.8% |
| Aging effect |
1.0% |
-0.8% |
-0.4% |
0.7% |
0.1% |
0.4% |
-0.2% |
-0.1% |
0.7% |
1.4% |
0.2% |
| Gender effect |
0.0% |
0.0% |
0.0% |
0.0% |
0.2% |
0.0% |
0.0% |
0.0% |
0.0% |
0.2% |
0.1% |
The table below the bar graph gives the corresponding changes in millions of dollars.
|
British Columbia |
Alberta |
Saskatchewan |
Manitoba |
Ontario |
New Brunswick |
Nova Scotia |
Prince Edward Island |
Newfoundland and Labrador |
Non-Insured Health Benefits |
Total |
| Drug cost 2012/13 |
$1,274.1 |
$695.8 |
$365.7 |
$469.9 |
$3,552.8 |
$165.1 |
$159.8 |
$31.7 |
$125.4 |
$291.8 |
$7,132.1 |
| Drug cost 2013/14 |
$1,216.5 |
$668.2 |
$380.8 |
$450.5 |
$3,813.5 |
$151.6 |
$159.9 |
$28.8 |
$111.3 |
$295.4 |
$7,276.6 |
| Absolute change |
-$57.6 |
-$27.7 |
$15.1 |
-$19.4 |
$260.7 |
-$13.4 |
$0.1 |
-$2.9 |
-$14.1 |
$3.6 |
$144.5 |
| Population effect |
$3.4 |
$18.7 |
$8.3 |
$3.1 |
$90.4 |
$0.3 |
$3.5 |
$1.1 |
-$3.3 |
$3.9 |
$129.3 |
| Aging effect |
$12.3 |
-$5.6 |
-$1.4 |
$3.5 |
$2.0 |
$0.7 |
-$0.3 |
$0.0 |
$0.9 |
$4.2 |
$16.2 |
| Gender effect |
$0.0 |
$0.0 |
$0.1 |
$0.0 |
$5.4 |
$0.0 |
$0.1 |
$0.0 |
$0.0 |
$0.6 |
$6.2 |
| Total demographic effect |
$15.7 |
$13.1 |
$6.9 |
$6.6 |
$97.8 |
$1.0 |
$3.3 |
$1.0 |
-$2.4 |
$8.6 |
$151.7 |
The increase in the size of the active beneficiary population pushed the overall drug plan cost upward by an estimated $129.3 million or 1.8%. This can be directly correlated to the increase in the active beneficiary population reported in Figure 4.2.2.
The aging and the gender effects had a negligible impact on the change in drug costs. Generally, the aging effect is expected to have a long-term impact on drug costs, and this is further discussed in Figure 4.2.3.
The results in this analysis report the aging of the active beneficiary in public drug plans, which is different than aging of the Canadian population. As the Canadian population ages, the number of people eligible for senior coverage (+65) grows, and this increases the
size of the beneficiary population in public plans. This latter trend is captured in the population effect reported in Figure 4.2.1.
The next two figures provide supporting statistical information on growth and aging in the beneficiary populations.
Figure 4.2.2 shows the rate of growth in the number of active beneficiaries from 2012/13 to 2013/14 (bar graph), while the associated table reports the total number of active beneficiaries for each fiscal year. Across plans, the active beneficiary populations grew at an average rate of 1.4%, ranging from -2.6% to 3.4%.
The number of active beneficiaries increased markedly in Prince Edward Island (3.4%), Alberta (2.7%), Ontario (2.5%), Saskatchewan (2.3%) and Nova Scotia (2.2%), while in Newfoundland and Labrador it declined (-2.6%)Footnote IV. The other plans had relatively low rates of increase in the active beneficiary population.
The increase in the active beneficiary population may be the result of growth in the overall population of a jurisdiction, the aging of the population (increasing the number of seniors eligible for coverage) and/or plan design changes that expanded coverage to new population or patient groups.
Figure 4.2.2 Rates of change in the active beneficiary populations, NPDUIS public drug plans, 2012/13 to 2013/14
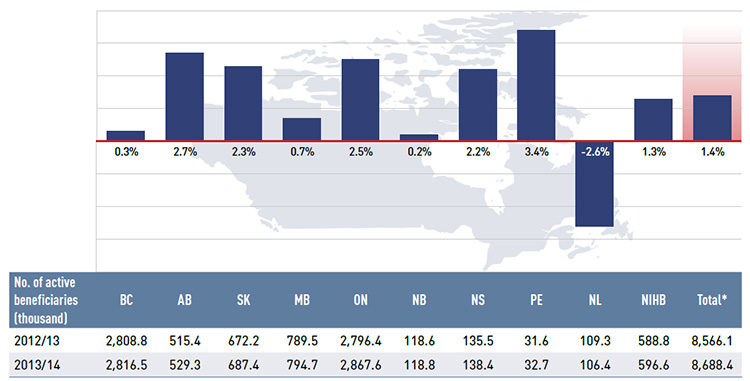
*Total results for the select public drug plans reported in this figure.
Data source: National Prescription Drug Utilization Information System Database, Canadian Institute for Health Information.
Figure description
This bar graph gives the rate of change in the beneficiary population for the NPDUIS public drug plans in 2013/14, along with the total number of active beneficiaries (thousands) from 2012/13 to 2013/14: British Columbia: 0.3% (2,808.8 to 2,816.5); Alberta: 2.7% (515.4 to 529.3); Saskatchewan: 2.3% (672.2 to 687.4); Manitoba: 0.7% (789.5 to 794.7); Ontario: 2.5% (2,796.4 to 2,867.6); New Brunswick: 0.2% (118.6 to 118.8); Nova Scotia: 2.2% (135.5 to 138.4); Prince Edward Island: 3.4% (31.6 to 32.7); Newfoundland and Labrador: -2.6% (109.3 to 106.2); Non-Insured Health Benefits: 1.3% (588.8 to 596.6); total for all plans: 1.4% (8,566.1 to 8,688.4).
Figure 4.2.3 reports the average age of the active beneficiary populations in the public drug plans from 2006/07 to 2013/14, along with the average age of the Canadian population in 2007 and 2013, as reported by Statistics Canada.Footnote 13
The average age of drug plan beneficiaries increased gradually from 2006/07 to 2013/14 in most jurisdictions; although in some public drug plans, the average age decreased due to the implementation of programs that expanded coverage to younger populations, such as
initiatives for high-cost or catastrophic drug coverage.
Figure 4.2.3 Average age of active beneficiary populations, NPDUIS public drug plans and Canada, 2006/07 to 2013/14
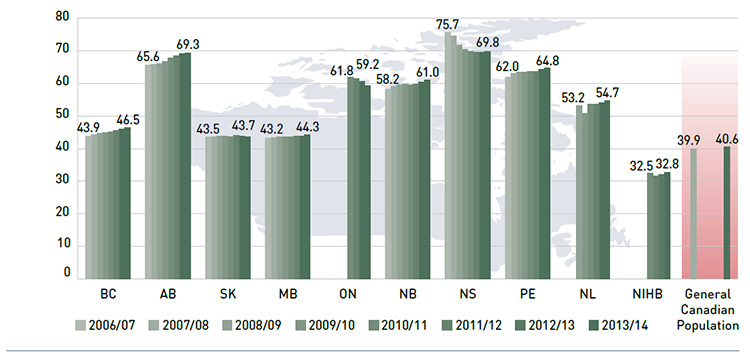
Note: Due to the lack of available data, limited results are reported for Ontario and the NIHB.
Data sources: National Prescription Drug Utilization Information System Database, Canadian Institute for Health Information; data for general Canadian population from Statistics Canada census data for 2006 and 2011.
Figure description
This bar graph describes the average age of beneficiary populations from 2006/07 to 2013/14 for the NPDUIS public drug plans. For comparison, the average age of the general Canadian population is given for 2007 and 2013.
- British Columbia – 2006/07: 43.9; 2013/14: 46.5
- Alberta - 2006/07: 65.6; 2013/14: 69.3
- Saskatchewan - 2006/07: 43.5; 2013/14: 43.7
- Manitoba - 2006/07: 43.2; 2013/14: 44.3
- Ontario - 2010/11: 61.8; 2013/14: 59.2
- New Brunswick - 2006/07: 58.2; 2013/14: 61.0
- Nova Scotia - 2006/07: 75.7; 2013/14: 69.8
- Prince Edward Island - 2006/07: 62.0; 2013/14: 64.8
- Newfoundland and Labrador - 2008/09: 53.2; 2013/14: 54.7
- Non-Insured Health Benefits - 2010/11: 32.5; 2013/14: 32.8
- General Canadian population - 2007: 39.9; 2013: 40.6
Senior-based public drug plans reimbursed beneficiaries that were on average older than the Canadian population. The average Canadian was 40.6 years old in 2013, while the average age of active beneficiaries ranged from 54.7 to 69.8 for the drug plans that
were principally senior-based.
The average age of beneficiaries in universal, income-based drug plans (British Columbia, 46.5; Saskatchewan, 43.7; and Manitoba, 44.3) was lower than in other plans, while NIHB beneficiaries were younger (32.8) than the Canadian average due to the unique demographic profile of the client population. Although many public drug plans are seniorbased, most reimburse a significant non-senior population with support for specialty drugs and diseases, and low-income residents. Hence, there is a wide variation in the average age
across the plans.
In the coming decades, the aging Canadian population is expected to gradually increase the pressure on drug expenditures. Statistics Canada forecasts that the proportion of Canada’s population that is 65 and older will increase from 15.7% in 2014 to between 24%
and 28% in 2063.Footnote 13 A previously published PMPRB NPDUIS study discusses this “baby-boomer effect” and its impact on drug expenditure.Footnote 14
4.3 Volume Effects
Volume effects include the prescription volume effect, the prescription size effect and the strength–form effect. In 2013/14, the combined volume effects had a slight push effect on drug cost levels.
The volume effects are controlled by assuming the number and the age–gender profile of the active beneficiary populations remain constant from 2012/13 to 2013/14. Thus, these effects are purely the result of increased exposure to drugs for a standardized active beneficiary group.
Prescription Volume Effect
This effect captures the impact of changes in the number of prescriptions dispensed to a standardized group of active beneficiaries (age, gender and size) over the two time periods analyzed. There are many factors that influence this effect, including the use of multiple
drugs, the presence of comorbidities and the persistency of treatment, among other things.
Prescription Size Effect
This effect captures the impact of changes in the average number of units dispensed per prescription for a given drug. An increase in this measure drives an increase in drug costs, unless it is offset by a reduction in the number of prescriptions (i.e., prescription volume effect).
Strength–Form Effect
This effect captures the impact of shifts in the use of different strengths or formulations of an ingredient. Drugs are typically available in a variety of strength–form combinations for which the cost per unit can vary substantially. Higher strength drugs are typically more
expensive, and an increase in their use could contribute positively to drug cost change.
Figure 4.3.1 reports the rate of change in drug costs for the NPDUIS public drug plans from 2012/13 to 2013/14 focusing on the three volume effects: the prescription volume, the prescription size and the strength–form effect. The bar graph and associated table show the
year-over-year impacts of each effect as a relative and absolute change in drug cost.
Figure 4.3.1 Rates of change in drug costs due to volume effect, NPDUIS public drug plans, 2012/13 to 2013/14
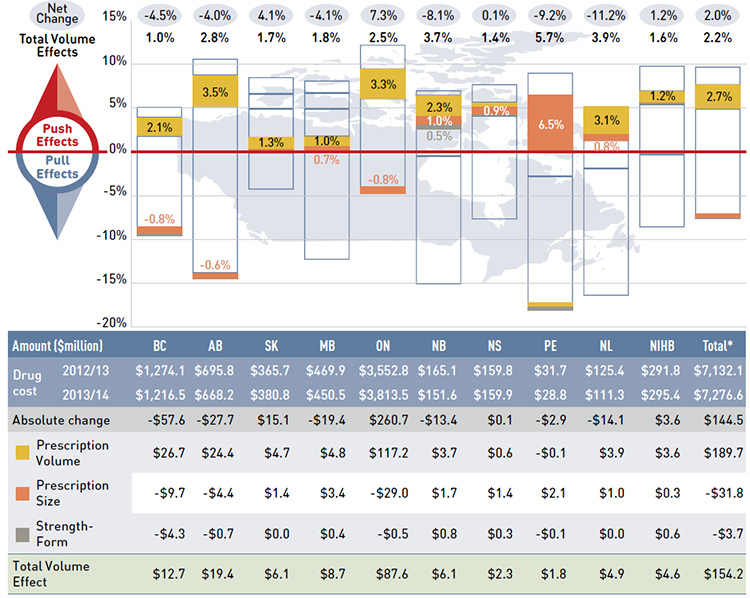
*Total results for the public drug plans reported in this figure.
Note: Values may not add to totals due to rounding.
Data source: National Prescription Drug Utilization Information System Database, Canadian Institute for Health Information.
Figure description
This figure describes the factors that impacted the rates of change in drug cost due to volume effects in the NPDUIS public drug plans from 2012/13 to 2013/14. It is composed of a bar graph with a table underneath.
The bar graph shows the percent change from 2012/13 to 2013/14 for each public drug plan. The results are as follows:
|
British Columbia |
Alberta |
Saskatchewan |
Manitoba |
Ontario |
New Brunswick |
Nova Scotia |
Prince Edward Island |
Newfoundland and Labrador |
Non-Insured Health Benefits |
Total |
| Net change |
-4.5% |
-4.0% |
4.1% |
-4.1% |
7.3% |
-8.1% |
0.1% |
-9.2% |
-11.2% |
1.2% |
2.0% |
| Total volume effects |
1.0% |
2.8% |
1.7% |
1.8% |
2.5% |
3.7% |
1.4% |
5.7% |
3.9% |
1.6% |
2.2% |
| Prescription volume effect |
2.1% |
3.5% |
1.3% |
1.0% |
3.3% |
2.3% |
0.4% |
-0.4% |
3.1% |
1.2% |
2.7% |
| Prescription size effect |
-0.8% |
-0.6% |
0.4% |
0.7% |
-0.8% |
1.0% |
0.9% |
6.5% |
0.8% |
0.1% |
-0.4% |
| Strength-form effect |
-0.3% |
-0.1% |
0.0% |
0.1% |
0.0% |
0.5% |
0.2% |
-0.5% |
0.0% |
0.2% |
-0.1% |
The table below the bar graph gives the corresponding changes in millions of dollars.
|
British Columbia |
Alberta |
Saskatchewan |
Manitoba |
Ontario |
New Brunswick |
Nova Scotia |
Prince Edward Island |
Newfoundland and Labrador |
Non-Insured Health Benefits |
Total |
| Drug cost 2012/13 |
$1,274.1 |
$695.8 |
$365.7 |
$469.9 |
$3,552.8 |
$165.1 |
$159.8 |
$31.7 |
$125.4 |
$291.8 |
$7,132.1 |
| Drug cost 2013/14 |
$1,216.5 |
$668.2 |
$380.8 |
$450.5 |
$3,813.5 |
$151.6 |
$159.9 |
$28.8 |
$111.3 |
$295.4 |
$7,276.6 |
| Absolute change |
-$57.6 |
-$27.7 |
$15.1 |
-$19.4 |
$260.7 |
-$13.4 |
$0.1 |
-$2.9 |
-$14.1 |
$3.6 |
$144.5 |
| Prescription volume effect |
$26.7 |
$24.4 |
$4.7 |
$4.8 |
$117.2 |
$3.7 |
$0.6 |
-$0.1 |
$3.9 |
$3.6 |
$189.7 |
| Prescription size effect |
-$9.7 |
-$4.4 |
$1.4 |
$3.4 |
-$29.0 |
$1.7 |
$1.4 |
$2.1 |
$1.0 |
$0.3 |
-$31.8 |
| Strength-form effect |
-$4.3 |
-$0.7 |
$0.0 |
$0.4 |
-$0.5 |
$0.8 |
$0.3 |
-$0.1 |
$0.0 |
$0.6 |
-$3.7 |
| Total volume effects |
$12.7 |
$19.4 |
$6.1 |
$8.7 |
$87.6 |
$6.1 |
$2.3 |
$1.8 |
$4.9 |
$4.6 |
$154.2 |
The volume effects pushed drug cost levels upwards by 2.2% in 2013/14, which translated into an increase of $154.2 million in drug costs in the NPDUIS public plans. This was mainly due to an increase in the number of prescriptions. The volume effects were higher
in 2013/14 (2.2%) than in 2012/13 (1.7%).Footnote 11
The prescription volume effect was an important driver in Alberta (3.5%), Ontario (3.3%), and British Columbia (2.1%). At the same time, prescription size had a small pull-down effect in these three plans: British Columbia
(-0.8%), Ontario (-0.8%), and Alberta (-0.6%). These results indicate that although the volume of use is increasing, the size of prescriptions in these plans is decreasing, pointing towards the potential influence of prescription frequency, which is further discussed in Section 5.
Prince Edward Island had an especially pronounced prescription size effect (6.5%), which was due to the implementation of a policy that increased the day supply allowed from 30 to 90 days for several drug categories. At the same time the volume of prescriptions
declined in this province, slightly pulling drug costs downward (0.4%). Figure 4.3.3, indicates that the average number of physical units per prescription for oral solid drugs markedly increased in this province, while Figure 4.3.2 shows that the average
number of prescriptions per active beneficiary slightly decreased.
The strength–form effect had a minimal impact on the change in drug costs across the plans.
Figures 4.3.2 and 4.3.3 provide supporting information on the average number of prescriptions per active beneficiary and trends in prescription size. For additional information on prescription size, see Section 5, Figure 5.3.
The prescription volume effect reported in Figure 4.3.1 reflects changes in the average number of prescriptions dispensed per active beneficiary. Figure 4.3.2 reports this measure for 2012/13 and 2013/14, along with the percent change over the two years.
Figure 4.3.2 Average number of prescriptions per active beneficiary, NPDUIS public drug plans, 2012/13 to 2013/14
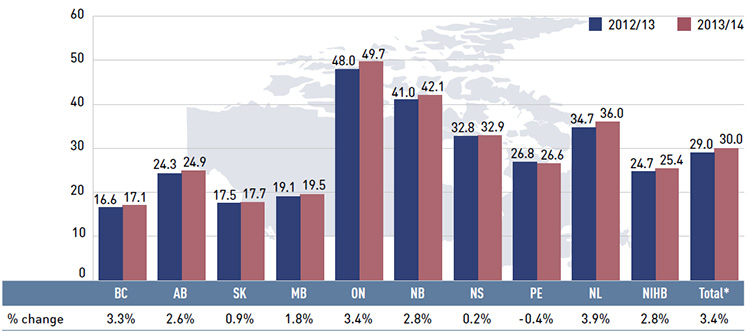
*Total results for the public drug plans reported in this figure.
Data source: National Prescription Drug Utilization Information System Database, Canadian Institute for Health Information.
Figure description
The bar graph gives the average number of prescriptions per beneficiary for the NPDUIS public drug plans in 2012/13 and 2013/14, along with the corresponding percent change.
- British Columbia: 16.6 in 2012/13 and 17.1 in 2013/14 (3.3% change)
- Alberta: 24.3 in 2012/13 and 24.9 in 2013/14 (2.6% change)
- Saskatchewan: 17.5 in 2012/13 and 17.7 in 2013/14 (0.9% change)
- Manitoba: 19.1 in 2012/13 and 19.5 in 2013/14 (1.8% change)
- Ontario: 48.0 in 2012/13 and 49.7 in 2013/14 (3.4% change)
- New Brunswick: 41.0 in 2012/13 and 42.1 in 2013/14 (2.8% change)
- Nova Scotia: 32.8 in 2012/13 and 32.9 in 2013/14 (0.2% change)
- Prince Edward Island: 26.8 in 2012/13 and 26.6 in 2013/14 (-0.4% change)
- Newfoundland and Labrador: 34.7 in 2012/13 and 36.0 in 2013/14 (3.9% change)
- Non-Insured Health Benefits: 24.7 in 2012/13 and 25.4 in 2013/14 (2.8% change)
- Total for all plans: 29.0 in 2012/13 and 30.0 in 2013/14 (3.4% change)
As with the results for the prescription volume effect, there has been an increase in the average number of prescriptions dispensed per beneficiary in many provinces, in the range of 1.8% to 3.9%. Saskatchewan, Nova Scotia and Prince Edward Island had small changes in the volume of use at beneficiary level.
Note that this rate of increase differs from that reported for the prescription volume effect because it includes demographic changes, such as aging and shifts in gender distribution.
Across the NPDUIS public drug plans, differences in the average number of prescriptions per active beneficiary are due to the demographic and therapeutic profile of the beneficiaries, as well as prescribing and dispensing practices.
Figure 4.3.3 shows the trend in the average prescription size in terms of physical units from 2009/10 to 2013/14. Note that the data reported is restricted to oral solid formulations.
Figure 4.3.3 Average number of physical units per prescription, NPDUIS public drug plans, oral solids, 2009/10 to 2013/14
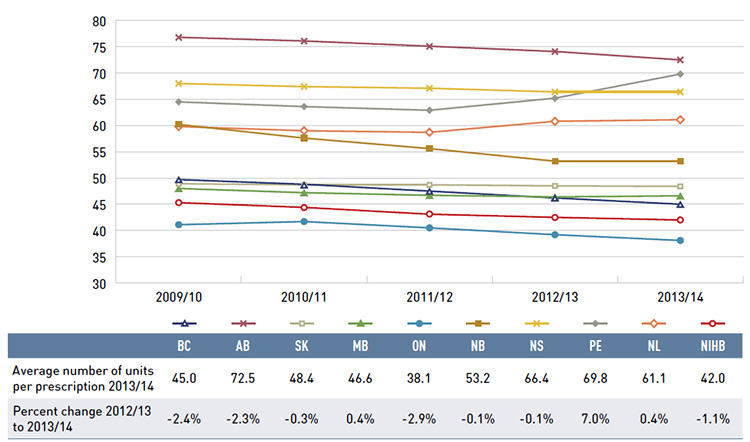
Data source: National Prescription Drug Utilization Information System Database, Canadian Institute for Health Information.
Figure description
This line graph shows the average number of units per prescription from 2009/10 to 2013/14 for the NPDUIS public drug plans. The analysis is limited to oral solids. The results show that the average prescription size has been either stable or trending slightly downward (with the exception of Prince Edward Island) over the past five years.
|
British Columbia |
Alberta |
Saskatchewan |
Manitoba |
Ontario |
New Brunswick |
Nova Scotia |
Prince Edward Island |
Newfoundland and Labrador |
Non-Insured Health Benefits |
| Average number of units per prescription 2013/14 |
45.0 |
72.5 |
48.4 |
46.6 |
38.1 |
53.2 |
66.4 |
69.8 |
61.1 |
42.0 |
| Percent change 2012/13 to 2013/14 |
-2.4% |
-2.3% |
-0.3% |
0.4% |
-2.9% |
-0.1% |
-0.1% |
7.0% |
0.4% |
-1.1% |
The prescription size effect measures the impact of changes in the average quantity of drugs dispensed per prescription.
The results suggest that the average prescription size has been either stable or trending slightly downward, with the exception of Prince Edward Island, which increased by 7.0%.
Similar to the findings for the cost driver model, there was a marked reduction in the prescription size in British Columbia, Alberta and Ontario (–2.4%, –2.3% and –2.9%, respectively) in 2013/14.
Note that the rate of decrease in the average number of units per prescription differs from that reported for the prescription size effect as the former includes demographic changes, such as aging and any shifts in gender distribution.
Prescription size is a two-way effect: it has the opposite impact on dispensing fee expenditures, with shorter prescriptions increasing the number of fees, and thus, pushing the dispensing costs upward. This topic is covered further in Section 5.
4.4 Drug-Mix Effects
Drug-mix effects are composed of the following individual sub-effects:
Existing Drug Effect
This effect captures the impact of shifts in market shares between ingredients that are available in both time periods analyzed (i.e., fiscal years 2012/13 and 2013/14). This driver may reflect changing treatments patterns, physician prescribing practices and/or the
prevalence of diseases in the population. The impact of switching between drugs and shifting market shares among therapeutic classes and subclasses is captured by this effect.
Entering Drug Effect
This effect captures the impact of shifts in utilization towards drugs that entered the market in the second time period (2013/14). Less expensive new drugs offer savings (pull effect) and more expensive new drugs result in cost increases (push effect). This driver
measures the net effect of these two opposing forces.
Exiting Drug Effect
This effect captures the impact of shifts in utilization away from drugs that exit the market in the second time period (2013/14). Its impact will be minimal unless high-use or expensive drugs are withdrawn.
The previous edition of the CompassRx provided a breakdown of the results by sub-effects. This additional detail provided limited insight, and thus, this edition of the report focuses on the drug-mix effect at a high-level and does not report on the sub-effects.
Figure 4.4.1 reports the rate of change in drug costs for the NPDUIS public drug plans from 2012/13 to 2013/14 focusing on the impact of the drug-mix effect. The bar graph and the associated table show the year-over-year impact of the drug-mix effect as a relative and absolute change in drug cost.
Figure 4.4.1 Rates of change in drug costs due to drug-mix effects, NPDUIS public drug plans, 2012/13 to 2013/14
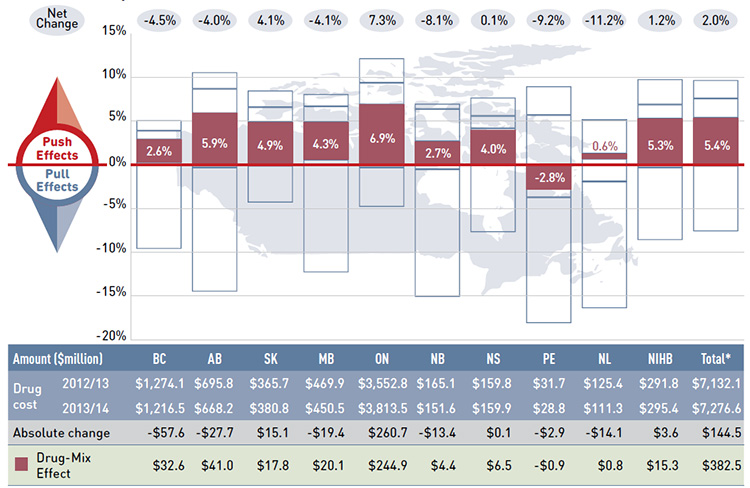
*Total results for the public drug plans reported in this figure.
Note: Values may not add to totals due to rounding.
Data source: National Prescription Drug Utilization Information System Database, Canadian Institute for Health Information.
Figure description
This figure describes the factors that impacted the rates of change in drug cost due to drug-mix effects in the NPDUIS public drug plans from 2012/13 to 2013/14. It is composed of a bar graph with a table underneath.
The bar graph shows the percent change from 2012/13 to 2013/14 for each public drug plan. The results are as follows:
|
British Columbia |
Alberta |
Saskatchewan |
Manitoba |
Ontario |
New Brunswick |
Nova Scotia |
Prince Edward Island |
Newfoundland and Labrador |
Non-Insured Health Benefits |
Total |
| Net change |
-4.5% |
-4.0% |
4.1% |
-4.1% |
7.3% |
-8.1% |
0.1% |
-9.2% |
-11.2% |
1.2% |
2.0% |
| Total drug-mix effects |
2.6% |
5.9% |
4.9% |
4.3% |
6.9% |
2.7% |
4.0% |
-2.8% |
0.6% |
5.3% |
5.4% |
The table below the bar graph gives the corresponding changes in millions of dollars.
|
British Columbia |
Alberta |
Saskatchewan |
Manitoba |
Ontario |
New Brunswick |
Nova Scotia |
Prince Edward Island |
Newfoundland and Labrador |
Non-Insured Health Benefits |
Total |
| Drug cost 2012/13 |
$1,274.1 |
$695.8 |
$365.7 |
$469.9 |
$3,552.8 |
$165.1 |
$159.8 |
$31.7 |
$125.4 |
$291.8 |
$7,132.1 |
| Drug cost 2013/14 |
$1,216.5 |
$668.2 |
$380.8 |
$450.5 |
$3,813.5 |
$151.6 |
$159.9 |
$28.8 |
$111.3 |
$295.4 |
$7,276.6 |
| Absolute change |
-$57.6 |
-$27.7 |
$15.1 |
-$19.4 |
$260.7 |
-$13.4 |
$0.1 |
-$2.9 |
-$14.1 |
$3.6 |
$144.5 |
| Drug-mix effect |
$32.6 |
$41.0 |
$17.8 |
$20.1 |
$244.9 |
$4.4 |
$6.5 |
-$0.9 |
$0.8 |
$15.3 |
$382.5 |
The drug-mix effect pushed drug cost levels upwards by 5.4% in 2013/14, which translated into an increase of $382.5 million in drug costs in the NPDUIS public plans. This was mainly due to the increased use of more expensive drugs. The drug-mix effect was more pronounced in 2013/14 (5.4%) than in 2012/13 (4.1%).Footnote 11
The results indicate that the drug-mix effect had an important push effect on the growth in expenditures in most plans, especially in Ontario (6.9%), Alberta (5.9%), and the NIHB (5.3%).
Figures 4.4.2 and 4.2.3 provide information on high-impact drugs and therapeutic classes that explain these results.
Figure 4.4.2 further decomposes the 5.4% growth in drug costs attributable to the drug-mix effect into the top ten and bottom five drugs that impacted this driver. These drugs have a relatively high average cost per prescription and important increases (top ten) or decreases (bottom five) in use in 2013/14, as measured by the number of prescriptions.
Figure 4.4.2 Top ten and bottom five drugs contributing to the drug-mix effect, NPDUIS public drug plans, 2013/14
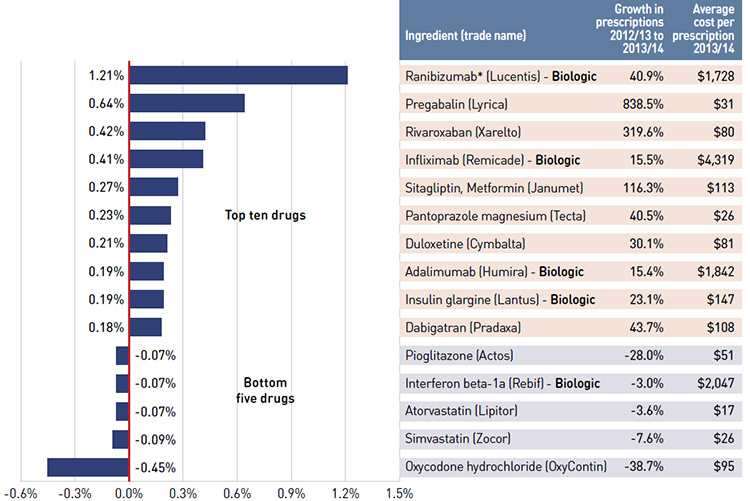
* This is a PMPRB Category 2 drug, indicating a breakthrough or substantial improvement. All the other drugs listed in the
table are either slight or no improvement, line extensions, or not categorized by the PMPRB. If a brand has several DINs,
the level of therapeutic improvement is for the DIN with highest utilization in 2013/14.
Note: The public drug plans include British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, New Brunswick,
Nova Scotia, Prince Edward Island, Newfoundland and Labrador, and the Non-Insured Health Benefits Program.
Data source: National Prescription Drug Utilization Information System Database, Canadian Institute for Health Information.
Figure description
This bar graph shows the top ten and bottom five drugs contributing to the drug-mix effect in the NPDUIS public drug plans in 2013/14. The largest pressure on drug cost in 2012/13 was by the new antithrombotic Pradaxa, which pushed costs up by 0.51%, while high-cost biologics Lucentis, Remicade and Lantus pushed costs up.
Ingredient (trade name) |
Contribution to the drug-mix effect |
Growth in prescriptions from 2012/13 to 2013/14 |
Average cost per prescription for 2013/14 |
| Ranibizumab (Lucentis*) - Biologic |
1.21% |
40.9% |
$1,728 |
| Pregabalin (Lyrica) |
0.64% |
838.5% |
$31 |
| Rivaroxaban (Xarelto) |
0.42% |
319.6% |
$80 |
| Infliximab (Remicade*) - Biologic |
0.41% |
15.5% |
$4,319 |
| Sitagliptin, Metformin (Janumet) |
0.27% |
116.3% |
$113 |
| Pantoprazole magnesium (Tecta) |
0.23% |
40.5% |
$26 |
| Duloxetine (Cymbalta) |
0.21% |
30.1% |
$81 |
| Adalimumab (Humira) - Biologic |
0.19% |
15.4% |
$1,842 |
| Insulin glargine (Lantus) - Biologic |
0.19% |
23.1% |
$147 |
| Dabigatran (Pradaxa) |
0.18% |
43.7% |
$108 |
| Pioglitazone (Actos) |
-0.07% |
-28.0% |
$51 |
| Interferon beta-1a (Rebif) - Biologic |
-0.07% |
-3.0% |
$2,047 |
| Atorvastatin (Lipitor) |
-0.07% |
-3.6% |
$17 |
| Simvastatin (Zocor) |
-0.09% |
-7.6% |
$26 |
| Oxycodone hydrochloride (OxyContin) |
-0.45% |
-38.7% |
$95 |
Ten drugs accounted for over half of the drug-mix
effect reported in Figure 4.4.1. Many of the
top contributors to drug costs in 2012/13 were
also top contributors in 2013/14, with Xarelto
and Humira being the new additions to the top
10 list in 2013/14. The largest cost pressure
was exerted by the biologic Lucentis, which
pushed costs up by 1.21%. Other high impact
drugs included Lyrica, Remicade, Janumet,
Tecta, Cymbalta, Lantus and Pradaxa.
Among the bottom five drugs, Oxycodone had
the most important pull-down effect on drug
costs, accounting for a 0.45% reduction in the
2013/14 drug cost level from 2012/13. The use
of the ingredient diminished when public
plans delisted OxyContin and launched the
tamper-resistant version, OxyNEO.
One of the limitations of this analysis is that
Ontario, as the largest public plan, carries a
large weight in the total results reported for
the plans.
Figure 4.4.3 reports the rates of change in the
drug costs for biologics compared to the rates
of change in the total drug costs from 2012/13
to 2013/14.
Figure 4.4.3 Rates of change in drug costs for biologic drugs compared with all drugs, NPDUIS public drug plans, 2012/13 to 2013/14
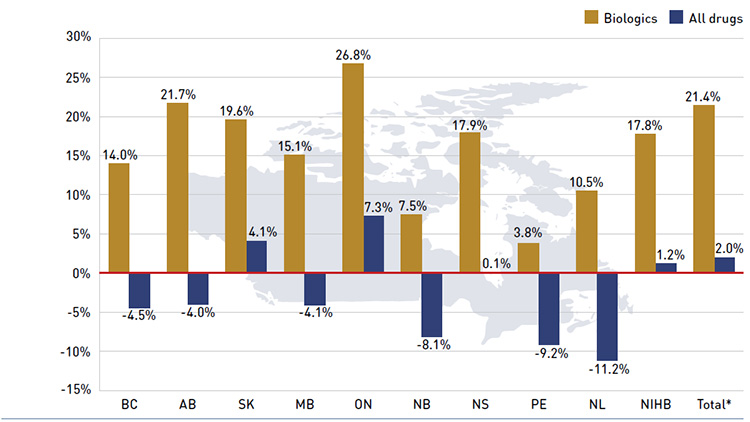
*Total results for the public drug plans reported in this figure.
Data source: National Prescription Drug Utilization Information System Database, Canadian Institute for Health Information.
Figure description
This bar graph describes the rates of change in biologic drug costs compared with all drugs for the NPDUIS public drug plans from 2012/13 to 2013/14. There has been a large increase in the cost for biologics compared to the rates of change in overall drug cost (given in parenthesis). British Columbia: 14.0% (-4.5%); Alberta: 21.7% (-4.0%); Saskatchewan: 19.6% (4.1%); Manitoba: 15.1% (-4.1%); Ontario: 26.8% (7.3%); New Brunswick: 7.5% (-8.1%); Nova Scotia: 17.9% (0.1%); Prince Edward Island: 3.8% (-9.2%); Newfoundland and Labrador: 10.5% (-11.2%); Non-Insured Health Benefits: 17.8% (1.2%); Total for all plans: 21.4% (2.0%).
There has been a large increase in the drug costs for biologics in 2013/14 (21.4%), contrasting with the low overall rate of change in drug costs (2.0%) in the public drug plans.
Jurisdictional differences in the rates of growth in biologics may be related to formulary listing decisions and the prevalence of diseases treated by this group of drugs, as well as demographic factors.
The relatively high rate of change in the cost of biologics compared to all drugs illustrated in Figure 4.4.3 has resulted in an increased market capture for biologics in recent years, which by 2013/14 accounted for 22.1% of total drug costs (Figure 4.4.4).
Alberta and Prince Edward Island had the highest levels of biologic-related costs relative to total drug costs in 2013/14 (34.6% and 28.3%, respectively).
Figure 4.4.4 Biologic share of total drug costs, NPDUIS public drug plans, 2009/10 to 2013/14
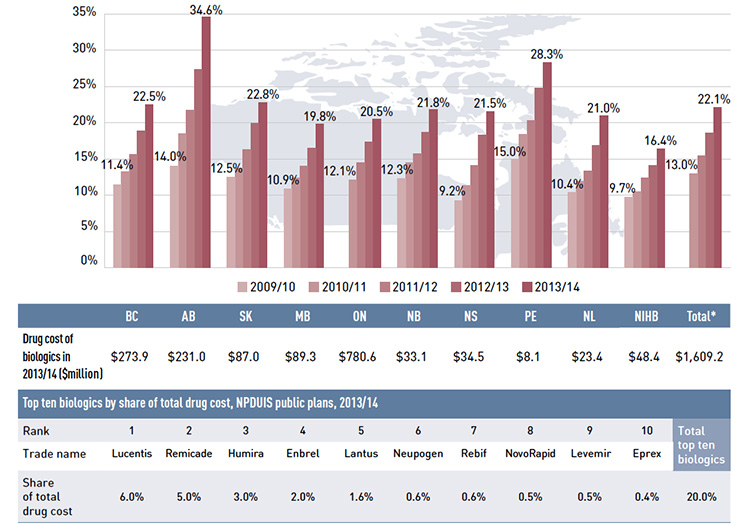
*Total results for the public drug plans reported in this figure.
Data source: National Prescription Drug Utilization Information System Database, Canadian Institute for Health Information.
Figure description
This bar graph shows the biologic share of total drug cost from 2009/10 to 2013/14 for the NPDUIS public drug plans. The results indicate that all plans had marked increases in biologic shares. Changes for the public drug plans from 2009/10 to 2013/14 are as follows:
- British Columbia: 11.4% to 22.5%
- Alberta: 14.0% to 34.6%
- Saskatchewan: 12.5% to 22.8%
- Manitoba: 10.9% to 19.8%
- Ontario: 12.1% to 20.5%
- New Brunswick: 12.3% to 21.8%
- Nova Scotia: 9.2% to 21.5%
- Prince Edward Island: 15.0% to 28.3%
- Newfoundland and Labrador: 10.4% to 21.0%
- Non-Insured Health Benefits: 9.7% to 16.4%
- Total for all plans: 13.0% to 22.1%
There are two tables below the bar graph. The first gives the cost of biologics in 2013/14 in millions of dollars. British Columbia: $273.9; Alberta: $231.0; Saskatchewan: $87.0; Manitoba: $89.3; Ontario: $780.6; New Brunswick: $33.1; Nova Scotia: $34.5; Prince Edward Island: $8.1; Newfoundland and Labrador: $23.4; Non-Insured Health Benefits: $48.4; Total select plans: $1,609.2.
The second table gives the top 10 biologics by share of total drug cost for the NPDUIS public drug plans in 2013/14. By rank from 1 to 10, they are as follows: Lucentis: 6.0%; Remicade: 5.0%; Humira: 3.0%; Enbrel: 2.0%; Lantus: 1.6%; Neupogen: 0.6%; Rebif: 0.6%; NovoRapid: 0.5%; Levemir: 0.5%; Eprex: 0.4%; Total top 10 biologics: 20.0%.
Figure 4.4.5 reports the shares of drug expenditure in 2013/14 for the top therapeutic classes as a total for all the plans. Level 1 of the World Health Organization’s Anatomical Therapeutic and Chemical (ATC) classification system is referenced, which refers to the main
anatomical group.
Figure 4.4.5 Top 10 level 1 ATC therapeutic classes by share of total drug costs, NPDUIS public drug plans, 2013/14
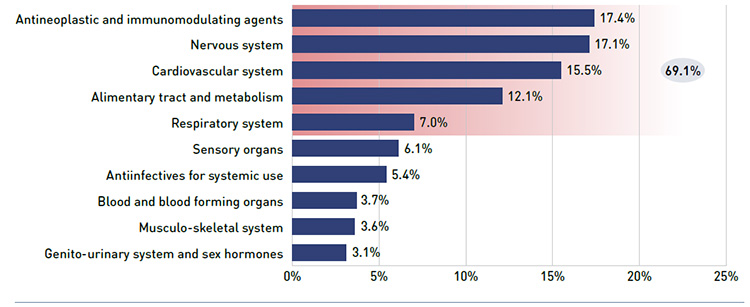
Note: The therapeutic classes reported are the level 1 category of the World Health Organization’s Anatomical Therapeutic and Chemical (ATC) classification system. The public drug plans include British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, New Brunswick, Nova Scotia, Prince Edward Island, Newfoundland and Labrador, and the Non-Insured Health Benefits Program.
Data source: National Prescription Drug Utilization Information System Database, Canadian Institute for Health Information.
Figure description
This bar graph shows the top 10 level one ATC therapeutic classes by share of total drug cost in 2013/14 for the NPDUIS public drug plans. Drug expenditure was concentrated in a few therapeutic classes, with antineoplastic and immunomodulating agents (17.4%), nervous system (17.1%), cardiovascular system (15.5%), alimentary tract and metabolism (12.1%), and respiratory system (7.0%) drugs accounting for 69.1% of the total drug expenditure. The other therapeutic classes and their share of drug cost include: sensory organs (6.1%); antiinfectives for systemic use (5.4%); blood and blood forming organs (3.7%); musculo-skeletal system (3.6%); and genito-urinary system and sex hormones (3.1%).
The results show that the drug cost expenditure was concentrated in a few therapeutic classes. Due to the increased use of biologic drugs, the antineoplastic and immunomodulating agents therapeutic class moved into the top position in terms of drug cost (17.4%) in 2013/14, followed by nervous system (17.1%), cardiovascular system (15.5%), alimentary tract and metabolism (12.1%) and respiratory system (7.0%). These five therapeutic classes accounted for over two-thirds (69.1%) of the total drug expenditure in 2013/14.
Figure 4.4.6 reports the top ten and bottom five therapeutic sub-classes contributing to the drug-mix effect from fiscal year 2012/13 to 2013/14, as a total for all plans. The ATC level 2 is referenced, which refers to the pharmacological/therapeutic subgroup.
Figure 4.4.6 Top ten and bottom five level 2 ATC therapeutic classes contributing to the drug-mix effect, NPDUIS public drug plans, 2013/14
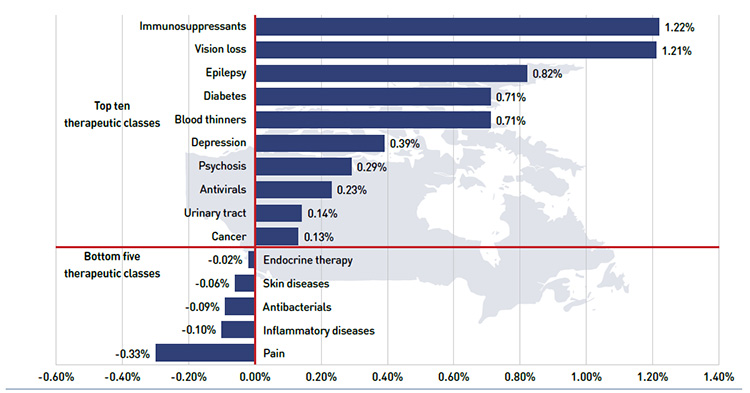
Note: The therapeutic classes reported are the level 2 category of the World Health Organization’s Anatomical Therapeutic and Chemical (ATC) classification system. The public drug plans include: British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, New Brunswick, Nova Scotia, Prince Edward Island, Newfoundland and Labrador, and the Non-Insured Health Benefits Program.
Data source: National Prescription Drug Utilization Information System Database, Canadian Institute for Health Information.
Figure description
This bar graph shows the top ten and bottom five level 2 ATC therapeutic classes contributing to the drug-mix effect from 2011/12 to 2012/13. The results suggest a significant push effect driven by immunosuppressant drugs, which increased the drug cost in 2012/13 by 0.71%. This class includes many fast-growing biologic drugs.
Top ten therapeutic classes:
- Immunosuppressants: 1.22%
- Vision loss: 1.21%
- Epilepsy: 0.82%
- Diabetes: 0.71%
- Blood thinners: 0.71%
- Depression: 0.39%
- Psychosis: 0.29%
- Antivirals: 0.23%
- Urinary tract: 0.14%
- Cancer: 0.13%
Bottom five therapeutic classes:
- Endocrine therapy: -0.02%
- Skin diseases: -0.06%
- Antibacterials: -0.09%
- Inflammatory diseases: -0.10%
- Pain: -0.33%
The results suggest a significant push effect in 2013/14 driven by two classes: immunosuppressants (1.22%) and vision loss (1.21%). These classes include some of the top biologic drugs reported in Figure 4.4.2 and 4.4.3. The bottom five therapeutic classes had
a downward pull effect of 0.61% on drug costs. Of these classes, the pain drugs had the most notable effect.
5 The Drivers of Dispensing Costs, 2012/13 to 2013/14
This section of the NPDUIS CompassRx report
provides a comprehensive analysis of the factors
that drive dispensing costs, measures their
impact, and delves into the factors determining
trends in use and fee levels in public drug plans.
This edition of the report focuses on the rates
of change in dispensing costs for the NPDUIS
drug plans from fiscal year 2012/13 to 2013/14.
Four effects are analyzed:
Demographic Effect
Similar to the demographic effect covered in the drivers of drug costs in Section 4.2, this effect encompasses changes in the size of the beneficiary population, as well as the aging and gender profile.
Fee Effect
This effect captures the impact of changes in the average dispensing fee per prescription.
Prescription Size Effect
This effect captures the impact of changes in the average number of units of a drug dispensed per prescription. This effect also drives drug costs, but has the opposite effect, as discussed in Section 4. A reduction in prescription size has an upward push effect on dispensing costs, as more prescriptions are required to dispense the same quantity of drugs.
Drug Volume Effect
This effect captures the impact of changes in the number of units dispensed to patients over the two periods analyzed (2012/13 and 2013/14). An increase in this measure has an upward push effect on dispensing costs, as more dispensing fees are claimed to dispense
an increased quantity of drugs.
Each of these effects was derived by assuming that all of the other factors remained constant over the period analyzed. The results provide an answer to the following question:
"How much would the dispensing costs have changed if only one factor (e.g., average dispensing fee per prescription) changed while the others remained the same?"
As with the drug costs analyzed in the previous section, multiple factors change simultaneously, creating a residual or a cross effect. The cross effect is also reported to account for the total change.
Figure 5.1 reports the rates of change in dispensing costs for the public drug plans from fiscal year 2012/13 to 2013/14, and isolates the change into four categories: demographic, fee, prescription size and drug volume effects. A cross effect is also reported. The bar graph and associated table below show the year-over-year impacts of each effect as a relative and absolute change in dispensing costs.
Figure 5.1 Rates of change in dispensing costs due to demographic, fee, prescription size and drug volume effects, NPDUIS public drug plans, 2012/13 to 2013/14
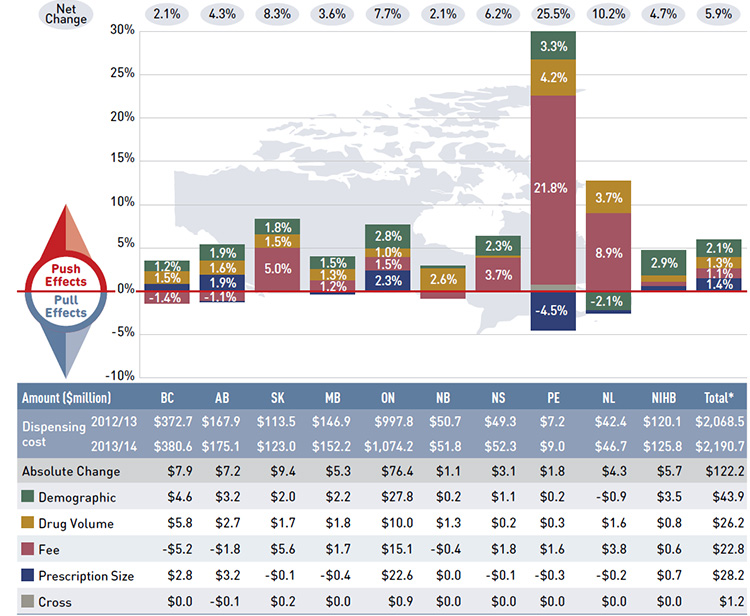
*Total results for the public drug plans reported in this figure.
Note: Values may not add to totals due to rounding.
Data source: National Prescription Drug Utilization Information System Database, Canadian Institute for Health Information.
Figure description
This figure describes the factors that impacted the rates of change in dispensing costs in the NPDUIS public drug plans from 2012/13 to 2013/14. It is composed of a bar graph with a table underneath.
The bar graph shows the percent change for each driver from 2012/13 to 2013/14 for the public plans. The results are as follows:
|
British Columbia |
Alberta |
Saskatchewan |
Manitoba |
Ontario |
New Brunswick |
Nova Scotia |
Prince Edward Island |
Newfoundland and Labrador |
Non-Insured Health Benefits |
Total |
| Net change |
2.1% |
4.3% |
8.3% |
3.6% |
7.7% |
2.1% |
6.2% |
25.5% |
10.2% |
4.7% |
5.9% |
| Demographic effect |
1.2% |
1.9% |
1.8% |
1.5% |
2.8% |
0.3% |
2.3% |
3.3% |
-2.1% |
2.9% |
2.1% |
| Drug volume effect |
1.5% |
1.6% |
1.5% |
1.3% |
1.0% |
2.6% |
0.3% |
4.2% |
3.7% |
0.7% |
1.3% |
| Fee effect |
-1.4% |
-1.1% |
5.0% |
1.2% |
1.5% |
-0.8% |
3.7% |
21.8% |
8.9% |
0.5% |
1.1% |
| Prescription size effect |
0.8% |
1.9% |
-0.1% |
-0.3% |
2.3% |
0.0% |
-0.1% |
-4.5% |
-0.4% |
0.6% |
1.4% |
| Cross effect |
0.0% |
-0.1% |
0.2% |
0.0% |
0.1% |
0.0% |
0.1% |
0.7% |
0.1% |
0.0% |
0.1% |
The table below the bar graph gives the corresponding changes in millions of dollars.
|
British Columbia |
Alberta |
Saskatchewan |
Manitoba |
Ontario |
New Brunswick |
Nova Scotia |
Prince Edward Island |
Newfoundland and Labrador |
Non-Insured Health Benefits |
Total select plans |
| Dispensing fee 2012/13 |
$372.7 |
$167.9 |
$113.5 |
$146.9 |
$997.8 |
$50.7 |
$49.3 |
$7.2 |
$42.4 |
$120.1 |
$2,068.5 |
| Dispensing fee 2013/14 |
$380.6 |
$175.1 |
$123.0 |
$152.2 |
$1,074.2 |
$51.8 |
$52.3 |
$9.0 |
$46.7 |
$125.8 |
$2,190.7 |
| Absolute change |
$7.9 |
$7.2 |
$9.4 |
$5.3 |
$76.4 |
$1.1 |
$3.1 |
$1.8 |
$4.3 |
$5.7 |
$122.2 |
| Demographic effect |
$4.6 |
$3.2 |
$2.0 |
$2.2 |
$27.8 |
$0.2 |
$1.1 |
$0.2 |
-$0.9 |
$3.5 |
$43.9 |
| Drug volume effect |
$5.8 |
$2.7 |
$1.7 |
$1.8 |
$10.0 |
$1.3 |
$0.2 |
$0.3 |
$1.6 |
$0.8 |
$26.2 |
| Fee effect |
-$5.2 |
-$1.8 |
$5.6 |
$1.7 |
$15.1 |
-$0.4 |
$1.8 |
$1.6 |
$3.8 |
$0.6 |
$22.8 |
| Prescription size effect |
$2.8 |
$3.2 |
-$0.1 |
-$0.4 |
$22.6 |
$0.0 |
-$0.1 |
-$0.3 |
-$0.2 |
$0.7 |
$28.2 |
| Cross effect |
$0.0 |
-$0.1 |
$0.2 |
$0.0 |
$0.9 |
$0.0 |
$0.0 |
$0.0 |
$0.0 |
$0.0 |
$1.2 |
Note that this edition of the CompassRx includes data for two additional public plans – British Columbia and Newfoundland and Labrador – that were not analyzed in the first report. Thus, the results reported for the total of all drug plans in the two editions
are not directly comparable. However, plan-by-plan comparisons are still relevant, and the interpretation of general trends is still appropriate.
The dispensing cost levels increased by $122.2 million (5.9%) in 2013/14, reaching $2.2 billion. This increase was mainly due to the increased use of drugs. The growth in dispensing costs in 2013/14 (5.9%) was comparable to the growth in 2012/13 (5.8%).Footnote 11
Overall, the results for most effects varied considerably across drug plans. Most notably, the fee effect had a large push effect on dispensing costs in Prince Edward Island (21.8%) and Newfoundland and Labrador (8.9%), with a more moderate positive effect in Saskatchewan (5.0%). Conversely, the fee effect had a negative or pull effect in a few jurisdictions, including British Columbia (-1.4%), Alberta (-1.1%) and New Brunswick (-0.8%). These results are directly related to the rates of change in the average dispensing
fee per prescription reported in Table 5.1.
Table 5.1 Average dispensing fee per prescription, NPDUIS public drug plans, 2009/10 to 2013/14
| Public drug plan |
2009/10 |
2010/11 |
2011/12 |
2012/13 |
2013/14 |
Growth rate
2012/13 to
2013/14 |
Compound annual
growth rate
2009/10 to 2013/14 |
| British Columbia |
$7.09 |
$7.49 |
$8.01 |
$8.01 |
$7.89 |
-1.4% |
2.7% |
| Alberta |
$13.07 |
$15.22 |
$14.50 |
$13.43 |
$13.29 |
-1.1% |
0.4% |
| Saskatchewan |
$8.54 |
$8.90 |
$9.29 |
$9.64 |
$10.12 |
5.0% |
4.3% |
| Manitoba |
$9.21 |
$9.39 |
$9.58 |
$9.73 |
$9.84 |
1.2% |
1.7% |
| Ontario |
$5.89 |
$7.00 |
$7.34 |
$7.43 |
$7.54 |
1.5% |
6.4% |
| New Brunswick |
$10.05 |
$10.21 |
$9.83 |
$10.45 |
$10.36 |
-0.8% |
0.8% |
| Nova Scotia |
$9.92 |
$10.08 |
$10.32 |
$11.08 |
$11.49 |
3.7% |
3.7% |
| Prince Edward Island |
$6.77 |
$6.84 |
$6.82 |
$8.46 |
$10.31 |
21.8% |
11.1% |
| Newfoundland and Labrador |
$4.63 |
$4.70 |
$4.76 |
$11.20 |
$12.20 |
8.9% |
27.4% |
| NIHB |
– |
– |
$8.16 |
$8.26 |
$8.30 |
0.5% |
0.9% |
Data source: National Prescription Drug Utilization Information System Database, Canadian Institute for Health Information.
Similarly, the prescription size effect had
a positive impact in a few public plans in
2013/14, such as Ontario (2.3%), Alberta
(1.9%) and British Columbia (0.8%). This
effect is related to declines in prescription size
(see Figure 5.2), as shorter, more frequent
prescriptions increase the cost of dispensing;
although shorter prescriptions result in some
savings by reducing drug waste. On the other
hand, there was a large “pull” effect in
Prince Edward Island (-4.5%) related to an
increase in the day supply per prescription
from 2012/13 to 2013/14. For other public
plans, the effect was very small or neutral.
While the cost driver model measures impacts
over one year, changes in prescription size –
and the corresponding impact on fee
expenditure – usually occur over several years.
To illustrate this, a case study for British
Columbia (Figure 5.3) analyses changes in the
day supply per claim over five years from
2009/10 to 2013/14.
The other main effect was drug volume, which
had a significant positive impact on dispensing
costs in Prince Edward Island (4.2%) and
Newfoundland and Labrador (3.7%). Drug
volume had a more moderate effect in New
Brunswick (2.6%). For other plans, the drug
volume effect was small, between 0.3%
and 1.6%.
With the exception of Newfoundland and
Labrador, the demographic effect was positive
across all plans, averaging 2.1% from 2012/13
to 2013/14. This result reflects changes in the
active beneficiary population (see Figure 4.2.2),
as well as the aging of the population (see
Figure 4.2.3).
The additional table and figures in this section
provide supporting statistical information
on the increase in the average dispensing fee
reimbursed per prescription, as well as trends
in prescription size.
The fee effect reported in Figure 5.1 is a direct
result of the increases in the average dispensing
fee per prescription from 2012/13 to 2013/14 reported in Table 5.1. This table also reports
the average dispensing fee per prescription
for the fiscal years 2009/10 to 2013/14, along
with the compound annual rate of change. The
results are an average across all prescriptions
and encompass a range of dispensing fees
reimbursed by the plans.
The variations in dispensing fee levels across
public plans reflect their differing reimbursement
policies (Appendix D) and appear to be correlated
with the average size of prescriptions reported
in Figure 5.2.
Figure 5.2 Average day supply per prescription by NPDUIS public drug plan, oral solids, 2009/10 to 2013/14
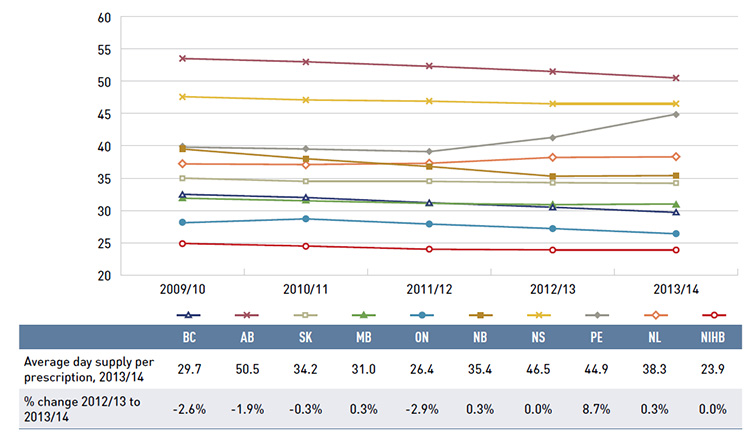
Data source: National Prescription Drug Utilization Information System Database, Canadian Institute for Health Information.
Figure description
This line graph shows the trend in average day supply per prescription from 2009/10 to 2013/14 in the NPDUIS public drug plans.
| |
2009/10 |
2010/11 |
2011/12 |
2012/13 |
2013/14 |
| British Columbia |
32.5 |
32.0 |
31.2 |
30.5 |
29.7 |
| Alberta |
53.5 |
53.0 |
52.3 |
51.5 |
50.5 |
| Saskatchewan |
35.0 |
34.5 |
34.5 |
34.3 |
34.2 |
| Manitoba |
31.9 |
31.5 |
31.1 |
30.9 |
31.0 |
| Ontario |
28.1 |
28.7 |
27.9 |
27.2 |
26.4 |
| New Brunswick |
39.5 |
38.0 |
36.8 |
35.3 |
35.4 |
| Nova Scotia |
47.6 |
47.1 |
46.9 |
46.5 |
46.5 |
| Prince Edward Island |
39.8 |
39.5 |
39.1 |
41.3 |
44.9 |
| Newfoundland and Labrador |
37.2 |
37.1 |
37.3 |
38.2 |
38.3 |
| Non-Insured Health Benefits |
24.9 |
24.5 |
24.0 |
23.9 |
23.9 |
This figure also includes a table with the average day supply per prescription in 2013/14 and the percent change in day supply from 2012/13 to 2013/14.
|
British Columbia |
Alberta |
Saskatchewan |
Manitoba |
Ontario |
New Brunswick |
Nova Scotia |
Prince Edward Island |
Newfoundland and Labrador |
Non-Insured Health Benefits |
| Average day supply per prescription for 2013/14 |
29.7 |
50.5 |
34.2 |
31 |
26.4 |
35.4 |
46.5 |
44.9 |
38.3 |
23.9 |
| Percent change from 2012/13 to 2013/14 |
-2.6% |
-1.9% |
-0.3% |
0.3% |
-2.9% |
0.3% |
0.0% |
8.7% |
0.3% |
0.0% |
For instance, British Columbia, Ontario and
the NIHB, which reimbursed some of the lowest
dispensing fees in 2013/14 ($7.89, $7.54 and
$8.30, respectively, on average), had some of
the smallest prescription sizes, as measured by
the number of average days supplied per
prescription for oral solids (29.7 days, 26.4
and 23.9 days, respectively).
On the other hand, Alberta, which had the
highest average dispensing fee per prescription
in 2013/14 ($13.29), had the largest
prescription size (50.5 days). Note that the
average dispensing fee in Alberta has been
declining in recent years, from a high of
$15.22 in 2010/11 to $13.29 in 2013/14. At
the same time, the prescription size has also
decreased from 53.5 in 2010/11 to 50.5
in 2013/14.
In Newfoundland and Labrador, the large
increase in average dispensing fees for 2012/13
was due to changes in the reimbursement policy
and a new agreement with the provincial
pharmacy association, which increased fee levels.
Despite the wide variations in the average
dispensing fee and prescription size across the
plans, the dispensing costs often represented
a comparable portion of the total prescription
cost (20.8% in Alberta and 20.7% in Ontario;
Figure 2.1).
The variations across plans may also reflect
differences in pharmacy reimbursement
determined by policies related to drug costs,
markups and dispensing costs. While the
amount reimbursed for dispensing fees and the
prescription size have a bearing on dispensing
costs, the levels may also be influenced by the
disease profile of the population and the type
of drugs predominantly used (e.g., acute versus
maintenance treatments).
The prescription size effect reported in Figure 5.1
is influenced by changes in the average number
of days supplied per prescription. The trend in
day supply per prescription is reported in Figure
5.2 for the fiscal years 2009/10 to 2013/14. The
results are an average across all prescriptions for
oral solid formulations and encompass all
therapy types (acute and maintenance).
Day supply per prescription and the number of
physical units of medication per prescription
(Figure 4.3.3) are measures of prescription size.
The latter is used in both the drug cost and
dispensing cost driver models.
Similarly to the results reported in Figure 4.3.3
on the average number of physical units of
medication per prescription, the results for the
average day supply per prescription suggest that
prescription size was either stable or declined
slightly in most public drug plans from 2009/10
to 2013/14. The exceptions were Prince Edward
Island and Newfoundland and Labrador, where
the average day supply increased in 2012/13
and 2013/14 due to plan design changes.
British Columbia, Alberta, Ontario and
New Brunswick had the most pronounced
reductions in average prescription size in
recent years. In New Brunswick, the rate
of decline stabilized in 2013/14, coinciding
with the implementation of a policy aimed
at addressing this issue. The reductions
in prescription size acted as a push effect
on dispensing costs, as more frequent
prescriptions were required to dispense a
given volume of drugs. The following section
investigates the recent changes in the size
of prescriptions for British Columbia.
Case Study on Prescription Size – British Columbia
Figure 5.3 illustrates a case study of changes in
the size of prescriptions in British Columbia.
Alberta and Ontario also had important
prescription size reductions in 2013/14. This
had a push effect on dispensing costs of 0.8%
in British Columbia, 1.9% in Alberta and
2.3% in Ontario (Figure 5.1).
For the case study, the top 350 highest
utilized ingredients in 2013/14 with oral solid
formulations were selected for analysis. The
percent change in the average day supply
from 2009/10 to 2013/14 was calculated. The
results are reported in a scatter diagram at the
ingredient level, with the percent change in the
average number of day supply per prescription
on the horizontal axis and the percent share of
total prescriptions on the vertical axis.
The figure also provides a table with the top
10 drugs by prescription volume, and their
corresponding change in prescription size
from 2009/10 to 2013/14.
In British Columbia, the prescription size
decreased for a large proportion of ingredients
(65.7%), while ingredients with the greatest
share of prescriptions had marked declines in
prescription length.
Figure 5.3 Percent change in prescription size by ingredient, British Columbia, 2009/10 to 2013/14
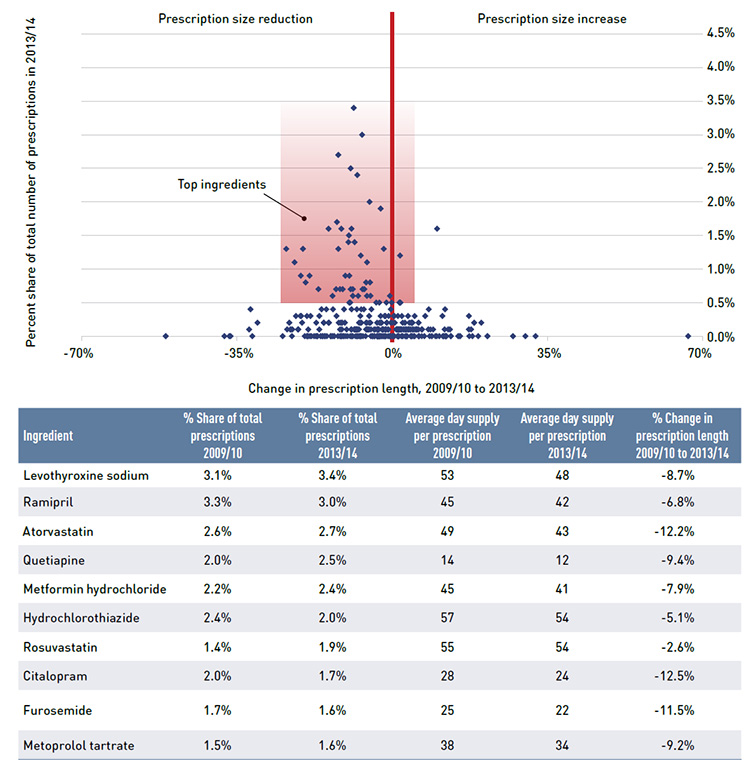
Note: Results are restricted to oral solid formulations (tablets and capsules).
Data source: National Prescription Drug Utilization Information System Database, Canadian Institute for Health Information.
Figure Description
This figure, composed of a scatter graph and an accompanying data table, shows the percent change in prescription size by ingredient for British Columbia.
The x-axis of the scatter graph plots the percent change in the average number of day supply per prescription from 2009/10 to 2013/14, while the y-axis gives the ingredient’s share of the total number of prescriptions for 2013/14. The results show that prescription size decreased for a large proportion of ingredients over the five years; ingredients with the largest market shares had marked declines.
The table gives data for select ingredients.
| Ingredient |
Percent share of total prescriptions 2009/10 |
Percent share of total prescriptions 2013/14 |
Average day supply per prescription 2009/10 |
Average day supply per prescription 2013/14 |
Percent change in prescription size 2009/10 to 2013/14 |
| Levothyroxine sodium |
3.1% |
3.4% |
53 |
48 |
-8.7% |
| Ramipril |
3.3% |
3.0% |
45 |
42 |
-6.8% |
| Atorvastatin |
2.6% |
2.7% |
49 |
43 |
-12.2% |
| Quetiapine |
2.0% |
2.5% |
14 |
12 |
-9.4% |
| Metformin hydrochloride |
2.2% |
2.4% |
45 |
41 |
-7.9% |
| Hydrochlorothiazide |
2.4% |
2.0% |
57 |
54 |
-5.1% |
| Rosuvastatin |
1.4% |
1.9% |
55 |
54 |
-2.6% |
| Citalopram |
2.0% |
1.7% |
28 |
24 |
-12.5% |
| Furosemide |
1.7% |
1.6% |
25 |
22 |
-11.5% |
| Metoprolol tartrate |
1.5% |
1.6% |
38 |
34 |
-9.2% |
Appendix A: Public Drug Plan Designs
Table A1 provides a summary of the NPDUIS public plan designs in 2013/14, as detailed in a Plan Information Document published by the Canadian Institute for Health Information.Footnote 2
Table A1: Public drug plan designs, 2013/14
British Columbia
Plans/Eligibility
British Columbia has a universal program with a variety of beneficiary groups and sub-plans: the Fair PharmaCare plan provides regular assistance to residents born in 1940 or later, with enhanced assistance provided to residents who are part of a family with at least one spouse born in 1939 or earlier; permanent residents of licenced residential care facilities; recipients of income assistance and children and youth in care; individuals with cystic fibrosis who are registered with a provincial cystic fibrosis clinic; severely handicapped children 18 years and under; psychiatric medication for individuals registered by a Mental Health Services Centre; medication management services provided by pharmacies such as publicly funded vaccinations and review of a patient’s medication; palliative care at home; patients enrolled at BC Centre for Excellence in HIV/AIDS; and a smoking cessation program.
Cost Sharing
British Columbia had income-based annual deductibles for its Fair PharmaCare and enhanced Fair PharmaCare assistance programs (see tables below). There were no deductibles for other programs/plans. After deductibles had been met, there were co-payments of 30% of the prescription drug costs for the Fair PharmaCare program and 25% for the enhanced program.
Fair PharmaCare
| Net family income |
Approximate deductible
(% of net income) |
| <$15,000 |
0% |
| $15,000–$30,000 |
2% |
| >$30,000 |
3% |
Fair PharmaCare – Enhanced Assistance
| Net family income |
Approximate deductible
(% of net income) |
| <$33,000 |
0% |
| $33,000–$50,000 |
1% |
| >$50,000 |
2% |
Alberta
Plans/Eligibility
Alberta has a Seniors Drug Plan that covers seniors 65 and older and eligible dependents; a Widows Plan for those who qualify and their dependents; Palliative Coverage for residents treated at home; and Non-Group Coverage for residents younger than 65. Claims dispensed to residents of long-term facilities, through Income Support, the Alberta Adult Health Benefit, the Assured Income for Severely Handicapped, the Alberta Child Health Benefit Child Intervention Services and Family Supports for Children with Disabilities programs are not submitted to NPDUIS. There are several other programs, including the Outpatient Cancer Drug Program and Specialized High Cost Drug Program (includes funding for transplant drugs and HIV/AIDS drugs, as well as several other drug costs) – information from these programs is also not submitted to NPDUIS.
Cost Sharing
Alberta set co-payments at 30% of the prescription to a maximum of $25 for seniors, widows, palliative care and non-group beneficiaries. Premiums for non-group beneficiaries were $118.00/month for families and $63.50/month for singles. Subsidized premiums for non-group beneficiaries were offered based on income as follows: $82.60/month for families and $44.45/month for singles. Palliative care has a maximum co-payment of $1,000.
Saskatchewan
Plans/Eligibility
Saskatchewan has a universal program with several plans and beneficiary groups (with the exception of those eligible under another agency, primarily federal programs). The universal program is the Special Support Program, which assists those whose benefit drug costs are high in relation to their income. Other beneficiary groups and plans include a Seniors’ Drug Plan for those who qualify based on income; seniors receiving the Guaranteed Income Supplement or the Saskatchewan Income Plan supplement; a Children’s Drug Program for children 14 or younger; Supplementary Health and Family Health Benefits for which eligibility is established through Social Services; a Saskatchewan Aids to Independent Living for paraplegics; cystic fibrosis and renal disease programs; Palliative Care; Emergency Assistance as a one-time assistance until the beneficiary can apply for Special Support. Beneficiaries who qualify under more than one program receive the better benefit as calculated by the system at the time of dispensing. Claims for Formulary and Exception Drug Status drugs are submitted to NPDUIS, while drugs covered under special programs such as the Saskatchewan Cancer Agency are not submitted to NPDUIS.
Cost Sharing
Saskatchewan had standard income-based annual deductibles for three plans/programs: Guaranteed Income Supplement (GIS): beneficiaries living in the community paid a semi-annual deductible of $200, while those living in special care homes paid $100 semi-annually. Saskatchewan Income Plan (SIP) and Family Health Benefits (FHB) beneficiaries paid a semi-annual deductible of $100.
Special Support Program: A family threshold (deductible) and a consumer co-payment were based on income information provided on the application form, income tax documentation and drug plan records. The threshold was based on 3.4% of the total family income (adjusted for the number of dependents), and the co-payment was calculated using total family income and actual benefit drug costs.
Co-payments were also made for the following plans/programs, including the Seniors Drug Plan: up to $20 per prescription; and FHB, SIP and GIS plans: after the deductible was met, 35% co-payment for prescriptions applied with certain conditions, for example, for FHB beneficiaries, the co-payment did not apply to children under 18, and for SIP and GIS recipients, the co-payment may have applied for income-tested coverage.
Manitoba
Plans/Eligibility
Manitoba Pharmacare covers all provincial residents who are eligible for benefits under The Prescription Drugs Cost Assistance Act, and includes residents as defined by The Health Services Insurance Act. To be eligible, the person must be a member of a family that has spent more on specified drugs in a benefit year than the allowed deductible amount. Other sub-plans cover those who receive benefits from the Employment and Income Assistance Program; residents in personal care homes who receive benefits from the Personal Care Home Drug Program; individuals who are terminally ill and wish to remain at home from the Palliative Care Drug Program; and individuals requiring out-patient cancer treatment with eligible oral cancer and specific supportive drugs from the Home Cancer Drug Program. Products available through Part 3 of the Manitoba Drug Formulary are not submitted to NPDUIS and are reported as exceptional status products in NPDUIS claims reports.
Cost Sharing
Manitoba had an annual deductible based on total family income, with a minimum deductible of $100 (see table below).
Deductible rates for range of family incomes
| Lower limit |
Upper limit |
Deductible |
| – |
≤$15,000 |
2.85% |
| >$15,000 |
≤$21,000 |
4.05% |
| >$21,000 |
≤$22,000 |
4.09% |
| >$22,000 |
≤$23,000 |
4.17% |
| >$23,000 |
≤$24,000 |
4.23% |
| >$24,000 |
≤$25,000 |
4.27% |
| >$25,000 |
≤$26,000 |
4.32% |
| >$26,000 |
≤$27,000 |
4.37% |
| >$27,000 |
≤$28,000 |
4.41% |
| >$28,000 |
≤$29,000 |
4.45% |
| >$29,000 |
≤$40,000 |
4.48% |
| >$40,000 |
≤$42,500 |
4.87% |
| >$42,500 |
≤$45,000 |
4.99% |
| >$45,000 |
≤$47,500 |
5.09% |
| >$47,500 |
≤$75,000 |
5.16% |
| >$75,000 |
– |
6.46% |
Ontario
Plans/Eligibility
The Ontario Drug Benefit (ODB) Program covers Ontario residents that are 65 and older, residents of long-term care homes and homes for special care, recipients of professional home services, recipients of social assistance, and recipients under the Trillium Drug Program, which provides drug benefits for Ontario residents who have high drug costs in relation to their household income. The Special Drugs Program covers expensive outpatient drugs used to treat specific diseases. The New Drug Funding Program covers drug benefits for intravenous cancer drugs, administered to outpatients at hospitals and cancer care facilities.
Cost Sharing
The Ontario Drug Benefit (ODB) Program had a $100 annual deductible for single seniors with an annual net income equal to or greater than $16,018; and senior couples with a combined annual income equal to or greater than $24,175.
Trillium Drug Program applicants paid a quarterly deductible that was based on income.
ODB recipients paid co-payments up to $2 per prescription if they were:
- A senior single person with an annual net income of less than $16,018; a senior couple with a combined annual net income of less than $24,175
- Receiving benefits under the Ontario Works Act or the Ontario Disability Support Program Act
- Receiving professional services under the Home Care Program
- Residents of long-term care facilities and homes for special care
- Eligible under the Trillium Drug Program (once their quarterly deductible is reached)
ODB recipients paid up to $6.11 toward the dispensing fee per prescription once they reached their $100 annual deductible if they were:
- A senior single person with an annual net income equal to or greater than $16,018
- A senior couple with a combined annual net income equal to or greater than $24,175
A co-payment of up to $2.83 was made for each prescription dispensed from an outpatient hospital pharmacy.
New Brunswick
Plans/Eligibility
The Seniors program is eligible to residents on a Guaranteed Income Supplement or who qualify based on an income test. Other programs/plans include Cystic Fibrosis; Individuals in Licensed Residential Facilities; Social Development clients; Children in the Care of the Minister of Social Development and Special Needs Children; Human Growth Hormone Deficiency; Multiple Sclerosis; Organ Transplant; HIV/AIDs and Nursing Home Residents.
Cost Sharing
The following plans had a $50 per year registration fee: Cystic Fibrosis, Multiple Sclerosis, Organ Transplant, Human Growth Hormone Deficiency and HIV/AIDS.
Co-payments varied across plan/programs as follows:
Co-payment per prescription for New Brunswick drug programs/plans
| Program/plan |
Co-payment per prescription |
Annual co-payment ceiling |
| Seniors Guaranteed Income Supplement |
$9.05 |
$500 |
| Seniors Non-Guaranteed Income Supplement |
$15.00 |
- |
| Adults in Licensed Residential Facilities |
$4.00 |
$250 per family |
| Department of Social Development |
$4 for adults 18 years and older
$2 for children younger than 18 |
$250 per family |
| Multiple Sclerosis |
Income tested annually |
- |
| Cystic Fibrosis, Organ Transplant, Human Growth Hormone Deficiency and HIV/AIDS |
20% of prescription to a maximum of $20 |
- |
Nova Scotia
Plans/Eligibility
The Family Pharmacare Program provides assistance with prescription drug coverage for residents of Nova Scotia with a valid Nova Scotia health card. Other programs/plans include Drug Assistance for Cancer Patients for families with a gross income no greater than $15,720 that do not have drug coverage under any other program, except Family Pharmacare; Diabetes Assistance Program (this program is closed to new enrollees); Seniors’ Pharmacare Program available for residents who are age 65 or older. Claims dispensed through the Department of Community Services programs for residents on income assistance are not submitted to NPDUIS.
Cost Sharing
For the Seniors’ Pharmacare Program, Nova Scotia had a maximum annual premium of $424. There was no premium for single seniors with an income lower than $18,000 or for seniors who are married and have a joint income less than $21,000. Seniors receiving the Guaranteed Income Supplement were also exempt from premiums. Other senior beneficiaries may have had a reduced premium: for singles with an income between $18,000 and $24,000 and those who were married and had a joint income between $21,000 and $28,000.
Nova Scotia’s Family Pharmacare and Diabetes Assistance programs had annual maximum deductibles based on sliding-scale percentages in relation to family size and income. The Nova Scotia Family Pharmacare program also had an annual maximum co-payment based on family size and income.
For co-payments, recipients of the Family Pharmacare and Diabetes Assistance programs paid 20% per prescription (to the maximum for Nova Scotia Family Pharmacare. There was no maximum for the Diabetes Assistance Program). Senior Pharmacare beneficiaries paid 30% of the prescription cost as a co-payment to a maximum of $382 per year.
Prince Edward Island
Plans/Eligibility
Seniors Drug Cost Assistance for persons age 65 or older; High-Cost Drug Program; Diabetes Control Drug Program; Family Health Benefit Program for families with income less than a threshold; Nursing Home Drug Program; Quit Smoking Program; Financial Assistance Drug Program; and a Catastrophic Drug Program (began on Oct. 1, 2013) for any permanent resident, with their annual out-of-pocket drug costs for eligible prescription medications capped at an amount not exceeding a set percentage of their household income, referred to as “household cap.”
To be eligible for the Catastrophic Drug Program: (i) the applicant must be a permanent resident who is present in the province for 6 months or more per year; (ii) the applicant and eligible household members must file a Prince Edward Island tax return for the previous year for which they are applying to the program to claim benefits; (iii) the applicant much have a valid Prince Edward Island Health Card.
A Sexually Transmitted Disease (STD) Drug Program is eligible to persons diagnosed with and an STD or had contact with a person diagnosed with an STD. Opioid Replacement Therapy (ORT) is a provincial addictions program that was linked to the STD program throughout 2013/14. (The two programs were separated in April 2015.)
Cost Sharing
Prince Edward Island had co-payments per prescription that varied for each program/plan and some medications.
Co-payment per prescription for PEI drug programs/plans or medication
| Program/plan |
Co-payment per prescription |
| Seniors Drug Cost Assistance Plan |
First $8.25 of the medication cost plus the professional fee |
| Family Health Benefit Program |
Professional fee |
| High-Cost Drug Program |
Income-based portion of the drug plus the professional fee |
| Insulin |
$10 per 10 mL or box of 1.5 mL cartridges or $20 per box of 3 mL cartridges |
| Blood glucose test strips |
$11 per prescription to a maximum of 100 strips every 30 days |
| Oral medications and urine testing materials |
$11 per prescription |
| High-cost diabetes medications |
An income-based portion of the drug cost plus the professional fee |
| Quit Smoking Program |
Patients were responsible for all medication costs approved, except for the first $75 per year, which was paid by the program. |
| Home Oxygen Program |
PEI Medicare program paid 50% of the eligible expenses up to $200 per month. |
| Catastrophic Drug Program |
This is an income-based program. Once an applicant’s out-of-pocket eligible drug expenses exceed the annual household limit the program covered any further eligible drug expenses in the program year. |
Newfoundland and Labrador
Plans/Eligibility
Newfoundland and Labrador has five drug plans under the Newfoundland and Labrador Prescription Drug Program:
- The 65Plus Plan for residents 65 years or older who receive old age security benefits and the Guaranteed Income Supplement.
- The Foundation Plan covers persons and families in receipt of Income Support benefits through the Department of Advanced Education and Skills, children in care of the Regional Health Authorities or the Department of Child, Youth and Family Services, as the case may be, individuals involved with Community Youth Corrections, persons in receipt of community supports, and persons who are subsidized residents in Long Term Care Homes and Personal Care Homes.
- The Access Plan covers residents with a low income determined by family net income level.
- The Assurance Plan covers residents with the financial burden of eligible high drug costs.
- The Needs Plan covers residents who have been diagnosed with cystic fibrosis and residents aged 18 years or younger with growth hormone deficiency.
Cost Sharing
Newfoundland and Labrador had co-payments per prescription that varied for each program/plan, as follows:
For the Seniors program (65Plus Plan) – the co-payment was up to $6 per prescription.
For the Access Plan, beneficiary co-payments per prescription varied based on income and family status, as follows:
Co-payments per prescription for the Newfoundland and Labrador Access Plan
Families with children
| Income |
Co-payment |
| <$30,009 |
20.0% |
| $31,000 |
23.9% |
| $32,000 |
27.7% |
| $33,000 |
31.6% |
| $34,000 |
35.5% |
| $35,000 |
39.4% |
| $36,000 |
43.3% |
| $37,000 |
47.2% |
| $38,000 |
51.1% |
| $39,000 |
55.0% |
| $40,000 |
58.8% |
| $41,000 |
62.7% |
| $42,000 |
66.6% |
| $42,870 |
70.0% |
Couples with no children
| Income |
Co-payment |
| <$21,435 |
20.0% |
| $22,000 |
23.3% |
| $23,000 |
29.1% |
| $24,000 |
35.0% |
| $25,000 |
40.8% |
| $26,000 |
46.6% |
| $27,000 |
52.4% |
| $28,000 |
58.3% |
| $29,000 |
64.1% |
| $30,000 |
69.9% |
| $30,009 |
70.0% |
| – |
– |
| – |
– |
| – |
– |
Single individuals
| Income |
Co-payment |
| <$18,577 |
20.0% |
| $19,000 |
22.5% |
| $20,000 |
28.3% |
| $21,000 |
34.1% |
| $22,000 |
40.0% |
| $23,000 |
45.8% |
| $24,000 |
51.6% |
| $25,000 |
57.5% |
| $26,000 |
63.3% |
| $27,000 |
69.1% |
| $27,151 |
70.0% |
| – |
– |
| – |
– |
| – |
– |
For the Assurance Plan, individuals and families had their annual out-of-pocket drug costs capped as per the following table:
| Annual net income (i.e., line 236 minus line 117 of income tax return) |
Maximum % of net income to spend on drug costs |
| $0–$39,999 |
5% |
| $40,000–$74,999 |
7.5% |
| $75,000–$149,999 |
10% |
NIHB
Plans/Eligibility
The Non-Insured Health Benefits Program provides registered First Nations and recognized Inuit with coverage for a limited range of medically necessary goods and services. To be eligible, an individual must be a resident of Canada and a registered First Nations according to the Indian Act; an Inuk recognized by one of the Inuit Land Claim organizations; or an infant of less than one year of age whose parent is an eligible recipient. Those individuals who are otherwise covered under a separate agreement (e.g., a self-government agreement) are not eligible for coverage.
Cost Sharing
–
Appendix B: Pricing Policies for Generic Drugs in Provincial Drug Plans
Table B1 provides a summary, as of December 31, 2015, of the generic price reduction policies across provinces with their respective dates of implementation.
Table B1. Provincial generic pricing policies, generic prices as a percentage of the brand-name price
| |
2010 |
2011 |
2012 |
2013 |
2014 |
2015 |
pCPA*
18% molecules |
|
|
|
April 1: 18% for six† of the most common generic drugs (the Council of the Federation) |
April 1: 18% for ten† of the most common generic drugs (the Council of the Federation) |
April 1: 18% for fourteen† of the most common generic drugs (the Council of the Federation) |
pCPA*
Tiered Pricing Framework |
|
|
|
|
- Tier 1 (single source) – one generic: 85% of brand reference price if a Product Listing Agreement (PLA) does not exist for the brand product; 75% if there is a PLA
- Tier 2 (dual source) – two generics: 50% of brand reference price
- Tier 3 (multi source) – three or more generics: 25% of brand reference price for oral solids; 35% for non-oral solids
|
| British Columbia
|
October 15:
50% existing generics
42% new generics |
July 4:
40% all generics |
April 2:
35% all generics |
April 1:
25% most generics |
April 1:
20% most generics |
|
| Alberta
|
April 1:
56% existing generics
45% new generics |
|
July 1:
35% all generics |
May 1:
18% |
April 1:
Lowest available price for existing generics; tiered pricing for new generics:
70% one generic
50% two generics
25% three generics
18% four or more generics |
|
| Saskatchewan
|
|
April 1:
40% new generics
May 1 and June 1:
45% existing generics
April 1 and October 1:
35% generics in former Standing Offer Contract categories |
April 1:
35% |
|
|
April 1:
25% |
| Manitoba
|
Generic drug pricing is subject to utilization management agreements with the manufacturers, which declare that the price of a generic is equal to that of other select provinces. |
| Ontario |
July 1:
25%‡ public
50% private & out-of-pocket |
April 1:
25%‡ public
35% private & out-of-pocket |
April 1:
25%‡ public, private & out-of-pocket |
|
|
May 15:
Tiered pricing for generics‡ |
| Quebec |
Quebec requires that generic manufacturers provide the province with the lowest price available in other provinces. |
| New Brunswick |
|
|
June 1: 40%
December 1: 35% |
June 1: 25% |
|
|
| Nova Scotia |
|
July 1: 45% |
January 1: 40%
July 1: 35% |
|
November 12:
25% |
|
| Prince Edward Island |
|
|
July 1: 35% |
December 1: 25% |
|
|
| Newfoundland & Labrador |
|
|
April 1: 45%
October 1: 40% |
April 1: 35%
July 1: 25% |
|
|
* pan-Canadian Pharmaceutical Alliance (pCPA): http://www.pmprovincesterritoires.ca/en/initiatives/358-pan-canadian-pharmaceutical-alliance. After April 1, 2013, the general provincial generic pricing policies no longer apply to the drugs subject to the 18% pricing policy as per the Council of the Federation. Quebec did not participate in the pCPA for generic drugs at this time, but benefited from it because of the lowest price policy.
† Drugs under the 18% rule by date of implementation:
- April 1, 2013: atorvastatin, ramipril, venlafaxine, amlodipine, omeprazole and rabeprazole.
- April 1, 2014: rosuvastatin, pantoprazole, citalopram, and simvastatin.
- April 1, 2015: clopidogrel, gabapentin, metformin, and olanzapine.
‡ Changes to regulations applicable to generics listed on the Ontario Drug Benefit (ODB) Formulary on or after April 1, 2013.
Note: Generic pricing policies apply to oral solid forms; all others are 35%.
Appendix C: Markup Policies in Public Drug Plans, 2013/14
Table C1 provides a summary of markup policies in 2013/14 for the public drug plans participating in the NPDUIS initiative.
Table C1: Public drug plan markup policies, 2013/14
British Columbia
- Most drugs maximum 8%.
- High-cost* drugs maximum 5%.
- Products subject to Actual Acquisition Cost (AAC) pricing maximum 7%.
* High-cost drugs are defined as those for which the expected daily cost of the typical dose is equal to or greater than $40 ($14,600 annual cost).
Alberta
Prices listed in the Alberta Health Drug Benefit List include a wholesaler markup, but only if the drug manufacturer distributes through a wholesaler. In such cases, the drug manufacturer is asked to include a distribution allowance of up to 7.5%. This applied only to single-source products in 2013/2014.
Saskatchewan
With a few exceptions, the maximum allowable pharmacy markup calculated on the prescription drug cost was as follows:
blank
| Drug cost |
Markup |
| $0.01–$6.30 |
30% |
| $6.31–$15.80 |
15% |
| $15.81–$200 |
10% |
| >$200.01 |
$20 max |
Saskatchewan also allowed a wholesale markup on specific products: insulin: 5%; standing offer contract (SOC) products: 6%; generic drugs: 6.5%; and most other drugs: 8.5%. Wholesale markup is capped at $50 per package size and is subject to the Actual Acquisition Cost (AAC).
Manitoba
No markup policy.
Ontario
Maximum 8% where permitted.
New Brunswick
Effective June 1, 2013, a markup on interchangeable drugs was increased to up to 8%.
Nova Scotia
Manufacturer list price plus 10.5% (maximum $250) including methadone, or the maximum reimbursable price (MRP) or the Pharmacare reimbursement price (PRP) plus 6.0% (maximum $250) plus $1.05 transition fee. Exceptions included: ostomy supplies— Actual Acquisition Cost (AAC) plus 10.0% (maximum $50) plus a $1.05 transition fee; and compounded extemporaneous products (except methadone and injectables)—Actual Acquisition Cost (AAC) plus 2.0% (maximum $50) plus $1.05 transition fee.
Prince Edward Island
A maximum 6% markup was allowed for drugs on a Maximum Reimbursable Price (MRP) list; and 10% on the ingredient cost for brand-name drugs for which the prescription cost was $2,702 or less, to a maximum of $250 per prescription, and 9.25% on the ingredient cost for brand-name drugs for which the prescription cost was $2,703 or more.
Newfoundland and Labrador
A markup of 8.5%, which was included in the list price on the benefit list.
NIHB
Pharmacy reimbursement, which may or may not include markup, was determined by the NIHB or negotiated between the NIHB and pharmacists’ associations, and differed by province.
Appendix D: Dispensing Fee Policies in Public Drug Plans, 2013/14
Table D1 provides a summary of dispensing fee reimbursement in 2013/14 for the public drug plans participating in the NPDUIS initiative, as detailed in a Plan Information Document published by the Canadian Institute for Health Information. Footnote 2
Table D1: Public drug plan dispensing fee reimbursement, 2013/14
British Columbia
In 2013/14, the maximum allowable dispensing fee was $10.00. No dispensing fee was reimbursed for insulins or needles and syringes for insulin therapy. Other reimbursements included pharmacies providing services to long-term care facilities, which received $43.75 per bed serviced. A rural incentive program provided a per claim subsidy ($3.00 to $10.50) to rural pharmacies with monthly claims volumes of less than 1,700. A vaccination administration program reimbursed pharmacies $10 for each publicly funded vaccination administered by an authorized pharmacist.
Alberta
Alberta reimbursed a dispensing fee to pharmacies and an additional inventory allowance. Fees charged varied based on the acquisition cost of the drug. From April 15, 2013, to March 31, 2014, the fees were as follows:
blank
| Actual Acquisition Cost (AAC) |
Additional inventory allowance |
Dispensing fee excluding the inventory allowance |
| $0.00-$74.99 |
$1.71 |
$10.22 |
| $75.00–$149.99 |
$2.00 |
$15.53 |
| $150.00 or greater |
$5.03 |
$20.94 |
Alberta also reimbursed an additional charge of up to 75 cents per minute in excess of seven minutes for compounded prescriptions. For some categories of drugs, such as insulin and oral contraceptives, the pharmacy reimbursement could not exceed the acquisition cost of the drug product multiplied by 5/3.
Saskatchewan
The maximum dispensing fee was set at $10.75 for 2013/14. Saskatchewan provided an additional reimbursement for trial prescriptions, methadone, compliance packaging and compounding drugs.
Manitoba
In Manitoba, pharmacy service providers were compensated by a market-based professional fee. The dispensing fee or professional fee is an all-inclusive fee that reimburses for the direct and indirect costs associated with dispensing, distribution, and cognitive service functions including patient counseling and profit. Dispensing fees are regulated under the Prescription Drugs Payment of Benefits Regulation, which defines the professional fee as “the amount regularly charged by a pharmacist to persons who are responsible for paying the fee without reimbursement”. The regulation ensures that pharmacy service providers establish a consistent market-based fee for which cash paying customers are provided equivalent services to that of Pharmacare beneficiaries. Other reimbursements included a maximum dispensing fee of $6.95 for the Employment and Income Assistance Program. For personal care homes, pharmacists were reimbursed $37.50 per bed per month in Winnipeg and $38.20 per bed per month for rural areas.
Ontario
Dispensing fees for non-rural pharmacies were $8.62, while the fees for rural pharmacies ranged from $9.69 to $12.92 for 2013/2014. Dispensing fees were set at a maximum of two fees per medication per patient per month; exceptions included patients in long-term care homes, homes for special care and/or drugs on the exemption medication list.
New Brunswick
The amounts paid for dispensing fees changed on June 1, 2013, as follows: $10.50 for each prescription for both interchangeable and non-interchangeable products: $9.50 for prescriptions for Methadone for Opioid Dependence and $15.75 for extemporaneous preparations (compounds).
A rural pharmacy incentive paid an additional $2 for the first 10,000 prescriptions filled in a fiscal year. This incentive applied to pharmacies that were 25 km or more apart.
Nova Scotia
Dispensing fees for drugs or supplies including methadone were reimbursed at $11.05. The exception was compounded extemporaneous products (except methadone and injectables), which were reimbursed at $16.58.
Prince Edward Island
Effective April 1, 2013, the maximum allowable dispensing fee increased from $11.65 to $12.00. The maximum allowable extemporaneous (compounding) fee was 1.5 times the maximum allowable dispensing fee to a maximum of $18. The private nursing home capitation fee was $73.55.
Newfoundland and Labrador
The dispensing fee schedule for the Foundation Plan, Access Plan and Assurance Plan from April 1, 2013, to March 31, 2014, was:
blank
| Drug cost |
Dispensing fee |
| $0.00–$49.99 |
$11.05 |
| $50.00–$249.99 |
$22.55 |
| $250.00 + |
$49.55 |
An extemporaneous preparations fee 1.5 times the dispensing fee was reimbursed for compound products. This applied to compounds that contain three or more ingredients.
The dispensing fee schedule for the 65Plus Plan from April 1, 2013, to March 31, 2014, was:
blank
| Drug cost |
Dispensing fee |
| $0.00–$249.99 |
$11.05 |
| $250.00 + |
$39.53 |
NIHB
Pharmacy reimbursement, which included dispensing fees, was determined by the NIHB or negotiated between the NIHB and pharmacists’ associations, and differed by province.
Appendix E: Common Drug Review Listing Recommendations by Drug and Indication, 2013/14
blank
| |
Trade name (ingredient) |
Indication |
Date recommendation issued |
| List with criteria/condition |
Actemra (tocilizumab) |
Polyarticular juvenile idiopathic arthritis |
19-Mar-14 |
| Fibristal (ulipristal acetate) |
Uterine fibroids (signs and symptoms) |
15-Nov-13 |
| Fycompa (perampanel) |
Epilepsy, partial onset seizures |
17-Oct-13 |
| Genotropin (somatropin) |
Growth hormone deficiency, adult |
20-Dec-13 |
| Genotropin (somatropin) |
Growth hormone deficiency, pediatric |
20-Dec-13 |
| Genotropin (somatropin) |
Turner syndrome |
20-Dec-13 |
| Incivek (telaprevir) |
Hepatitis C infection, chronic |
13-Jun-13 |
| Incivek (telaprevir) |
Hepatitis C, chronic |
13-Jun-13 |
| Jentadueto (linagliptin-metformin) |
Diabetes mellitus (Type 2) |
17-Oct-13 |
| Jetrea (ocriplasmin) |
Vitreomacular adhesion |
20-Dec-13 |
| Latuda (lurasidone) |
Schizophrenia |
20-Dec-13 |
| Onglyza (saxagliptin) |
Diabetes mellitus (Type 2) |
15-Nov-13 |
| Seebri (glycopyrronium bromide) |
Chronic obstructive pulmonary disease (COPD), maintenance bronchodilator treatment |
15-May-13 |
| Stribild (elvitegravir, cobicistat, emtricitabine, tenofovir disoproxil fumarate) |
HIV-1 infection |
15-May-13 |
| Tecfidera (dimethyl fumarate) |
Multiple sclerosis, relapsing |
25-Sep-13 |
| Victrelis (boceprevir) |
Hepatitis C infection, chronic |
13-Jun-13 |
| Victrelis (boceprevir) |
Hepatitis C, chronic |
13-Jun-13 |
| Xarelto (rivaroxaban) |
Thromboembolic events (venous), pulmonary embolism |
26-Mar-14 |
| List with clinical criteria and/or conditions |
Humira (adalimumab) |
Arthritis, juvenile idiopathic |
18-Jul-13 |
| Orencia (abatacept) |
Arthritis, rheumatoid |
18-Jul-13 |
| Pradaxa (dabigatran etexilate) |
Atrial fibrillation prevention of stroke and systemic embolism |
18-Jul-13 |
| Xarelto (rivaroxaban) |
Stroke prevention in patients with atrial fibrillation |
18-Jul-13 |
| Do not list at the submitted price |
Aloxi – injection (palonosetron hydrochloride) |
Nausea and vomiting (chemotherapy induced) prevention |
15-May-13 |
| Bystolic (nebivolol) |
Hypertension, essential |
18-Jul-13 |
| Edarbi (azilsartan medoxomil) |
Hypertension, essential |
17-Oct-13 |
| Simponi (golimumab) |
Ulcerative colitis |
19-Mar-14 |
| Do not list |
Afinitor (everolimus) |
Renal angiomyolipoma associated with tuberous sclerosis complex (TSC) |
25-Sep-13 |
| Aloxi – capsule (palonosetron hydrochloride) |
Nausea and vomiting (chemotherapy induced) prevention |
24-Apr-13 |
| Edarbyclor (azilsartan medoxomil, chlorthalidone) |
Hypertension, essential |
17-Oct-13 |
| Esbriet (pirfenidone) |
Pulmonary fibrosis (idiopathic, mild to moderate) |
18-Apr-13 |
| Picato (ingenol mebutate) |
Keratosis, actinic |
22-Jan-14 |
| Rebif (interferon beta-1a) |
Clinically isolated syndrome |
19-Aug-13 |
| Soliris (eculizumab) |
Hemolytic uremic syndrome, atypical |
18-Jul-13 |
| Sublinox (zolpidem tartrate) |
Insomnia, short-term treatment |
25-Sep-13 |
Appendix F: Top 100 Patented Drugs by Drug Cost, NPDUIS Public Drug Plans, 2013/14 ($million)
Table: Appendix F
| Rank |
Trade name (ingredient) |
Manufacturer |
Total* |
BC |
AB |
SK |
MB |
ON |
NB |
NS |
PE |
NL |
NIHB |
| 1 |
Lucentis (ranibizumab) |
Novartis Pharmaceuticals Canada Inc |
$337.42 |
— |
$56.23 |
$4.15 |
— |
$270.12 |
$3.66 |
— |
$0.22 |
$2.00 |
$1.04 |
| 2 |
Remicade (infliximab) |
Janssen Inc. |
$280.87 |
$67.22 |
$49.20 |
$25.08 |
$24.96 |
$87.18 |
$6.36 |
$10.07 |
$2.21 |
$3.87 |
$4.70 |
| 3 |
Advair (salmeterol , fluticasone propionate) |
GlaxoSmithKline Inc. |
$162.37 |
$20.84 |
$20.56 |
$5.27 |
$11.41 |
$90.52 |
$4.40 |
$3.25 |
$0.27 |
$1.50 |
$4.35 |
| 4 |
Humira (adalimumab) |
AbbVie Corporation |
$139.79 |
$49.44 |
$26.69 |
$12.43 |
$16.23 |
$17.27 |
$2.57 |
$5.89 |
$1.65 |
$3.04 |
$4.58 |
| 5 |
Enbrel (etanercept) |
Immunex Corporation |
$112.04 |
$27.08 |
$18.50 |
$8.12 |
$14.14 |
$28.82 |
$2.80 |
$3.70 |
$0.85 |
$1.32 |
$6.72 |
| 6 |
Spiriva (tiotropium) |
Boehringer Ingelheim |
$102.44 |
$7.51 |
$14.36 |
$3.45 |
$1.83 |
$68.99 |
$2.18 |
$2.23 |
$0.22 |
$0.60 |
$1.08 |
| 7 |
Cipralex (escitalopram) |
Lundbeck Canada Inc. |
$100.42 |
$39.62 |
$5.63 |
— |
$0.01 |
$52.65 |
— |
$0.02 |
$0.00 |
— |
$2.48 |
| 8 |
Aricept (donepezil hydrochloride) |
Pfizer Canada Inc. |
$95.76 |
$9.96 |
$7.71 |
$1.31 |
$2.60 |
$69.08 |
$1.64 |
$1.87 |
$0.39 |
$0.98 |
$0.23 |
| 9 |
Lantus (insulin glargine) |
Sanofi-Aventis Canada Inc. |
$90.76 |
$15.46 |
$7.88 |
$5.93 |
$2.26 |
$50.83 |
$0.74 |
$0.79 |
$0.00 |
$0.00 |
$6.86 |
| 10 |
Coversyl (perindopril erbumine) |
Servier Canada Inc. |
$87.28 |
$13.05 |
$8.06 |
$5.16 |
$4.20 |
$48.50 |
$2.52 |
$2.11 |
$0.32 |
$1.20 |
$2.18 |
| 11 |
Ezetrol (ezetimibe) |
Merck Canada Inc. |
$84.58 |
$0.03 |
$7.07 |
$6.53 |
$1.39 |
$63.12 |
$1.97 |
$1.96 |
$0.16 |
$1.01 |
$1.35 |
| 12 |
Januvia (sitagliptin) |
Merck Canada Inc. |
$75.83 |
$4.10 |
$2.02 |
$1.42 |
$1.00 |
$63.01 |
$0.56 |
$0.62 |
— |
$0.09 |
$3.02 |
| 13 |
Cymbalta (duloxetine) |
Eli Lilly Canada Inc. |
$71.28 |
$0.55 |
$6.62 |
$2.35 |
$2.78 |
$57.67 |
$0.13 |
$0.21 |
$0.00 |
$0.02 |
$0.94 |
| 14 |
Revlimid (lenalidomide) |
Celgene Inc. |
$63.49 |
— |
— |
— |
$6.42 |
$49.65 |
$2.30 |
$2.66 |
— |
$2.18 |
$0.27 |
| 15 |
Symbicort (budesonide, formoterol fumarate dihydrate) |
AstraZeneca Canada Inc. |
$61.87 |
$8.04 |
$10.31 |
$2.67 |
$4.34 |
$32.80 |
$0.85 |
$1.11 |
$0.11 |
$0.52 |
$1.13 |
| 16 |
Tecta (pantoprazole) |
Takeda Canada Inc. |
$61.57 |
$8.20 |
$4.16 |
$1.65 |
— |
$34.82 |
$5.99 |
$2.64 |
$0.73 |
$1.92 |
$1.44 |
| 17 |
Flovent HFA (fluticasone propionate) |
GlaxoSmithKline Inc. |
$53.24 |
$10.84 |
$1.46 |
$3.29 |
$2.92 |
$22.76 |
$2.15 |
$1.53 |
$0.31 |
$2.01 |
$5.96 |
| 18 |
Pradaxa (dabigatran etexilate) |
Boehringer Ingelheim |
$46.77 |
$2.68 |
$5.65 |
$1.31 |
$1.32 |
$34.26 |
$0.71 |
$0.61 |
$0.04 |
$0.03 |
$0.16 |
| 19 |
Celebrex (celecoxib) |
Pfizer Canada Inc. |
$46.43 |
$1.10 |
$4.59 |
$3.31 |
$2.73 |
$30.65 |
$1.74 |
$0.37 |
$0.00 |
$0.61 |
$1.34 |
| 20 |
Copaxone (glatiramer acetate) |
Teva Pharmaceutical Industries Ltd. |
$41.63 |
$7.06 |
$13.03 |
$4.85 |
$4.14 |
$9.35 |
$1.34 |
— |
$0.52 |
$0.67 |
$0.68 |
| 21 |
Janumet (sitagliptin , metformin hydrochloride) |
Merck Canada Inc. |
$38.42 |
$2.39 |
$1.23 |
$0.47 |
— |
$33.04 |
$0.22 |
$0.12 |
— |
$0.00 |
$0.94 |
| 22 |
Abilify (aripiprazole) |
Bristol-Myers Squibb Canada |
$34.00 |
$6.46 |
$0.62 |
$0.46 |
$2.13 |
$23.10 |
$0.36 |
$0.14 |
$0.00 |
$0.04 |
$0.68 |
| 23 |
Invega Sustenna (paliperidone) |
Janssen Inc. |
$33.81 |
$10.94 |
$0.48 |
$1.00 |
$0.05 |
$19.49 |
$0.36 |
$0.02 |
— |
$0.00 |
$1.47 |
| 24 |
Oxyneo (oxycodone hydrochloride) |
Purdue Pharma |
$33.05 |
$1.60 |
$7.42 |
$1.48 |
$3.07 |
$16.86 |
$0.79 |
$0.25 |
$0.04 |
$0.26 |
$1.27 |
| 25 |
Xarelto (rivaroxaban) |
Bayer Inc. |
$32.82 |
$3.54 |
$3.94 |
$1.26 |
$0.92 |
$22.00 |
$0.46 |
$0.38 |
$0.03 |
$0.05 |
$0.25 |
| 26 |
Risperdal Consta (risperidone) |
Janssen Inc. |
$32.77 |
$5.83 |
$0.44 |
$1.45 |
$1.76 |
$17.97 |
$1.91 |
$0.22 |
$0.28 |
$0.38 |
$2.54 |
| 27 |
Neupogen (filgrastim) |
Amgen Canada Inc. |
$31.80 |
$7.54 |
$1.57 |
$0.16 |
$0.02 |
$20.02 |
$0.61 |
— |
$0.24 |
$1.00 |
$0.65 |
| 28 |
Truvada (tenofovir disoproxil fumarate, emtricitabine) |
Gilead Sciences Canada Inc. |
$30.93 |
— |
— |
$1.24 |
$2.42 |
$22.68 |
$0.96 |
— |
— |
$0.24 |
$3.40 |
| 29 |
Atripla (emtricitabine, tenofovir disoproxil fumarate, efavirenz) |
Bristol-Myers Squibb and Gilead Sciences LLC |
$30.77 |
— |
— |
$1.77 |
$1.16 |
$24.63 |
$0.94 |
— |
— |
$0.26 |
$2.01 |
| 30 |
Novorapid (insulin aspart) |
Novo Nordisk Canada Inc. |
$26.78 |
$3.01 |
$2.22 |
$0.83 |
$1.97 |
$15.56 |
$0.39 |
$0.69 |
$0.38 |
$0.10 |
$1.62 |
| 31 |
Levemir Penfill (insulin detemir) |
Novo Nordisk Canada Inc. |
$25.81 |
$2.09 |
$3.43 |
$1.19 |
$0.00 |
$17.89 |
$0.13 |
$0.42 |
— |
— |
$0.67 |
| 32 |
Viread (tenofovir disoproxil fumarate) |
Gilead Sciences Canada Inc. |
$25.72 |
$5.01 |
$2.29 |
$0.26 |
$1.01 |
$16.87 |
$0.08 |
$0.08 |
— |
$0.02 |
$0.12 |
| 33 |
Tiazac XC (diltiazem hydrochloride) |
Valeant Canada LP Valeant Canada S.E.C. |
$25.71 |
$2.89 |
$3.56 |
$0.66 |
$1.45 |
$15.05 |
$0.66 |
$0.57 |
$0.11 |
$0.48 |
$0.29 |
| 34 |
Eprex (epoetin alfa) |
Janssen Inc. |
$24.97 |
— |
$1.92 |
$2.47 |
$0.58 |
$16.44 |
$0.84 |
$0.13 |
— |
$1.17 |
$1.42 |
| 35 |
Simponi (golimumab) |
Janssen Inc. |
$24.85 |
$5.05 |
$4.14 |
$1.51 |
$0.31 |
$10.02 |
$0.51 |
$1.04 |
$0.11 |
$0.60 |
$1.56 |
| 36 |
Botox (onabotulinumtoxina) |
Allergan Inc. |
$24.11 |
$5.14 |
$2.41 |
$0.78 |
$1.29 |
$12.81 |
$0.47 |
$0.54 |
— |
— |
$0.67 |
| 37 |
Avonex (interferon beta-1a) |
Biogen Canada Inc. |
$23.31 |
$4.01 |
$3.12 |
$1.39 |
$3.36 |
$7.57 |
$2.32 |
— |
$0.39 |
$0.73 |
$0.41 |
| 38 |
Stelara (ustekinumab) |
Janssen Inc. |
$23.18 |
$4.30 |
$4.21 |
$0.93 |
$1.12 |
$9.37 |
$0.38 |
$1.22 |
— |
$1.19 |
$0.46 |
| 39 |
Detrol LA (tolterodine tartrate) |
Pfizer Canada Inc. |
$21.34 |
$0.07 |
$2.22 |
$1.04 |
$0.76 |
$16.03 |
$0.40 |
$0.33 |
$0.02 |
$0.18 |
$0.31 |
| 40 |
Kivexa (abacavir , lamivudine) |
ViiV Healthcare ULC |
$20.97 |
— |
— |
$0.61 |
$2.31 |
$15.69 |
$0.36 |
— |
— |
$0.21 |
$1.78 |
| 41 |
Actonel DR (risedronate sodium) |
Warner Chilcott Canada Co. |
$20.52 |
— |
— |
$0.09 |
— |
$20.42 |
— |
$0.00 |
— |
— |
— |
| 42 |
Lupron Depot (leuprolide acetate) |
AbbVie Corporation |
$19.75 |
$0.82 |
$0.07 |
$0.25 |
$0.27 |
$15.89 |
$0.54 |
$0.69 |
$0.09 |
$0.69 |
$0.44 |
| 43 |
Prolia (denosumab) |
Amgen Canada Inc. |
$18.48 |
$0.28 |
$0.12 |
$0.05 |
$0.24 |
$17.61 |
$0.05 |
$0.10 |
— |
$0.00 |
$0.02 |
| 44 |
Concerta (methylphenidate hydrochloride) |
Janssen Inc. |
$17.90 |
$4.36 |
— |
$4.35 |
$2.94 |
$3.83 |
$0.08 |
$0.03 |
$0.01 |
— |
$2.30 |
| 45 |
Prezista (darunavir) |
Janssen Inc. |
$17.89 |
— |
— |
$0.72 |
$1.83 |
$12.53 |
$0.55 |
— |
— |
$0.21 |
$2.04 |
| 46 |
Orencia (abatacept) |
Bristol-Myers Squibb Canada |
$17.35 |
$4.28 |
$2.72 |
$1.06 |
$0.84 |
$6.33 |
$0.14 |
$0.64 |
$0.12 |
$0.23 |
$1.00 |
| 47 |
Wellbutrin XL (bupropion hydrochloride) |
Valeant Canada LP Valeant Canada S.E.C. |
$17.14 |
$5.15 |
$1.51 |
$1.57 |
$1.65 |
$6.03 |
$0.23 |
$0.20 |
$0.00 |
$0.15 |
$0.65 |
| 48 |
Crestor (rosuvastatin) |
AstraZeneca Canada Inc. |
$17.10 |
$3.44 |
$0.74 |
$0.53 |
$0.11 |
$11.54 |
$0.05 |
$0.37 |
$0.03 |
$0.09 |
$0.20 |
| 49 |
Rituxan (rituximab) |
Hoffmann-La Roche Limited |
$17.02 |
$5.08 |
$1.97 |
$0.85 |
$1.31 |
$5.43 |
$0.14 |
$0.82 |
$0.04 |
$0.07 |
$1.31 |
| 50 |
Seroquel XR (quetiapine) |
AstraZeneca Canada Inc. |
$17.01 |
$7.67 |
$0.45 |
$2.12 |
$0.41 |
$6.04 |
$0.26 |
$0.00 |
— |
— |
$0.05 |
| 51 |
Victrelis Triple (peginterferon alfa-2b, boceprevir, ribavirin) |
Merck Canada Inc. |
$15.89 |
$6.69 |
$1.23 |
$0.65 |
$1.33 |
$5.05 |
$0.11 |
$0.35 |
— |
— |
$0.49 |
| 52 |
Pegasys RBV (ribavirin, peginterferon alfa-2a) |
Hoffmann-La Roche Limited |
$15.85 |
$5.16 |
$2.07 |
$0.94 |
$0.83 |
$5.56 |
$0.22 |
$0.15 |
— |
— |
$0.92 |
| 53 |
Soliris (eculizumab) |
Alexion Pharma International Sarl |
$15.65 |
— |
$2.51 |
— |
$0.55 |
$12.59 |
— |
— |
— |
— |
— |
| 54 |
Sutent (sunitinib) |
Pfizer Canada Inc. |
$15.06 |
— |
— |
— |
$1.64 |
$10.93 |
$0.73 |
$0.70 |
$0.17 |
$0.39 |
$0.50 |
| 55 |
Gleevec (imatinib) |
Novartis Pharmaceuticals Canada Inc. |
$14.88 |
— |
— |
— |
$3.06 |
$9.18 |
$0.59 |
$0.83 |
$0.13 |
$0.67 |
$0.41 |
| 56 |
Tracleer (bosentan) |
Actelion Pharmaceuticals Ltd. |
$14.69 |
$4.02 |
— |
$0.02 |
$0.76 |
$9.68 |
— |
$0.06 |
— |
$0.09 |
$0.07 |
| 57 |
Reyataz (atazanavir) |
Bristol-Myers Squibb Canada |
$14.66 |
— |
— |
$0.58 |
$1.31 |
$10.32 |
$0.42 |
— |
— |
$0.16 |
$1.87 |
| 58 |
Mavik (trandolapril) |
BGP Pharma ULC |
$14.37 |
$3.79 |
$1.29 |
$0.58 |
$0.71 |
$6.88 |
$0.08 |
$0.41 |
$0.03 |
$0.29 |
$0.31 |
| 59 |
Victrelis (boceprevir) |
Merck Canada Inc. |
$14.16 |
$2.32 |
$0.40 |
$0.39 |
$0.66 |
$10.02 |
$0.04 |
$0.02 |
— |
— |
$0.31 |
| 60 |
Isentress (raltegravir) |
Merck Canada Inc. |
$14.04 |
— |
— |
$0.36 |
$0.67 |
$12.08 |
$0.40 |
— |
— |
$0.14 |
$0.39 |
| 61 |
Onglyza (saxagliptin) |
AstraZeneca Canada Inc. |
$13.90 |
— |
$1.67 |
$0.49 |
$0.65 |
$10.75 |
— |
— |
— |
— |
$0.34 |
| 62 |
Incivek (telaprevir) |
Vertex Pharmaceuticals |
$13.52 |
$6.55 |
$2.68 |
$0.90 |
$1.00 |
$0.79 |
$0.30 |
$0.27 |
— |
$0.01 |
$1.02 |
| 63 |
Accupril (quinapril) |
Pfizer Canada Inc. |
$13.38 |
$5.43 |
$1.19 |
$0.70 |
$0.51 |
$4.84 |
$0.04 |
$0.14 |
$0.03 |
$0.25 |
$0.26 |
| 64 |
Humalog (insulin lispro) |
Eli Lilly Canada Inc. |
$13.35 |
$3.76 |
$2.06 |
$1.43 |
$1.87 |
$0.95 |
$0.54 |
$0.37 |
$0.50 |
$0.15 |
$1.71 |
| 65 |
Coversyl Plus HD (perindopril erbumine, indapamide) |
Servier Canada Inc. |
$13.30 |
— |
$1.70 |
$1.55 |
$1.07 |
$7.17 |
$0.66 |
$0.41 |
$0.04 |
$0.25 |
$0.44 |
| 66 |
Nexium (esomeprazole) |
AstraZeneca Canada Inc. |
$13.20 |
$9.58 |
— |
$3.06 |
$0.55 |
— |
— |
$0.00 |
— |
— |
$0.00 |
| 67 |
Diamicron MR (gliclazide) |
Servier Canada Inc. |
$13.09 |
$0.86 |
$0.47 |
$0.62 |
$1.10 |
$8.56 |
$0.13 |
$0.46 |
$0.11 |
$0.06 |
$0.73 |
| 68 |
Coversyl Plus (perindopril erbumine, indapamide) |
Servier Canada Inc. |
$13.05 |
— |
$1.66 |
$1.56 |
$1.22 |
$6.91 |
$0.55 |
$0.43 |
$0.05 |
$0.23 |
$0.43 |
| 69 |
Lumigan RC (bimatoprost) |
Allergan Inc. |
$12.84 |
$4.15 |
$2.00 |
$0.67 |
$1.48 |
$3.20 |
$0.27 |
$0.49 |
$0.11 |
$0.20 |
$0.26 |
| 70 |
Mirena (levonorgestrel) |
Bayer Inc. |
$12.76 |
$5.35 |
$0.12 |
$1.27 |
$1.16 |
$1.94 |
$0.15 |
$0.03 |
$0.01 |
$0.13 |
$2.60 |
| 71 |
Betaseron (interferon beta-1b) |
Bayer Inc. |
$12.75 |
$2.75 |
$1.57 |
$1.65 |
$1.63 |
$4.21 |
$0.52 |
— |
$0.06 |
$0.30 |
$0.06 |
| 72 |
Actemra (tocilizumab) |
Hoffmann-La Roche Limited |
$12.24 |
$2.22 |
$1.40 |
$1.09 |
$0.92 |
$5.34 |
$0.15 |
$0.30 |
$0.00 |
$0.26 |
$0.55 |
| 73 |
Exjade (deferasirox) |
Novartis Pharmaceuticals Canada Inc. |
$12.17 |
$2.89 |
$1.57 |
$0.33 |
$0.17 |
$6.58 |
$0.35 |
$0.16 |
— |
$0.07 |
$0.05 |
| 74 |
Myfortic (mycophenolic acid) |
Novartis Pharmaceuticals Canada Inc. |
$11.68 |
$0.07 |
— |
$0.52 |
$0.12 |
$10.26 |
$0.38 |
$0.01 |
— |
— |
$0.32 |
| 75 |
Trajenta (linagliptin) |
Boehringer Ingelheim |
$11.67 |
$0.78 |
$0.61 |
$0.33 |
$0.23 |
$9.45 |
$0.12 |
$0.01 |
— |
$0.01 |
$0.12 |
| 76 |
Novomix (insulin aspart protamine, insulin aspart) |
Novo Nordisk Canada Inc. |
$11.61 |
$0.82 |
— |
— |
$0.00 |
$10.78 |
— |
$0.00 |
— |
— |
$0.00 |
| 77 |
Enbrel (etanercept, water) |
Immunex Corporation |
$11.60 |
$2.99 |
$2.08 |
$0.43 |
$0.78 |
$3.38 |
$0.13 |
$0.90 |
$0.10 |
$0.04 |
$0.78 |
| 78 |
Atrovent HFA (ipratropium bromide) |
Boehringer Ingelheim |
$11.40 |
$3.44 |
$0.54 |
$0.46 |
$1.20 |
$2.38 |
$0.90 |
$1.02 |
$0.19 |
$0.65 |
$0.62 |
| 79 |
Vesicare (solifenacin succinate) |
Astellas Pharma Canada Inc. |
$11.35 |
$0.06 |
$1.18 |
$0.25 |
$0.49 |
$8.56 |
$0.34 |
$0.27 |
$0.03 |
$0.03 |
$0.16 |
| 80 |
Advagraf (tacrolimus) |
Astellas Pharma Canada Inc. |
$11.24 |
— |
— |
$0.16 |
$0.25 |
$9.80 |
$0.62 |
$0.00 |
— |
— |
$0.40 |
| 81 |
Prograf (tacrolimus) |
Astellas Pharma Canada Inc. |
$11.20 |
$0.11 |
— |
$1.15 |
$2.01 |
$6.43 |
$0.63 |
$0.00 |
— |
— |
$0.86 |
| 82 |
Aranesp HSA free (darbepoetin alfa) |
Amgen Canada Inc. |
$10.68 |
— |
$7.26 |
$0.17 |
$0.01 |
$0.29 |
$1.16 |
$0.01 |
— |
$0.53 |
$1.25 |
| 83 |
Fosavance (alendronic acid , vitamin d3 (cholecalciferol)) |
Merck Canada Inc. |
$10.55 |
$0.31 |
$0.50 |
$0.32 |
— |
$9.27 |
$0.03 |
$0.06 |
$0.00 |
$0.01 |
$0.05 |
| 84 |
Champix (varenicline) |
Pfizer Canada Inc. |
$10.52 |
$3.17 |
$0.44 |
$1.04 |
$1.29 |
$3.75 |
— |
— |
— |
— |
$0.83 |
| 85 |
Novolin ge NPH Penfill (insulin isophane human biosynthetic) |
Novo Nordisk Canada Inc. |
$10.40 |
$3.39 |
$1.32 |
$0.63 |
$1.44 |
— |
$0.49 |
$1.41 |
$0.21 |
$0.59 |
$0.92 |
| 86 |
Avelox (moxifloxacin) |
Bayer Inc. |
$9.61 |
$3.30 |
$0.64 |
$0.18 |
$0.37 |
$4.67 |
$0.20 |
$0.13 |
$0.01 |
$0.02 |
$0.09 |
| 87 |
RAN-pantoprazole (pantoprazole) |
Ranbaxy Pharmaceuticals Canada Inc. |
$9.56 |
$0.37 |
$2.34 |
$0.38 |
$0.18 |
$6.07 |
$0.05 |
$0.01 |
— |
$0.00 |
$0.16 |
| 88 |
Asacol (mesalazine) |
Warner Chilcott Canada Co. |
$9.43 |
$2.75 |
$1.44 |
$0.98 |
$0.81 |
$2.44 |
$0.26 |
$0.18 |
$0.03 |
$0.26 |
$0.28 |
| 89 |
Biaxin XL (clarithromycin) |
BGP Pharma ULC |
$9.40 |
$3.81 |
$0.63 |
$0.43 |
$0.46 |
$3.23 |
$0.09 |
$0.13 |
$0.00 |
$0.12 |
$0.50 |
| 90 |
Travatan Z (travoprost) |
Alcon Canada Inc. |
$9.27 |
$2.29 |
$1.15 |
$0.60 |
$0.76 |
$3.40 |
$0.29 |
$0.37 |
$0.08 |
$0.15 |
$0.16 |
| 91 |
Omnaris (ciclesonide) |
Takeda Canada Inc. |
$9.11 |
— |
— |
$0.28 |
— |
$8.83 |
— |
— |
— |
— |
— |
| 92 |
Zytiga (abiraterone acetate) |
Janssen Inc. |
$8.82 |
— |
— |
— |
$0.65 |
$6.49 |
$0.73 |
$0.34 |
— |
$0.58 |
$0.03 |
| 93 |
Sprycel (dasatinib) |
Bristol-Myers Squibb Canada |
$8.63 |
— |
— |
— |
$0.92 |
$6.05 |
$0.38 |
$0.69 |
$0.05 |
$0.41 |
$0.13 |
| 94 |
Alvesco (ciclesonide) |
Takeda Canada Inc. |
$8.59 |
$2.14 |
$0.51 |
$0.46 |
$0.47 |
$3.99 |
$0.26 |
$0.22 |
$0.03 |
$0.15 |
$0.38 |
| 95 |
Tysabri (natalizumab) |
Biogen Canada Inc. |
$8.32 |
$3.15 |
$0.80 |
$0.61 |
$0.42 |
$2.06 |
$1.13 |
— |
— |
— |
$0.15 |
| 96 |
Lipitor (atorvastatin) |
Pfizer Canada Inc. |
$7.66 |
$1.36 |
$0.37 |
$0.13 |
$0.04 |
$5.64 |
$0.01 |
$0.03 |
$0.01 |
$0.02 |
$0.06 |
| 97 |
Novolin ge (insulin injection human biosynthetic, insulin isophane human biosynthetic) |
Novo Nordisk Canada Inc. |
$7.64 |
$2.41 |
$0.64 |
$0.57 |
$0.82 |
$0.57 |
$0.45 |
$0.60 |
$0.03 |
$0.67 |
$0.88 |
| 98 |
Myozyme (alglucosidase alfa) |
Genzyme Canada a Division of Sanofi-Aventis Canada Inc. |
$7.58 |
— |
— |
$0.54 |
$0.88 |
$6.17 |
— |
— |
— |
— |
— |
| 99 |
Humalog Mix (insulin lispro, insulin lispro protamine suspension) |
Eli Lilly Canada Inc. |
$7.49 |
$0.61 |
$0.89 |
— |
$0.59 |
$4.77 |
$0.25 |
$0.00 |
$0.10 |
$0.00 |
$0.28 |
| 100 |
Cimzia (certolizumab pegol) |
UCB Canada Inc. |
$7.43 |
$1.55 |
— |
$0.00 |
$0.01 |
$5.59 |
— |
— |
$0.02 |
— |
$0.25 |
| |
|
Total |
$3,442.37 |
$498.16 |
$371.45 |
$160.30 |
$180.04 |
$1,938.62 |
$74.94 |
$62.64 |
$12.01 |
$39.80 |
$104.41 |
| |
|
Share of all patented drugs |
87% |
85% |
92% |
84% |
87% |
87% |
88% |
87% |
89% |
87% |
84% |
Note: Drug costs of less than $5,000 appear as $0.00 million due to rounding.
*Total results for the public drug plans reported in this figure.
Data source: National Prescription Drug Utilization Information System Database, Canadian Institute for Health Information.
Appendix G: Top 100 Non-Patented Single Source Drugs by Drug Cost, NPDUIS Public Drug Plans, 2013/14 ($thousand)
Table: Appendix G
| Rank |
Trade name (ingredient) |
Manufacturer |
Total* |
BC |
AB |
SK |
MB |
ON |
NB |
NS |
PE |
NL |
NIHB |
| 1 |
Avodart (dutasteride) |
GlaxoSmithKline Inc. |
$37,036 |
$1,940 |
$5,109 |
$1,672 |
$1,513 |
$25,962 |
$426 |
$109 |
$16 |
$39 |
$250 |
| 2 |
Fragmin anti-Xa (dalteparin sodium) |
Pfizer Canada Inc. |
$33,390 |
$7,613 |
$3,031 |
$845 |
$2,738 |
$17,132 |
$325 |
$1,010 |
$2 |
$0 |
$694 |
| 3 |
Rebif (interferon beta-1a) |
EMD Serono a Division of EMD Inc. Canada |
$31,510 |
$7,402 |
$6,964 |
$3,472 |
$1,981 |
$8,788 |
$1,601 |
— |
$394 |
$471 |
$436 |
| 4 |
Olmetec (olmesartan medoxomil) |
Merck Canada Inc. |
$21,422 |
$1,596 |
$2,502 |
$2,071 |
$1 |
$12,824 |
$757 |
$621 |
$123 |
$434 |
$493 |
| 5 |
Zoladex LA inj depot (goserelin) |
AstraZeneca Canada Inc. |
$20,171 |
$16 |
— |
— |
$58 |
$17,889 |
$656 |
$941 |
$202 |
$249 |
$160 |
| 6 |
Humulin (insulin isophane) |
Eli Lilly Canada Inc. |
$11,695 |
$3,343 |
$840 |
$1,310 |
$891 |
$821 |
$856 |
$803 |
$104 |
$1,511 |
$1,217 |
| 7 |
Innohep multi-dose vial (tinzaparin sodium) |
Leo Pharma Inc. |
$10,565 |
$320 |
$3,848 |
$1,355 |
$11 |
$4,721 |
$64 |
$3 |
— |
$12 |
$230 |
| 8 |
Aggrenox (dipyridamole, acetylsalicylic acid) |
Boehringer Ingelheim |
$8,101 |
$41 |
$963 |
$127 |
$96 |
$6,639 |
$45 |
$66 |
— |
$41 |
$83 |
| 9 |
Suboxone (buprenorphine , naloxone (naloxone hydrochloride dihydrate)) |
RB Pharmaceuticals Limited |
$7,013 |
$1,944 |
$190 |
$11 |
$57 |
$4,640 |
$22 |
$8 |
$30 |
$0 |
$111 |
| 10 |
Olestyr (cholestyramine resin) |
Pendopharm Division of Pharmascience Inc. |
$6,926 |
$1,600 |
$673 |
$385 |
$283 |
$3,250 |
$187 |
$220 |
$47 |
$143 |
$139 |
| 11 |
Tri-Cyclen (norgestimate, norgestimate, norgestimate) |
Janssen Inc. |
$6,702 |
$3,047 |
$90 |
$1,113 |
$1,088 |
$561 |
$66 |
$37 |
$10 |
$147 |
$542 |
| 12 |
Trelstar (triptorelin) |
Actavis Specialty Pharmaceuticals Co. |
$4,960 |
— |
— |
— |
— |
$4,222 |
$96 |
$485 |
$143 |
$3 |
$11 |
| 13 |
Remodulin (treprostinil) |
United Therapeutics Corporation |
$4,617 |
$1,460 |
— |
— |
$199 |
$1,974 |
$722 |
— |
— |
— |
$262 |
| 14 |
HP-Pac (lansoprazole, amoxicillin, clarithromycin) |
Takeda Pharmaceuticals America Inc. |
$3,870 |
$1,653 |
$189 |
$98 |
$59 |
$1,105 |
$6 |
$45 |
— |
$54 |
$662 |
| 15 |
Elmiron (pentosan polysulfate sodium) |
Janssen Inc. |
$3,449 |
$1,856 |
$306 |
$61 |
$346 |
$282 |
$139 |
$247 |
$14 |
$58 |
$141 |
| 16 |
Fucidin (fusidic acid) |
Leo Pharma Inc. |
$3,338 |
$608 |
$162 |
$364 |
$139 |
$1,506 |
$113 |
$50 |
$6 |
$173 |
$218 |
| 17 |
Act Temozolomide (temozolomide) |
Actavis Pharma Company |
$2,956 |
— |
— |
— |
$798 |
$1,823 |
$57 |
$197 |
— |
— |
$81 |
| 18 |
Codeine Contin controlled release (codeine) |
Purdue Pharma |
$2,835 |
$340 |
— |
$58 |
$169 |
$1,681 |
$117 |
$76 |
$4 |
$20 |
$371 |
| 19 |
Cerezyme (imiglucerase) |
Genzyme Canada a Division of Sanofi-Aventis Canada Inc. |
$2,773 |
— |
— |
— |
$911 |
— |
— |
— |
— |
$1,193 |
$669 |
| 20 |
Humatrope (somatropin, diluent) |
Eli Lilly Canada Inc. |
$2,739 |
$555 |
$238 |
$11 |
$321 |
$1,025 |
$107 |
$24 |
— |
$324 |
$134 |
| 21 |
Bezalip SR (bezafibrate) |
Actavis Group PTC ehf |
$2,667 |
— |
$253 |
$8 |
$439 |
$1,829 |
$14 |
$35 |
— |
$16 |
$72 |
| 22 |
Serevent Diskus (salmeterol) |
GlaxoSmithKline Inc. |
$2,601 |
$433 |
$249 |
$150 |
$187 |
$1,342 |
$92 |
$27 |
$5 |
$7 |
$109 |
| 23 |
Elaprase (idursulfase) |
Shire Human Genetic Therapies Inc. |
$2,523 |
— |
— |
— |
— |
$2,523 |
— |
— |
— |
— |
— |
| 24 |
Micronor (norethindrone) |
Janssen Inc. |
$2,454 |
$1,263 |
$22 |
$323 |
$319 |
$233 |
$18 |
$17 |
$2 |
$20 |
$237 |
| 25 |
Cytomel (liothyronine) |
Pfizer Canada Inc. |
$2,038 |
$1,623 |
$188 |
— |
$150 |
$7 |
$27 |
$16 |
— |
$11 |
$16 |
| 26 |
Apo-Entecavir (entecavir) |
Apotex Inc. |
$1,921 |
$635 |
$1,025 |
— |
— |
$221 |
$15 |
$14 |
— |
$9 |
$2 |
| 27 |
One-Alpha (alfacalcidol) |
Leo Pharma Inc. |
$1,828 |
$790 |
$59 |
$51 |
$13 |
$735 |
$53 |
$30 |
$1 |
$19 |
$76 |
| 28 |
Prolopa (levodopa, benserazide) |
Hoffmann-La Roche Limited |
$1,711 |
$125 |
$123 |
$42 |
$78 |
$1,295 |
$5 |
$28 |
— |
$10 |
$5 |
| 29 |
Imipramine (imipramine hydrochloride) |
AA Pharma Inc. |
$1,679 |
$419 |
$154 |
$122 |
$133 |
$655 |
$29 |
$64 |
$15 |
$52 |
$38 |
| 30 |
Soriatane (acitretin) |
Actavis Group PTC ehf |
$1,676 |
$318 |
$98 |
$88 |
$115 |
$815 |
$36 |
$63 |
$5 |
$49 |
$89 |
| 31 |
Thyrogen (thyrotropin alfa) |
Genzyme Canada a Division of Sanofi-Aventis Canada Inc. |
$1,658 |
— |
$401 |
— |
— |
$1,131 |
$8 |
$56 |
— |
$5 |
$57 |
| 32 |
Lotriderm (clotrimazole, betamethasone) |
Merck Canada Inc. |
$1,624 |
— |
$510 |
$410 |
$39 |
— |
$131 |
$241 |
— |
$28 |
$264 |
| 33 |
Delatestryl (testosterone enanthate) |
Valeant Canada LP Valeant Canada S.E.C. |
$1,608 |
$229 |
$236 |
$138 |
$131 |
$742 |
$8 |
$34 |
$2 |
$15 |
$74 |
| 34 |
Triquilar (levonorgestrel) |
Bayer Inc. |
$1,581 |
$678 |
$24 |
$246 |
$288 |
$102 |
$15 |
$6 |
$2 |
$48 |
$172 |
| 35 |
Nplate (romiplostim) |
Amgen Canada Inc. |
$1,529 |
— |
— |
— |
— |
$1,480 |
— |
— |
— |
$49 |
— |
| 36 |
Cyclen (norgestimate, ethinyl estradiol) |
Janssen Inc. |
$1,485 |
$664 |
$31 |
$326 |
$235 |
$119 |
$0 |
$10 |
$1 |
$18 |
$80 |
| 37 |
pms-Testosterone (testosterone undecanoate) |
Pharmascience Inc. |
$1,481 |
$7 |
$4 |
$36 |
$26 |
$1,255 |
$23 |
$76 |
$1 |
$3 |
$49 |
| 38 |
Lomotil (diphenoxylate hydrochloride, atropine sulfate) |
Pfizer Canada Inc. |
$1,428 |
$140 |
$291 |
$91 |
$27 |
$753 |
$29 |
$35 |
$1 |
$55 |
$6 |
| 39 |
Mestinon USP (pyridostigmine bromide) |
Valeant Canada LP Valeant Canada S.E.C. |
$1,392 |
$243 |
$166 |
$107 |
$107 |
$633 |
$28 |
$44 |
$5 |
$15 |
$44 |
| 40 |
Ratio-IPRA SAL UDV (salbutamol, ipratropium bromide) |
Teva Canada Limited |
$1,282 |
$98 |
$271 |
$392 |
$99 |
$223 |
$1 |
$79 |
$7 |
$62 |
$50 |
| 41 |
Locacorten Vioform Eardrops (flumethasone pivalate, clioquinol) |
Paladin Labs Inc. |
$1,258 |
$310 |
$43 |
$76 |
$73 |
$612 |
$23 |
$22 |
$5 |
$28 |
$66 |
| 42 |
Teva-Alendronate/Cholecalciferol (alendronic acid , vitamin D3 (cholecalciferol)) |
Teva Canada Limited |
$1,194 |
— |
$14 |
— |
— |
$1,169 |
$2 |
$8 |
$0 |
— |
$2 |
| 43 |
Clopixol Depot (zuclopenthixol decanoate) |
Lundbeck Canada Inc. |
$1,168 |
$375 |
$32 |
$106 |
$25 |
$412 |
$50 |
$6 |
— |
$52 |
$111 |
| 44 |
Apo-Valganciclovir (valganciclovir) |
Apotex Inc. |
$1,105 |
$44 |
$205 |
— |
$79 |
$604 |
$30 |
$22 |
— |
$2 |
$118 |
| 45 |
Efudex (fluorouracil) |
Valeant Canada LP Valeant Canada S.E.C. |
$1,072 |
$288 |
$105 |
$61 |
$75 |
$489 |
$25 |
$17 |
$7 |
$3 |
$3 |
| 46 |
Trizivir (abacavir , lamivudine, zidovudine) |
ViiV Healthcare ULC |
$1,019 |
— |
— |
$7 |
$10 |
$927 |
$38 |
— |
— |
— |
$38 |
| 47 |
Teva-Capecitabine (capecitabine) |
Teva Canada Limited |
$1,011 |
— |
— |
— |
$97 |
$780 |
$59 |
$37 |
$21 |
— |
$17 |
| 48 |
Apo-Quinapril/HCTZ (quinapril , hydrochlorothiazide) |
Apotex Inc. |
$957 |
$13 |
$19 |
— |
$35 |
$811 |
$7 |
$31 |
$2 |
$24 |
$16 |
| 49 |
Betoptic S oph (betaxolol) |
Alcon Canada Inc. |
$945 |
$195 |
$78 |
$40 |
$52 |
$481 |
$23 |
$40 |
$9 |
$19 |
$9 |
| 50 |
Desipramine (desipramine) |
AA Pharma Inc. |
$907 |
$237 |
$67 |
$61 |
$80 |
$363 |
$18 |
$29 |
$6 |
$20 |
$27 |
| 51 |
Benzaclin topical (benzoyl peroxide, clindamycin) |
Valeant Canada LP Valeant Canada S.E.C. |
$865 |
— |
$6 |
$357 |
— |
$493 |
— |
— |
$0 |
$10 |
— |
| 52 |
Midodrine (midodrine hydrochloride) |
AA Pharma Inc. |
$844 |
$152 |
$55 |
$13 |
$62 |
$506 |
$15 |
$14 |
$1 |
$5 |
$21 |
| 53 |
Linessa (desogestrel) |
Aspen Pharma Trading Limited |
$842 |
$414 |
$11 |
$146 |
$138 |
$60 |
$8 |
$5 |
$0 |
$7 |
$53 |
| 54 |
Tapazole (thiamazole) |
Paladin Labs Inc. |
$831 |
$360 |
$80 |
$86 |
$146 |
$1 |
$12 |
$20 |
$4 |
$29 |
$95 |
| 55 |
Minestrin (norethindrone acetate, ethinyl estradiol) |
Warner Chilcott Canada Co. |
$790 |
$393 |
$13 |
$133 |
$121 |
$66 |
$6 |
$5 |
$0 |
$9 |
$43 |
| 56 |
Suprefact Depot (buserelin) |
Sanofi-Aventis Canada Inc. |
$761 |
— |
— |
$1 |
$4 |
$679 |
$4 |
$40 |
— |
$27 |
$6 |
| 57 |
Flagystatin vaginal ovule (nystatin, metronidazole) |
Sanofi-Aventis Canada Inc. |
$728 |
$238 |
$37 |
— |
$4 |
$347 |
$5 |
$12 |
$1 |
$17 |
$68 |
| 58 |
Sandoz latanoprost/Timolol (timolol , latanoprost) |
Sandoz Canada Incorporated |
$716 |
$71 |
$26 |
$19 |
$40 |
$511 |
$6 |
$23 |
$9 |
$9 |
$2 |
| 59 |
Robaxisal-C (codeine phosphate, methocarbamol, acetylsalicylic acid) |
Pfizer Consumer Healthcare a Division of Pfizer Canada Inc. |
$688 |
$623 |
— |
— |
$1 |
— |
$1 |
$4 |
$1 |
$55 |
$3 |
| 60 |
Mepron (atovaquone) |
GlaxoSmithKline Inc. |
$630 |
$37 |
$138 |
$2 |
$73 |
$316 |
$13 |
$5 |
— |
$1 |
$45 |
| 61 |
Methyldopa (methyldopa) |
AA Pharma Inc. |
$596 |
$132 |
$34 |
$55 |
$33 |
$259 |
$17 |
$16 |
$2 |
$30 |
$17 |
| 62 |
Thyroid (thyroid) |
ERFA Canada 2012 Inc. |
$563 |
$308 |
$98 |
$43 |
$2 |
$96 |
$4 |
$4 |
— |
— |
$8 |
| 63 |
Demulen (ethynodiol diacetate, ethinyl estradiol) |
Pfizer Canada Inc. |
$560 |
$264 |
$9 |
$51 |
$122 |
$62 |
$7 |
$3 |
$2 |
$7 |
$33 |
| 64 |
Trifluoperazine (trifluoperazine) |
AA Pharma Inc. |
$557 |
$106 |
$23 |
$40 |
$56 |
$252 |
$24 |
$27 |
$6 |
$14 |
$9 |
| 65 |
Tears Naturale II drop (hypromellose, dextran) |
Alcon Canada Inc. |
$550 |
$29 |
— |
— |
$4 |
$359 |
— |
$94 |
— |
$13 |
$52 |
| 66 |
Duvoid (bethanechol chloride) |
Paladin Labs Inc. |
$545 |
$89 |
— |
$17 |
$39 |
$321 |
$32 |
$30 |
$2 |
$3 |
$12 |
| 67 |
Resonium Calcium (calcium polystyrene sulfonate) |
Sanofi-Aventis Canada Inc. |
$541 |
$347 |
$168 |
$2 |
$0 |
$2 |
$3 |
$1 |
— |
— |
$18 |
| 68 |
Xtandi (enzalutamide) |
Astellas Pharma Canada Inc. |
$512 |
— |
— |
— |
— |
$371 |
$126 |
— |
— |
— |
$15 |
| 69 |
Cuprimine (D-penicillamine) |
Valeant Canada LP Valeant Canada S.E.C. |
$488 |
$173 |
$58 |
$10 |
$17 |
$182 |
$4 |
$16 |
$1 |
$18 |
$10 |
| 70 |
Revia (naltrexone hydrochloride) |
Teva Canada Limited |
$482 |
$145 |
— |
— |
$57 |
$125 |
$102 |
$5 |
$5 |
— |
$43 |
| 71 |
Modafinil (modafinil) |
Apotex Inc. |
$480 |
$110 |
$52 |
$26 |
$106 |
$97 |
$22 |
$3 |
$2 |
$7 |
$56 |
| 72 |
Sufentanil Citrate Injection USP (sufentanil) |
Sandoz Canada Incorporated |
$469 |
$419 |
|
— |
$49 |
— |
— |
$0 |
— |
— |
$1 |
| 73 |
Ortho (norethindrone) |
Janssen Inc. |
$465 |
$201 |
$11 |
$54 |
$76 |
$62 |
$5 |
$2 |
$1 |
$9 |
$45 |
| 74 |
Novo-Mexiletine (mexiletine hydrochloride) |
Teva Canada Limited |
$458 |
$186 |
$35 |
$37 |
$40 |
$126 |
$3 |
$7 |
$2 |
$4 |
$16 |
| 75 |
Min-Ovral (ethinyl estradiol, levonorgestrel) |
Pfizer Canada Inc. |
$449 |
$301 |
$15 |
$78 |
$17 |
$18 |
$1 |
$1 |
$0 |
$3 |
$15 |
| 76 |
Dantrium (dantrolene sodium) |
Par Pharmaceutical Companies |
$449 |
$77 |
$21 |
$67 |
$20 |
$202 |
$26 |
$4 |
$2 |
$6 |
$24 |
| 77 |
Propyl-Thyracil (propylthiouracil) |
Paladin Labs Inc. |
$431 |
$61 |
$12 |
$38 |
$37 |
$242 |
$5 |
$6 |
$1 |
$9 |
$20 |
| 78 |
Dapsone (dapsone) |
Jacobus Pharmaceutical Company, Inc. |
$429 |
$124 |
$67 |
$61 |
$39 |
$80 |
$11 |
$9 |
— |
$6 |
$32 |
| 79 |
Apo Lamivudine Zidovudine (lamivudine , zidovudine) |
Apotex Inc. |
$426 |
— |
— |
— |
$63 |
$271 |
$18 |
— |
— |
$18 |
$57 |
| 80 |
Apo-Enalapril Maleate/HCTZ (enalapril maleate, hydrochlorothiazide) |
Apotex Inc. |
$411 |
$106 |
$63 |
$97 |
$42 |
— |
$11 |
$32 |
$0 |
$39 |
$22 |
| 81 |
Viskazide (pindolol, hydrochlorothiazide) |
Novartis Pharmaceuticals Canada Inc. |
$406 |
$77 |
$31 |
$38 |
— |
$210 |
$10 |
$22 |
— |
$13 |
$4 |
| 82 |
Fludara (fludarabine phosphate) |
Sanofi-Aventis Canada Inc. |
$379 |
— |
— |
— |
— |
$367 |
$2 |
— |
$8 |
— |
$1 |
| 83 |
Ultravate (halobetasol propionate) |
Valeant Canada LP Valeant Canada S.E.C. |
$357 |
$196 |
$45 |
$0 |
$87 |
— |
— |
— |
— |
$0 |
$28 |
| 84 |
Sandoz Opium & Belladonna (opium, belladonna) |
Sandoz Canada Incorporated |
$343 |
$267 |
$49 |
— |
$2 |
$2 |
$2 |
$3 |
— |
$1 |
$17 |
| 85 |
Sabril (vigabatrin) |
Lundbeck LLC |
$329 |
$74 |
$19 |
$19 |
$16 |
$140 |
$16 |
$3 |
$3 |
$15 |
$25 |
| 86 |
Rougier Magnesium (magnesium glucoheptonate) |
Rougier Pharma Division of Ratiopharm Inc. |
$328 |
$128 |
$130 |
— |
$3 |
$25 |
$5 |
$9 |
$3 |
$3 |
$22 |
| 87 |
Carnitor oral (levocarnitine) |
Sigma-Tau Pharmaceuticals Inc. |
$321 |
$22 |
$17 |
— |
$109 |
$104 |
$3 |
$4 |
— |
$8 |
$53 |
| 88 |
Flamazine (silver sulfadiazine) |
Smith & Nephew Inc. |
$321 |
$125 |
$31 |
— |
$26 |
$75 |
$16 |
$13 |
$2 |
$5 |
$28 |
| 89 |
Flunarizine (flunarizine) |
AA Pharma Inc. |
$317 |
$103 |
$36 |
$5 |
$28 |
$101 |
$6 |
$14 |
$1 |
$7 |
$17 |
| 90 |
Piportil (pipotiazine palmitate) |
Sanofi-Aventis Canada Inc. |
$312 |
$58 |
$2 |
$34 |
$4 |
$187 |
$8 |
$3 |
— |
$7 |
$9 |
| 91 |
Trosec (trospium chloride) |
Sunovion Pharmaceuticals Canada Inc. |
$311 |
— |
$47 |
$14 |
$12 |
$197 |
$2 |
$21 |
$0 |
$14 |
$3 |
| 92 |
Differin topical (adapalene) |
Galderma Canada Inc. |
$307 |
— |
— |
$195 |
$13 |
— |
$0 |
— |
— |
$0 |
$99 |
| 93 |
Sterile Diluent for Flolan injection sol (glycine, sodium chloride, water) |
GlaxoSmithKline Inc. |
$305 |
$87 |
— |
$8 |
— |
$152 |
$1 |
— |
$9 |
$28 |
$19 |
| 94 |
Matulane (procarbazine) |
Sigma-Tau Pharmaceuticals Inc. |
$305 |
— |
— |
— |
$130 |
$135 |
$18 |
— |
— |
$13 |
$9 |
| 95 |
Primidone (primidone) |
AA Pharma Inc. |
$298 |
$43 |
$23 |
$16 |
$13 |
$177 |
$10 |
$6 |
$1 |
$6 |
$4 |
| 96 |
Loniten (minoxidil) |
Pfizer Canada Inc. |
$298 |
$63 |
$28 |
$11 |
$18 |
$159 |
$3 |
$7 |
— |
$1 |
$9 |
| 97 |
Parnate (tranylcypromine) |
GlaxoSmithKline Inc. |
$296 |
$70 |
$13 |
$16 |
$77 |
$92 |
$4 |
$13 |
$4 |
$5 |
$2 |
| 98 |
Cafergot (ergotamine tartrate, caffeine) |
Novartis Pharmaceuticals Canada Inc. |
$296 |
$72 |
$25 |
— |
$0 |
$178 |
$2 |
$4 |
— |
$8 |
$8 |
| 99 |
K Lyte (potassium bicarbonate, citric acid) |
WellSpring Pharmaceutical Canada Corp. |
$290 |
$115 |
$85 |
— |
$42 |
$11 |
— |
$13 |
— |
$12 |
$12 |
| 100 |
Proctofoam HC (hydrocortisone acetate, pramoxine hydrochloride) |
Duchesnay Inc. |
$274 |
$156 |
$58 |
— |
$0 |
— |
$14 |
$40 |
— |
$5 |
$1 |
| |
|
Total |
$296,823 |
$51,583 |
$31,441 |
$17,621 |
$16,555 |
$148,004 |
$7,784 |
$6,590 |
$1,267 |
$6,020 |
$9,958 |
| |
|
Share of all non-patented single source drugs |
96% |
95% |
97% |
96% |
94% |
97% |
96% |
95% |
96% |
92% |
87% |
Note: Drug costs of less than $500 will appear as $0 thousand due to rounding.
*Total results for the public drug plans reported in this figure.
Data source: National Prescription Drug Utilization Information System Database, Canadian Institute for Health Information.
Appendix H: Top 100 Multi-Source Generic Drugs by Drug Cost, NPDUIS Public Drug Plans, 2013/14 ($million)
Table: Appendix H
| Rank |
Ingredient |
Total* |
BC |
AB |
SK |
MB |
ON |
NB |
NS |
PE |
NL |
NIHB |
| 1 |
Atorvastatin |
$110.87 |
$19.90 |
$10.73 |
$4.86 |
$6.99 |
$58.44 |
$1.97 |
$2.58 |
$0.50 |
$1.58 |
$3.31 |
| 2 |
Rosuvastatin |
$92.27 |
$14.50 |
$8.49 |
$6.89 |
$4.23 |
$46.23 |
$2.31 |
$4.09 |
$0.69 |
$2.56 |
$2.29 |
| 3 |
Amlodipine |
$55.08 |
$8.31 |
$5.70 |
$2.59 |
$3.02 |
$31.28 |
$0.83 |
$1.38 |
$0.35 |
$0.30 |
$1.33 |
| 4 |
Gabapentin |
$51.33 |
$13.30 |
$4.79 |
$3.50 |
$5.36 |
$15.44 |
$1.24 |
$1.60 |
$0.29 |
$0.20 |
$5.61 |
| 5 |
Pantoprazole |
$49.59 |
$1.91 |
$10.73 |
$2.45 |
$1.98 |
$30.20 |
$0.23 |
$0.08 |
$0.00 |
$0.02 |
$1.99 |
| 6 |
Metformin hydrochloride |
$44.78 |
$10.28 |
$4.37 |
$0.03 |
$3.97 |
$19.60 |
$0.92 |
$1.45 |
$0.44 |
$1.00 |
$2.73 |
| 7 |
Ramipril |
$44.47 |
$13.20 |
$3.55 |
$2.01 |
$2.34 |
$18.72 |
$0.82 |
$0.85 |
$0.22 |
$0.77 |
$1.99 |
| 8 |
Olanzapine |
$40.50 |
$7.37 |
$1.40 |
$1.22 |
$2.30 |
$22.57 |
$1.77 |
$0.79 |
$0.29 |
$0.84 |
$1.95 |
| 9 |
Simvastatin |
$39.08 |
$8.98 |
$3.71 |
$2.71 |
$2.00 |
$16.88 |
$0.91 |
$1.74 |
$0.31 |
$1.01 |
$0.83 |
| 10 |
Clopidogrel |
$37.84 |
$4.87 |
$3.12 |
$1.92 |
$3.08 |
$20.76 |
$0.90 |
$1.53 |
$0.17 |
$0.62 |
$0.87 |
| 11 |
Quetiapine |
$32.51 |
$5.43 |
$0.99 |
$1.57 |
$2.67 |
$17.23 |
$1.30 |
$0.65 |
$0.14 |
$1.04 |
$1.49 |
| 12 |
Fentanyl |
$31.05 |
$3.28 |
$1.37 |
$1.60 |
$1.75 |
$21.71 |
$0.33 |
$0.38 |
$0.02 |
$0.06 |
$0.55 |
| 13 |
Citalopram |
$30.85 |
$7.08 |
$1.64 |
$3.02 |
$4.31 |
$9.94 |
$1.06 |
$1.39 |
$0.30 |
$0.59 |
$1.53 |
| 14 |
Rabeprazole sodium |
$30.15 |
$4.78 |
$0.66 |
$2.13 |
$1.22 |
$16.84 |
$0.26 |
$1.38 |
$0.11 |
$1.04 |
$1.74 |
| 15 |
Risedronate sodium |
$28.07 |
$0.30 |
$1.62 |
$0.36 |
$0.22 |
$24.41 |
$0.24 |
$0.70 |
$0.03 |
$0.08 |
$0.11 |
| 16 |
Venlafaxine |
$27.67 |
$7.69 |
$1.99 |
$2.38 |
$2.58 |
$9.59 |
$0.74 |
$0.58 |
$0.10 |
$0.68 |
$1.34 |
| 17 |
Omeprazole |
$27.66 |
$0.83 |
$4.05 |
$1.43 |
$2.94 |
$12.07 |
$1.20 |
$1.52 |
$0.28 |
$0.77 |
$2.57 |
| 18 |
Nifedipine |
$21.92 |
$2.61 |
$1.95 |
$0.91 |
$2.45 |
$10.10 |
$1.21 |
$0.72 |
$0.12 |
$0.90 |
$0.96 |
| 19 |
Lansoprazole |
$21.42 |
$0.82 |
$5.01 |
$0.46 |
$0.21 |
$14.10 |
$0.21 |
$0.11 |
$0.00 |
$0.01 |
$0.48 |
| 20 |
Diltiazem hydrochloride |
$20.66 |
$4.58 |
$1.53 |
$1.14 |
$1.22 |
$9.89 |
$0.69 |
$0.70 |
$0.19 |
$0.32 |
$0.40 |
| 21 |
Metoprolol tartrate |
$20.37 |
$3.67 |
$2.28 |
$1.35 |
$2.18 |
$7.13 |
$0.61 |
$1.34 |
$0.20 |
$0.96 |
$0.65 |
| 22 |
Paroxetine |
$18.67 |
$3.99 |
$1.12 |
$1.83 |
$2.37 |
$6.22 |
$0.56 |
$0.69 |
$0.11 |
$0.73 |
$1.05 |
| 23 |
Sertraline |
$18.41 |
$4.63 |
$1.07 |
$1.67 |
$1.56 |
$7.00 |
$0.48 |
$0.60 |
$0.09 |
$0.49 |
$0.82 |
| 24 |
Amoxicillin |
$17.27 |
$5.61 |
$0.69 |
$1.77 |
$1.61 |
$5.07 |
$0.17 |
$0.18 |
$0.05 |
$0.36 |
$1.77 |
| 25 |
Ranitidine |
$17.17 |
$3.44 |
$0.49 |
$1.49 |
$1.32 |
$6.59 |
$0.51 |
$0.93 |
$0.17 |
$1.02 |
$1.24 |
| 26 |
Risperidone |
$17.10 |
$3.21 |
$0.49 |
$1.32 |
$1.33 |
$8.03 |
$0.83 |
$0.36 |
$0.13 |
$0.51 |
$0.89 |
| 27 |
Fluoxetine |
$16.78 |
$5.51 |
$1.14 |
$1.81 |
$1.73 |
$4.58 |
$0.30 |
$0.36 |
$0.08 |
$0.34 |
$0.92 |
| 28 |
Levodopa, carbidopa |
$16.42 |
$2.44 |
$1.31 |
$0.73 |
$1.26 |
$9.48 |
$0.30 |
$0.38 |
$0.08 |
$0.19 |
$0.23 |
| 29 |
Zopiclone |
$15.01 |
$3.05 |
$5.56 |
— |
$3.48 |
$0.07 |
$1.24 |
$0.60 |
$0.09 |
$0.92 |
— |
| 30 |
Acetaminophen, oxycodone hydrochloride |
$14.98 |
$1.80 |
$0.64 |
— |
$0.85 |
$10.24 |
$0.14 |
$0.10 |
$0.06 |
$0.38 |
$0.77 |
| 31 |
Valsartan |
$14.86 |
$1.66 |
$2.37 |
$1.35 |
$0.87 |
$7.50 |
$0.11 |
$0.48 |
$0.09 |
$0.11 |
$0.32 |
| 32 |
Candesartan cilexetil |
$14.59 |
$2.02 |
$1.53 |
$1.11 |
$0.62 |
$7.68 |
$0.31 |
$0.53 |
$0.09 |
$0.42 |
$0.29 |
| 33 |
Salbutamol |
$14.40 |
$3.68 |
$0.47 |
$0.88 |
$1.07 |
$5.90 |
$0.49 |
$0.39 |
$0.08 |
$0.29 |
$1.15 |
| 34 |
Warfarin sodium |
$13.99 |
$2.70 |
$1.45 |
$1.10 |
$1.49 |
$5.67 |
$0.33 |
$0.59 |
$0.13 |
$0.33 |
$0.21 |
| 35 |
Cephalexin |
$13.68 |
$4.12 |
$0.75 |
$1.36 |
$1.30 |
$3.96 |
$0.17 |
$0.22 |
$0.05 |
$0.23 |
$1.52 |
| 36 |
Irbesartan |
$13.32 |
$1.00 |
$1.92 |
$0.84 |
$1.22 |
$7.18 |
$0.19 |
$0.41 |
$0.07 |
$0.13 |
$0.35 |
| 37 |
Atenolol |
$12.89 |
$2.25 |
$0.76 |
$1.06 |
$0.63 |
$6.53 |
$0.34 |
$0.53 |
$0.15 |
$0.36 |
$0.28 |
| 38 |
Clozapine |
$12.75 |
$6.92 |
$0.31 |
$0.74 |
$2.43 |
$0.00 |
$0.80 |
$0.00 |
$0.02 |
$0.35 |
$1.17 |
| 39 |
Methotrexate |
$11.90 |
$2.86 |
$1.63 |
$0.56 |
$0.80 |
$4.58 |
$0.21 |
$0.36 |
$0.04 |
$0.16 |
$0.69 |
| 40 |
Valproic acid |
$11.82 |
$2.67 |
$0.33 |
$0.86 |
$1.13 |
$5.30 |
$0.40 |
$0.18 |
$0.08 |
$0.36 |
$0.52 |
| 41 |
Pravastatin sodium |
$11.71 |
$1.83 |
$1.58 |
$0.91 |
$0.55 |
$5.64 |
$0.25 |
$0.46 |
$0.08 |
$0.17 |
$0.22 |
| 42 |
Tamsulosin hydrochloride |
$11.46 |
$2.21 |
$2.04 |
$0.91 |
$0.93 |
$4.38 |
$0.38 |
$0.09 |
$0.12 |
$0.17 |
$0.23 |
| 43 |
Morphine sulfate |
$11.37 |
$0.44 |
$0.77 |
$0.48 |
$1.16 |
$6.87 |
$0.26 |
$0.15 |
$0.06 |
$0.40 |
$0.78 |
| 44 |
Hydromorphone hydrochloride |
$11.31 |
$2.99 |
$0.71 |
$0.94 |
$0.59 |
$4.95 |
$0.23 |
$0.43 |
$0.06 |
$0.03 |
$0.38 |
| 45 |
Epinephrine |
$11.19 |
$4.17 |
$0.59 |
$1.13 |
$1.31 |
$2.35 |
$0.14 |
$0.06 |
$0.00 |
$0.04 |
$1.39 |
| 46 |
Valacyclovir |
$11.03 |
$5.63 |
$1.09 |
$1.50 |
$0.91 |
$0.77 |
$0.11 |
$0.18 |
$0.02 |
$0.09 |
$0.74 |
| 47 |
Ondansetron |
$10.76 |
$1.68 |
$2.09 |
— |
$1.26 |
$4.37 |
$0.15 |
$0.26 |
$0.00 |
$0.06 |
$0.90 |
| 48 |
Topiramate |
$10.42 |
$2.76 |
$0.75 |
$1.12 |
$1.44 |
$3.49 |
$0.14 |
$0.07 |
$0.02 |
$0.05 |
$0.59 |
| 49 |
Galantamine |
$10.38 |
— |
$0.76 |
$0.09 |
$0.15 |
$8.38 |
$0.32 |
$0.49 |
$0.08 |
$0.10 |
$0.02 |
| 50 |
Pregabalin |
$10.37 |
$0.16 |
— |
$0.77 |
$0.00 |
$9.03 |
$0.02 |
$0.22 |
— |
$0.00 |
$0.16 |
| 51 |
Alendronic acid |
$10.15 |
$0.60 |
$1.75 |
$0.56 |
$0.61 |
$5.24 |
$0.42 |
$0.54 |
$0.05 |
$0.14 |
$0.25 |
| 52 |
Losartan potassium |
$9.88 |
$1.28 |
$1.02 |
$0.80 |
$0.60 |
$4.99 |
$0.19 |
$0.46 |
$0.09 |
$0.15 |
$0.29 |
| 53 |
Lamotrigine |
$9.84 |
$2.86 |
$0.43 |
$1.39 |
$1.66 |
$2.54 |
$0.18 |
$0.17 |
$0.03 |
$0.19 |
$0.40 |
| 54 |
Fenofibrate |
$9.70 |
$1.24 |
$0.93 |
$0.30 |
$1.26 |
$4.95 |
$0.12 |
$0.26 |
$0.03 |
$0.22 |
$0.38 |
| 55 |
Nabilone |
$9.70 |
$2.33 |
$0.54 |
$0.03 |
$1.11 |
$5.09 |
$0.20 |
$0.11 |
— |
$0.06 |
$0.21 |
| 56 |
Furosemide |
$9.35 |
$1.25 |
$0.82 |
$0.52 |
$0.69 |
$5.06 |
$0.26 |
$0.28 |
$0.05 |
$0.22 |
$0.21 |
| 57 |
Naproxen |
$9.34 |
$2.44 |
$0.50 |
$0.63 |
$0.73 |
$3.39 |
$0.18 |
$0.21 |
$0.06 |
$0.09 |
$1.12 |
| 58 |
Telmisartan |
$9.01 |
$1.00 |
$1.00 |
$0.47 |
$0.48 |
$5.26 |
$0.17 |
$0.27 |
$0.13 |
$0.06 |
$0.17 |
| 59 |
Levetiracetam |
$8.83 |
$2.19 |
$0.99 |
$1.10 |
$0.83 |
$2.41 |
$0.20 |
$0.21 |
$0.03 |
$0.14 |
$0.74 |
| 60 |
Ciprofloxacin |
$8.23 |
$2.29 |
$0.86 |
$0.09 |
$0.76 |
$2.99 |
$0.05 |
$0.22 |
$0.00 |
$0.39 |
$0.56 |
| 61 |
Enalapril maleate |
$8.21 |
$0.47 |
$0.64 |
$0.01 |
$1.51 |
$3.91 |
$0.20 |
$0.26 |
$0.04 |
$0.19 |
$0.99 |
| 62 |
Carvedilol |
$8.14 |
$1.11 |
$1.54 |
$0.66 |
$0.38 |
$3.56 |
$0.21 |
$0.37 |
$0.04 |
$0.02 |
$0.23 |
| 63 |
Bisoprolol fumarate |
$8.09 |
$1.62 |
$0.87 |
$0.10 |
$0.11 |
$4.79 |
$0.17 |
$0.20 |
$0.02 |
$0.09 |
$0.13 |
| 64 |
Gliclazide |
$8.04 |
$0.39 |
$1.06 |
$0.26 |
$0.77 |
$3.58 |
$0.25 |
$0.54 |
$0.12 |
$0.40 |
$0.67 |
| 65 |
Valsartan, hydrochlorothiazide |
$7.91 |
$0.54 |
$1.44 |
$1.16 |
$0.58 |
$3.64 |
$0.04 |
$0.25 |
$0.03 |
$0.06 |
$0.18 |
| 66 |
Mirtazapine |
$7.77 |
$1.55 |
$0.44 |
$0.45 |
$0.97 |
$3.34 |
$0.18 |
$0.29 |
$0.05 |
$0.24 |
$0.28 |
| 67 |
Finasteride |
$7.60 |
$0.40 |
$0.77 |
$0.45 |
$0.64 |
$4.68 |
$0.13 |
$0.35 |
$0.01 |
$0.08 |
$0.09 |
| 68 |
Azithromycin |
$7.47 |
$1.27 |
$0.37 |
$0.83 |
$1.43 |
$2.58 |
$0.11 |
$0.05 |
$0.00 |
$0.18 |
$0.65 |
| 69 |
Baclofen |
$7.07 |
$1.10 |
$0.40 |
$0.58 |
$0.57 |
$3.48 |
$0.12 |
$0.11 |
$0.02 |
$0.12 |
$0.58 |
| 70 |
Leflunomide |
$7.04 |
$0.94 |
$0.69 |
$0.56 |
$0.43 |
$3.60 |
$0.04 |
$0.14 |
$0.02 |
$0.03 |
$0.59 |
| 71 |
Clonazepam |
$6.91 |
$1.33 |
$0.39 |
$0.23 |
$1.11 |
$2.66 |
$0.29 |
$0.21 |
$0.03 |
$0.24 |
$0.41 |
| 72 |
Terazosin |
$6.70 |
$1.11 |
$0.35 |
$0.14 |
$0.22 |
$3.79 |
$0.14 |
$0.50 |
$0.02 |
$0.32 |
$0.12 |
| 73 |
Lisinopril |
$6.57 |
$0.62 |
$0.84 |
$0.47 |
$1.14 |
$2.48 |
$0.15 |
$0.23 |
$0.07 |
$0.29 |
$0.27 |
| 74 |
Codeine phosphate, acetaminophen, caffeine |
$6.56 |
$1.45 |
$0.53 |
$0.42 |
$1.00 |
$2.33 |
$0.02 |
$0.01 |
$0.03 |
$0.02 |
$0.75 |
| 75 |
Verapamil hydrochloride |
$6.51 |
$1.55 |
$0.71 |
$0.44 |
$0.75 |
$2.26 |
$0.25 |
$0.23 |
$0.07 |
$0.12 |
$0.13 |
| 76 |
Carbamazepine |
$6.33 |
$1.22 |
$0.28 |
$0.31 |
$0.73 |
$2.91 |
$0.14 |
$0.10 |
$0.03 |
$0.27 |
$0.35 |
| 77 |
Mometasone furoate |
$6.31 |
$1.06 |
$0.29 |
$0.03 |
$1.45 |
$2.20 |
$0.06 |
$0.07 |
$0.08 |
$0.29 |
$0.76 |
| 78 |
Pioglitazone |
$6.25 |
$0.47 |
$0.94 |
$0.39 |
$0.31 |
$3.28 |
$0.08 |
$0.11 |
$0.00 |
$0.08 |
$0.59 |
| 79 |
Clarithromycin |
$6.08 |
$1.32 |
$0.52 |
$0.60 |
$0.85 |
$1.41 |
$0.12 |
$0.14 |
$0.00 |
$0.25 |
$0.87 |
| 80 |
Sumatriptan |
$6.01 |
$3.63 |
$0.67 |
$0.10 |
$0.73 |
$0.36 |
$0.03 |
$0.06 |
$0.00 |
$0.02 |
$0.41 |
| 81 |
Hydroxychloroquine sulfate |
$5.99 |
$1.69 |
$0.58 |
$0.24 |
$0.44 |
$2.28 |
$0.10 |
$0.13 |
$0.02 |
$0.06 |
$0.44 |
| 82 |
Esomeprazole |
$5.97 |
— |
— |
— |
$5.92 |
— |
— |
$0.05 |
— |
$0.00 |
— |
| 83 |
Irbesartan, hydrochlorothiazide |
$5.95 |
$0.33 |
$1.16 |
$0.74 |
$0.92 |
$2.29 |
$0.04 |
$0.24 |
$0.02 |
$0.05 |
$0.17 |
| 84 |
Trazodone hydrochloride |
$5.86 |
$1.32 |
$0.40 |
$0.19 |
$0.97 |
$2.41 |
$0.06 |
$0.17 |
$0.01 |
$0.04 |
$0.27 |
| 85 |
Cyclobenzaprine hydrochloride |
$5.85 |
$3.62 |
$0.28 |
$0.00 |
$0.39 |
— |
$0.32 |
$0.16 |
$0.00 |
$0.32 |
$0.76 |
| 86 |
Amlodipine , atorvastatin (atorvastatin calcium) |
$5.82 |
— |
— |
$0.38 |
$0.00 |
$5.09 |
$0.05 |
$0.27 |
— |
$0.00 |
$0.03 |
| 87 |
Enalapril sodium |
$5.68 |
$0.91 |
$0.80 |
$0.54 |
$1.47 |
$0.95 |
$0.10 |
$0.34 |
$0.04 |
$0.22 |
$0.30 |
| 88 |
Levonorgestrel, ethinyl estradiol |
$5.53 |
$2.08 |
$0.04 |
$0.74 |
$1.14 |
$0.69 |
$0.04 |
$0.02 |
$0.01 |
$0.11 |
$0.66 |
| 89 |
Diclofenac sodium |
$5.48 |
$0.83 |
$0.59 |
$0.62 |
$1.14 |
$1.49 |
$0.05 |
$0.10 |
$0.05 |
$0.14 |
$0.47 |
| 90 |
Domperidone |
$5.12 |
$0.84 |
$0.30 |
$0.29 |
$0.53 |
$2.40 |
$0.15 |
$0.28 |
$0.05 |
$0.10 |
$0.17 |
| 91 |
Imatinib |
$5.02 |
— |
— |
— |
$0.70 |
$3.72 |
$0.11 |
$0.19 |
$0.05 |
$0.05 |
$0.20 |
| 92 |
Methylphenidate hydrochloride |
$4.96 |
$0.61 |
$0.05 |
$0.17 |
$1.10 |
$2.05 |
$0.12 |
$0.05 |
$0.03 |
$0.23 |
$0.55 |
| 93 |
Latanoprost |
$4.89 |
$0.72 |
$0.44 |
$0.15 |
$0.30 |
$2.77 |
$0.10 |
$0.19 |
$0.05 |
$0.09 |
$0.07 |
| 94 |
Amiodarone hydrochloride |
$4.77 |
$0.85 |
$0.35 |
$0.33 |
$0.46 |
$2.33 |
$0.11 |
$0.14 |
$0.03 |
$0.11 |
$0.08 |
| 95 |
Meloxicam |
$4.77 |
$0.11 |
— |
$0.03 |
$0.10 |
$4.21 |
$0.11 |
$0.10 |
— |
$0.01 |
$0.11 |
| 96 |
Bupropion hydrochloride |
$4.75 |
$0.62 |
$0.25 |
$0.20 |
$0.70 |
$2.50 |
$0.12 |
$0.10 |
$0.02 |
$0.06 |
$0.19 |
| 97 |
Prednisolone acetate |
$4.73 |
$1.32 |
$0.29 |
$0.08 |
$0.28 |
$2.20 |
$0.12 |
$0.19 |
$0.04 |
$0.07 |
$0.15 |
| 98 |
Glyburide |
$4.68 |
$1.24 |
$0.18 |
$0.24 |
$0.56 |
$1.85 |
$0.06 |
$0.08 |
$0.02 |
$0.15 |
$0.31 |
| 99 |
Lorazepam |
$4.64 |
$0.56 |
$0.20 |
$0.13 |
$0.38 |
$2.63 |
$0.19 |
$0.20 |
$0.03 |
$0.14 |
$0.18 |
| 100 |
Clindamycin |
$4.63 |
$1.22 |
$0.26 |
$0.53 |
$0.58 |
$1.20 |
$0.06 |
$0.06 |
$0.01 |
$0.06 |
$0.65 |
| |
Total |
$1,594.81 |
$288.58 |
$142.83 |
$92.55 |
$134.87 |
$740.74 |
$36.32 |
$45.83 |
$8.48 |
$31.13 |
$73.47 |
| |
Share of all multi-source generic drugs |
85% |
82% |
84% |
83% |
82% |
87% |
83% |
83% |
85% |
81% |
82% |
Note: Drug costs of less than $5,000 appear as $0.00 million due to rounding.
*Total results for the public drug plans reported in this figure.
Data source: National Prescription Drug Utilization Information System Database, Canadian Institute for Health Information.
Appendix I: Top 100 Manufacturers by Drug Cost, NPDUIS Public Drug Plans, 2013/14 ($million)
Table: Appendix I
| Rank |
Company |
Total* |
BC |
AB |
SK |
MB |
ON |
NB |
NS |
PE |
NL |
NIHB |
| 1 |
Apotex Inc. |
$543.90 |
$75.48 |
$44.85 |
$20.07 |
$50.42 |
$289.99 |
$12.13 |
$11.18 |
$3.08 |
$8.92 |
$27.77 |
| 2 |
Janssen Inc. |
$517.27 |
$116.53 |
$62.44 |
$40.83 |
$38.88 |
$199.74 |
$12.70 |
$13.96 |
$2.66 |
$8.73 |
$20.80 |
| 3 |
Novartis Pharmaceuticals Canada Inc. |
$473.69 |
$18.60 |
$70.53 |
$9.48 |
$8.77 |
$346.38 |
$8.36 |
$3.11 |
$0.60 |
$3.96 |
$3.90 |
| 4 |
Teva Canada Limited |
$429.33 |
$63.29 |
$27.18 |
$25.32 |
$37.22 |
$221.97 |
$9.08 |
$13.28 |
$2.11 |
$9.15 |
$20.73 |
| 5 |
Pfizer Canada Inc. |
$358.74 |
$54.80 |
$28.95 |
$17.16 |
$19.70 |
$209.82 |
$7.01 |
$5.83 |
$0.84 |
$4.11 |
$10.54 |
| 6 |
Merck Canada Inc. |
$350.46 |
$32.58 |
$23.19 |
$19.34 |
$10.41 |
$239.75 |
$5.61 |
$6.05 |
$0.67 |
$2.95 |
$9.91 |
| 7 |
GlaxoSmithKline Inc. |
$290.39 |
$42.10 |
$30.37 |
$12.41 |
$18.14 |
$156.15 |
$7.78 |
$5.76 |
$0.94 |
$4.29 |
$12.44 |
| 8 |
Pharmascience Inc. |
$213.63 |
$46.76 |
$16.50 |
$18.63 |
$16.00 |
$81.50 |
$7.01 |
$8.32 |
$1.38 |
$6.21 |
$11.31 |
| 9 |
Sandoz Canada Incorporated |
$197.08 |
$40.20 |
$20.20 |
$12.02 |
$14.26 |
$88.96 |
$3.44 |
$5.71 |
$0.89 |
$2.83 |
$8.57 |
| 10 |
Boehringer Ingelheim |
$189.73 |
$15.50 |
$23.80 |
$6.41 |
$5.00 |
$126.37 |
$4.07 |
$4.09 |
$0.50 |
$1.51 |
$2.48 |
| 11 |
AstraZeneca Canada Inc. |
$175.54 |
$34.13 |
$15.87 |
$11.22 |
$7.38 |
$96.79 |
$2.30 |
$3.23 |
$0.46 |
$1.27 |
$2.88 |
| 12 |
AbbVie Corporation |
$168.38 |
$50.26 |
$26.81 |
$12.98 |
$17.29 |
$39.84 |
$3.25 |
$6.59 |
$1.74 |
$3.77 |
$5.87 |
| 13 |
Sanofi-Aventis Canada Inc. |
$135.99 |
$21.76 |
$11.00 |
$7.75 |
$3.67 |
$78.24 |
$1.42 |
$1.69 |
$0.23 |
$0.88 |
$9.34 |
| 14 |
Servier Canada Inc. |
$126.90 |
$13.92 |
$11.89 |
$8.90 |
$7.59 |
$71.29 |
$3.86 |
$3.41 |
$0.52 |
$1.74 |
$3.78 |
| 15 |
Eli Lilly Canada Inc. |
$125.81 |
$13.90 |
$11.87 |
$6.97 |
$7.66 |
$71.02 |
$2.67 |
$2.23 |
$0.97 |
$2.92 |
$5.58 |
| 16 |
Immunex Corporation |
$123.64 |
$30.07 |
$20.57 |
$8.55 |
$14.92 |
$32.20 |
$2.93 |
$4.60 |
$0.95 |
$1.35 |
$7.50 |
| 17 |
Mylan Pharmaceuticals ULC |
$123.62 |
$21.87 |
$10.87 |
$5.42 |
$15.31 |
$52.38 |
$3.68 |
$4.28 |
$0.57 |
$3.31 |
$5.93 |
| 18 |
Bayer Inc. |
$123.05 |
$25.79 |
$10.42 |
$9.46 |
$9.44 |
$54.87 |
$2.78 |
$2.40 |
$0.32 |
$2.88 |
$4.69 |
| 19 |
Purdue Pharma |
$106.45 |
$10.27 |
$11.33 |
$6.47 |
$7.78 |
$60.81 |
$2.75 |
$2.16 |
$0.18 |
$0.77 |
$3.93 |
| 20 |
Lundbeck Canada Inc. |
$105.40 |
$40.76 |
$5.99 |
$0.33 |
$0.25 |
$54.98 |
$0.12 |
$0.07 |
$0.00 |
$0.10 |
$2.79 |
| 21 |
Ranbaxy Pharmaceuticals Canada Inc. |
$105.30 |
$21.97 |
$11.08 |
$4.35 |
$9.61 |
$50.35 |
$2.01 |
$1.83 |
$0.45 |
$1.55 |
$2.11 |
| 22 |
Actavis Pharma Company |
$103.06 |
$24.57 |
$12.04 |
$4.38 |
$12.87 |
$39.10 |
$1.27 |
$3.02 |
$0.35 |
$1.61 |
$3.86 |
| 23 |
Novo Nordisk Canada Inc. |
$98.82 |
$13.77 |
$10.80 |
$4.29 |
$5.30 |
$51.41 |
$1.80 |
$3.91 |
$0.74 |
$2.18 |
$4.62 |
| 24 |
Bristol-Myers Squibb Canada |
$88.72 |
$12.04 |
$4.09 |
$2.51 |
$6.19 |
$55.24 |
$1.60 |
$1.69 |
$0.18 |
$0.94 |
$4.23 |
| 25 |
Sanis Health Inc. |
$87.60 |
$36.33 |
$17.46 |
$11.74 |
$2.93 |
— |
$3.88 |
$5.35 |
$0.76 |
$3.39 |
$5.77 |
| 26 |
Hoffmann-La Roche Limited |
$82.28 |
$17.39 |
$6.43 |
$4.68 |
$6.41 |
$38.05 |
$1.48 |
$2.21 |
$0.23 |
$1.09 |
$4.31 |
| 27 |
Takeda Canada Inc. |
$81.13 |
$10.58 |
$4.95 |
$2.50 |
$0.53 |
$48.65 |
$6.26 |
$2.91 |
$0.76 |
$2.07 |
$1.92 |
| 28 |
Gilead Sciences Canada Inc. |
$70.73 |
$7.52 |
$2.74 |
$2.21 |
$3.78 |
$48.83 |
$1.19 |
$0.10 |
— |
$0.36 |
$4.00 |
| 29 |
Valeant Canada LP Valeant Canada S.E.C. |
$69.55 |
$14.11 |
$7.44 |
$6.49 |
$5.13 |
$29.56 |
$1.50 |
$1.39 |
$0.21 |
$1.05 |
$2.68 |
| 30 |
Amgen Canada Inc. |
$69.13 |
$8.16 |
$11.51 |
$0.38 |
$0.31 |
$41.29 |
$2.97 |
$0.21 |
$0.24 |
$1.58 |
$2.47 |
| 31 |
Celgene Inc. |
$63.55 |
— |
— |
— |
$6.42 |
$49.69 |
$2.30 |
$2.66 |
— |
$2.18 |
$0.30 |
| 32 |
BGP Pharma ULC |
$57.56 |
$16.08 |
$5.70 |
$3.07 |
$4.14 |
$23.22 |
$0.91 |
$1.56 |
$0.23 |
$0.91 |
$1.74 |
| 33 |
AA Pharma Inc. |
$52.27 |
$11.26 |
$4.27 |
$2.72 |
$3.29 |
$24.45 |
$1.25 |
$1.35 |
$0.25 |
$1.52 |
$1.91 |
| 34 |
Allergan Inc. |
$43.64 |
$11.63 |
$5.27 |
$1.84 |
$3.12 |
$17.85 |
$0.93 |
$1.24 |
$0.16 |
$0.33 |
$1.27 |
| 35 |
Teva Pharmaceutical Industries Ltd. |
$42.36 |
$7.06 |
$13.03 |
$5.06 |
$4.14 |
$9.86 |
$1.34 |
— |
$0.52 |
$0.67 |
$0.68 |
| 36 |
Warner Chilcott Canada Co. |
$39.52 |
$5.28 |
$1.99 |
$2.77 |
$1.17 |
$26.52 |
$0.38 |
$0.34 |
$0.03 |
$0.36 |
$0.68 |
| 37 |
Astellas Pharma Canada Inc. |
$36.58 |
$0.25 |
$1.22 |
$1.60 |
$2.83 |
$26.91 |
$1.73 |
$0.28 |
$0.03 |
$0.04 |
$1.69 |
| 38 |
Leo Pharma Inc. |
$34.12 |
$4.18 |
$5.29 |
$2.83 |
$0.75 |
$18.24 |
$0.38 |
$0.25 |
$0.03 |
$0.35 |
$1.82 |
| 39 |
EMD Serono, a Division of EMD Inc. Canada |
$33.53 |
$8.02 |
$7.00 |
$3.48 |
$2.35 |
$9.55 |
$1.70 |
— |
$0.39 |
$0.51 |
$0.53 |
| 40 |
Alcon Canada Inc. |
$33.02 |
$6.64 |
$3.30 |
$2.15 |
$2.71 |
$13.97 |
$0.83 |
$1.41 |
$0.19 |
$0.52 |
$1.29 |
| 41 |
Biogen Canada Inc. |
$31.64 |
$7.16 |
$3.92 |
$2.00 |
$3.79 |
$9.63 |
$3.45 |
— |
$0.39 |
$0.73 |
$0.57 |
| 42 |
Bristol-Myers Squibb and Gilead Sciences, LLC |
$30.77 |
— |
— |
$1.77 |
$1.16 |
$24.63 |
$0.94 |
— |
— |
$0.26 |
$2.01 |
| 43 |
Taro Pharmaceuticals Inc. |
$30.41 |
$5.85 |
$2.06 |
$1.62 |
$2.41 |
$14.79 |
$0.52 |
$0.84 |
$0.19 |
$0.59 |
$1.54 |
| 44 |
ViiV Healthcare ULC |
$24.71 |
— |
— |
$0.94 |
$2.56 |
$18.36 |
$0.47 |
$0.00 |
— |
$0.31 |
$2.07 |
| 45 |
Pendopharm, a Division of Pharmascience Inc. |
$23.58 |
$5.51 |
$2.00 |
$1.54 |
$1.40 |
$10.23 |
$0.64 |
$0.54 |
$0.13 |
$0.44 |
$1.15 |
| 46 |
Paladin Labs Inc. |
$23.43 |
$8.09 |
$2.35 |
$1.25 |
$1.69 |
$6.74 |
$0.43 |
$0.70 |
$0.36 |
$0.36 |
$1.47 |
| 47 |
Cobalt Pharmaceuticals Company |
$20.87 |
$3.25 |
$1.36 |
$1.64 |
$1.78 |
$11.08 |
$0.18 |
$0.37 |
$0.03 |
$0.35 |
$0.83 |
| 48 |
Actelion Pharmaceuticals Ltd. |
$17.01 |
$4.02 |
— |
$0.02 |
$1.00 |
$11.49 |
— |
$0.06 |
— |
$0.09 |
$0.34 |
| 49 |
Shire Pharma Canada ULC |
$16.25 |
$2.50 |
$0.21 |
$1.04 |
$0.25 |
$11.56 |
$0.05 |
$0.07 |
$0.01 |
$0.06 |
$0.50 |
| 50 |
Alexion Pharma International Sarl |
$15.65 |
— |
$2.51 |
— |
$0.55 |
$12.59 |
— |
— |
— |
— |
— |
| 51 |
Vertex Pharmaceuticals |
$13.52 |
$6.55 |
$2.68 |
$0.90 |
$1.00 |
$0.79 |
$0.30 |
$0.27 |
— |
$0.01 |
$1.02 |
| 52 |
UCB Canada Inc. |
$12.97 |
$2.46 |
$0.14 |
$0.26 |
$0.22 |
$9.36 |
$0.06 |
$0.04 |
$0.04 |
$0.05 |
$0.34 |
| 53 |
Mint Pharmaceuticals Inc. |
$12.38 |
$7.54 |
$1.27 |
$0.32 |
$0.59 |
$1.70 |
$0.14 |
$0.45 |
$0.01 |
$0.04 |
$0.32 |
| 54 |
Genzyme Canada a Division of Sanofi-Aventis Canada Inc. |
$12.02 |
— |
$0.40 |
$0.54 |
$1.79 |
$7.30 |
$0.01 |
$0.06 |
— |
$1.20 |
$0.73 |
| 55 |
Genmed a Division of Pfizer Canada Inc. |
$11.45 |
$1.75 |
$0.20 |
$1.63 |
$0.53 |
$6.65 |
$0.10 |
$0.36 |
$0.02 |
$0.03 |
$0.17 |
| 56 |
Aptalis Pharma Canada Inc. |
$11.20 |
$3.39 |
$1.05 |
$0.54 |
$0.56 |
$4.27 |
$0.33 |
$0.28 |
$0.06 |
$0.32 |
$0.38 |
| 57 |
Mylan Specialty L.P. |
$11.18 |
$4.17 |
$0.59 |
$1.13 |
$1.31 |
$2.34 |
$0.14 |
$0.06 |
$0.00 |
$0.04 |
$1.39 |
| 58 |
Hospira Healthcare Corporation |
$10.81 |
$2.43 |
$1.64 |
$0.43 |
$0.70 |
$4.18 |
$0.23 |
$0.38 |
$0.04 |
$0.14 |
$0.65 |
| 59 |
Actavis Specialty Pharmaceuticals Co. |
$9.59 |
— |
$0.08 |
— |
$0.01 |
$8.75 |
$0.10 |
$0.49 |
$0.14 |
$0.00 |
$0.02 |
| 60 |
Ferring Inc. |
$9.53 |
$1.93 |
$0.70 |
$0.70 |
$0.60 |
$4.75 |
$0.22 |
$0.17 |
$0.03 |
$0.13 |
$0.30 |
| 61 |
RB Pharmaceuticals Limited |
$7.01 |
$1.94 |
$0.19 |
$0.01 |
$0.06 |
$4.64 |
$0.02 |
$0.01 |
$0.03 |
— |
$0.11 |
| 62 |
Duchesnay Inc. |
$6.98 |
$2.54 |
$0.11 |
$0.76 |
$0.82 |
$1.36 |
$0.10 |
$0.06 |
$0.01 |
$0.09 |
$1.14 |
| 63 |
Shire Human Genetic Therapies Inc. |
$6.39 |
— |
— |
— |
— |
$6.39 |
— |
— |
— |
— |
— |
| 64 |
Marcan Pharmaceuticals Inc. |
$6.39 |
$1.71 |
$0.62 |
$0.39 |
$0.26 |
$2.99 |
$0.10 |
$0.13 |
$0.03 |
$0.05 |
$0.09 |
| 65 |
Takeda Pharmaceuticals America Inc. |
$6.21 |
$2.18 |
$0.53 |
$0.29 |
$0.11 |
$2.00 |
$0.12 |
$0.11 |
$0.01 |
$0.08 |
$0.78 |
| 66 |
Grifols Therapeutics Inc. |
$6.20 |
$4.20 |
— |
— |
$0.89 |
$1.11 |
— |
— |
— |
— |
— |
| 67 |
Ethypharm Inc. |
$6.06 |
$2.96 |
$0.35 |
$0.05 |
$0.10 |
$1.94 |
$0.08 |
$0.08 |
$0.03 |
$0.11 |
$0.37 |
| 68 |
McNeil Consumer Healthcare Division of Johnson & Johnson Inc. |
$5.22 |
$1.62 |
$0.26 |
$0.07 |
$0.14 |
$0.19 |
$0.00 |
$0.01 |
$0.01 |
$0.04 |
$2.86 |
| 69 |
United Therapeutics Corporation |
$4.62 |
$1.46 |
— |
— |
$0.20 |
$1.97 |
$0.72 |
— |
— |
— |
$0.26 |
| 70 |
Aspen Pharma Trading Limited |
$4.42 |
$0.82 |
$0.30 |
$0.42 |
$0.33 |
$2.34 |
$0.02 |
$0.03 |
$0.00 |
$0.02 |
$0.13 |
| 71 |
Actavis Group PTC ehf |
$4.34 |
$0.32 |
$0.35 |
$0.10 |
$0.55 |
$2.64 |
$0.05 |
$0.10 |
$0.00 |
$0.07 |
$0.16 |
| 72 |
Euro-Pharm International Canada Inc. |
$3.93 |
$0.27 |
$0.02 |
$0.04 |
$0.04 |
$3.38 |
$0.00 |
$0.00 |
— |
$0.00 |
$0.17 |
| 73 |
Abbott Laboratories, Limited |
$3.82 |
$0.87 |
$0.46 |
$0.39 |
$0.43 |
$1.33 |
$0.24 |
$0.02 |
— |
$0.00 |
$0.09 |
| 74 |
Taropharma, a Division of Taro Pharmaceuticals Inc. |
$3.61 |
$0.07 |
$0.19 |
$0.09 |
$0.05 |
$2.97 |
$0.04 |
$0.04 |
$0.01 |
$0.03 |
$0.11 |
| 75 |
Auro Pharma Inc. |
$3.46 |
$0.94 |
$0.22 |
$0.41 |
$0.02 |
$1.38 |
$0.07 |
$0.17 |
$0.03 |
$0.04 |
$0.18 |
| 76 |
Odan Laboratories Ltd. |
$3.41 |
$1.25 |
$0.53 |
$0.14 |
$0.14 |
$0.61 |
$0.17 |
$0.17 |
$0.02 |
$0.21 |
$0.17 |
| 77 |
Jamp Pharma Corporation |
$3.18 |
$0.56 |
$0.17 |
$0.46 |
$0.16 |
$1.30 |
$0.05 |
$0.05 |
$0.04 |
$0.05 |
$0.33 |
| 78 |
Galderma Canada Inc. |
$3.12 |
$0.66 |
$0.14 |
$0.33 |
$0.22 |
$1.22 |
$0.04 |
$0.06 |
$0.01 |
$0.07 |
$0.37 |
| 79 |
Sunovion Pharmaceuticals Canada Inc. |
$3.03 |
$0.07 |
$0.25 |
$0.10 |
$0.03 |
$2.37 |
$0.04 |
$0.08 |
$0.00 |
$0.04 |
$0.04 |
| 80 |
Accel Pharma Inc. |
$2.92 |
$0.40 |
$0.80 |
$0.40 |
$0.63 |
$0.38 |
$0.02 |
$0.01 |
— |
$0.03 |
$0.27 |
| 81 |
Patriot a Division of Janssen Inc. |
$2.82 |
$0.08 |
$0.21 |
$0.06 |
$0.05 |
$2.04 |
$0.09 |
$0.19 |
$0.03 |
$0.05 |
$0.02 |
| 82 |
Sivem Pharmaceuticals ULC |
$2.69 |
— |
$1.19 |
$0.37 |
$0.04 |
— |
$0.02 |
$0.02 |
$0.01 |
— |
$1.04 |
| 83 |
GlaxoSmithKline Consumer Healthcare Inc. |
$2.62 |
$0.62 |
$0.09 |
$0.09 |
$0.03 |
$0.94 |
$0.06 |
$0.01 |
$0.01 |
$0.09 |
$0.68 |
| 84 |
Aurobindo Pharma Limited |
$2.29 |
$0.42 |
$0.12 |
$0.23 |
— |
$1.25 |
$0.07 |
$0.04 |
$0.00 |
$0.01 |
$0.14 |
| 85 |
Mayne Pharma International Pty Ltd. |
$2.26 |
$0.28 |
$0.08 |
$0.18 |
$0.04 |
$1.38 |
$0.04 |
$0.03 |
— |
$0.02 |
$0.22 |
| 86 |
Accord Healthcare Inc. |
$2.18 |
$0.08 |
$0.10 |
$0.00 |
$0.21 |
$1.51 |
$0.07 |
$0.12 |
$0.01 |
$0.05 |
$0.05 |
| 87 |
Merus Labs Luxco S.a.R.L. |
$2.07 |
$0.01 |
$0.28 |
$0.03 |
$0.00 |
$1.68 |
$0.00 |
$0.03 |
— |
$0.01 |
$0.03 |
| 88 |
Dr. Reddy's Laboratories Inc. |
$2.04 |
$0.85 |
$0.28 |
$0.19 |
$0.36 |
$0.27 |
$0.00 |
$0.01 |
$0.00 |
$0.01 |
$0.08 |
| 89 |
Merz Pharmaceuticals GmbH |
$1.57 |
$0.33 |
$0.14 |
$0.03 |
$0.13 |
$0.89 |
$0.01 |
$0.02 |
— |
— |
$0.02 |
| 90 |
ERFA Canada 2012 Inc. |
$1.55 |
$0.64 |
$0.15 |
$0.08 |
$0.10 |
$0.29 |
$0.11 |
$0.08 |
$0.01 |
$0.04 |
$0.06 |
| 91 |
Trimel Pharmaceuticals Corporation |
$1.29 |
$0.74 |
$0.12 |
$0.06 |
$0.15 |
$0.03 |
$0.04 |
$0.07 |
$0.01 |
$0.01 |
$0.06 |
| 92 |
Merus Labs International Inc. |
$1.28 |
$0.43 |
$0.15 |
$0.11 |
$0.02 |
$0.54 |
$0.00 |
$0.00 |
$0.00 |
$0.00 |
$0.02 |
| 93 |
Pfizer Consumer Healthcare a Division of Pfizer Canada Inc. |
$1.12 |
$0.65 |
— |
$0.00 |
$0.01 |
$0.00 |
$0.01 |
$0.01 |
$0.01 |
$0.15 |
$0.28 |
| 94 |
Septa Pharmaceuticals, Inc. |
$1.06 |
$0.79 |
$0.03 |
$0.00 |
— |
$0.23 |
— |
— |
— |
— |
$0.01 |
| 95 |
Dominion Pharmacal |
$1.01 |
$0.01 |
$0.00 |
$0.13 |
$0.00 |
$0.19 |
— |
— |
— |
— |
$0.69 |
| 96 |
Ratiopharm Inc. Division of Teva Canada Limited |
$0.94 |
$0.10 |
$0.06 |
$0.08 |
$0.11 |
$0.45 |
$0.04 |
$0.01 |
$0.00 |
$0.03 |
$0.06 |
| 97 |
SteriMax Inc. |
$0.93 |
$0.56 |
$0.15 |
$0.01 |
$0.03 |
$0.04 |
$0.01 |
$0.01 |
$0.00 |
$0.02 |
$0.09 |
| 98 |
Pro Doc Limitee |
$0.86 |
— |
— |
— |
— |
— |
— |
— |
— |
— |
$0.86 |
| 99 |
Omega Laboratories Ltd. |
$0.79 |
$0.15 |
$0.09 |
$0.01 |
$0.06 |
$0.40 |
$0.02 |
$0.03 |
— |
$0.00 |
$0.02 |
| 100 |
Medtech Products Inc |
$0.78 |
— |
— |
$0.05 |
$0.05 |
$0.25 |
$0.01 |
— |
$0.00 |
$0.01 |
$0.40 |
| |
Total |
$6,646.91 |
$1,103.59 |
$652.15 |
$353.14 |
$424.58 |
$3,431.90 |
$149.47 |
$146.60 |
$27.16 |
$101.46 |
$256.88 |
Note: Drug costs of less than $5,000 appear as $0.00 million due to rounding.
*Total results for the public drug plans reported in this figure.
Data source: National Prescription Drug Utilization Information System Database, Canadian Institute for Health Information.
Appendix J: Glossary
Active beneficiaryFootnote 1:
An individual with at least one claim accepted by a public drug program, either for reimbursement or applied toward a deductible. In Manitoba and Saskatchewan, claimants are also individuals with accepted claims who are eligible for coverage under a provincial drug program but who have not submitted an application and, therefore, do not have a defined deductible.
Anatomical Therapeutic Chemical (ATC):
A classification system that divides drugs into different groups according to the organ or system on which they act and/or their therapeutic and chemical characteristics. It is maintained by the World Health Organization Collaborating Centre for Drug Statistics Methodology. The ATC system is divided into five different levels. The level 1 and 2 are reported in this study, and reflect the anatomical and therapeutic main groups, respectively.
Co-paymentFootnote 1:
The portion of the claim cost that individuals must pay each time they make a claim. This may be a fixed amount or a percentage of the total claim cost. When calculated as a percentage of the total cost, it is also known as “co-insurance.”
DeductibleFootnote 1:
The amount of total drug spending an individual must pay in a given year (or other defined time period) before any part of his or her drug costs will be paid by the drug program. A deductible may be a fixed amount or a percentage of income (income-based deductible).
Dispensing fee:
A professional fee charged by a pharmacist for the dispensing of a prescription and accepted for reimbursement by a public drug plan.
Drivers of drug expenditure:
The level of drug expenditure is determined by many factors or determinants, such as the size and age of the population, the volume and type of drugs used, the price levels, etc. A change in any factor becomes a driver. For example, the changes in the brand versus generic market shares due to the launch of generic products are expected to drive a decline in the level of prescription drug expenditures. On the other hand, expensive emerging therapies are expected to fuel the upward pressure on costs.
Drug cost:
An amount accepted for reimbursement by a public drug plan that reflects the acquisition cost to the pharmacy for a drug, including the wholesale markups, and excluding markups and dispensing fees.
Drug Identification Number (DIN):
A computer-generated eight digit number assigned by Health Canada to a drug product prior to being marketed in Canada. A DIN uniquely identifies the following product characteristics: manufacturer; product name; active ingredient(s); strength(s) of active ingredient(s); pharmaceutical form; route of administration.
Generic drug:
A drug product which is equivalent to a reference or brand-name drug in active ingredient, dosage, form, strength and performance characteristics.
Markup:
An amount accepted for reimbursement by a public drug plan that reflects the difference between the pharmacy retail price and the drug cost.
Multi-Source drug:
A drug product manufactured by two or more companies. Multi-source drugs are available as the original brand-name drug or its generic equivalent.
Patented drug:
A drug product with one or more patents issued by the Commissioner of Patents. A patent may be assigned to the active ingredient, a process to manufacture the drug or another aspect such as a timed-release coating or inhaler mechanism. A patent provides its holder with a monopoly or market exclusivity over the invention for a limited time.
Plan-paid:
An amount that a public drug plan reimburses an eligible beneficiary towards the prescription drug expenditure. It reflects the government–patient cost sharing structure specific to each plan.
Prescription:
A claimFootnote 1 where the drug program accepts at least a portion of the cost, either toward a deductible or for reimbursement. Claims reimbursed by a public drug plan and that relate to pharmacy professional services other than the dispensing of medications (such as the medication review or administration of vaccines) are not included in the analysis.
Prescription drug expenditures:
The sum of the three components of a prescription: drug costs, markups (if applicable) and dispensing fees. These are amounts accepted by a public drug plan towards the deductible or for reimbursement of eligible beneficiaries. Submitted amounts that were not accepted for reimbursement (drug not reimbursed, unit cost above the accepted price, etc.) are not captured in these amounts. The expenditure totals include both the plan-paid and beneficiary-paid amounts, such as co-payments and deductibles.
Prescription size:
The physical quantity of drugs or the number of day supply for which the prescribed drug was dispensed to an eligible beneficiary. The day supply can be used to measure the prescription length.
Public drug plan:
This is a general term used to describe drug plans that are administered by provincial, territorial or federal governments. Examples include the public drug plans analyzed in this report. Public drug plans establish eligibility requirements, cost sharing structures as well as drugs and prices accepted for reimbursement.
Rate of change:
The percent change from one year to another in a drug utilization or expenditure metric. The annual rate of change is calculated over two consecutive years as follows:
[(Value in year 1)/(Value in year 0)] - 1
The compound annual rate of change is calculated over three or more consecutive years as follows:
[(Value in year n)/(Value in year 0)]1/n - 1
Single-source drug:
A drug product manufactured by one company. With a few exceptions, patented drugs are single-source. Some generic drugs are also single-source: a regulatory body may grant a generic drug manufacturer market exclusivity for a period of time; or there may be insufficient demand for more than one market entrant in a therapeutic area with a small patient population.