The Use of Gabapentin in Public Drug Plans
Gabapentin is an anti-epileptic agent, which is also used off-label in Canada to treat peripheral neuropathic pain and a number of other indications. Gabapentin has the potential to be used inappropriately, and anecdotal reports of its misuse have been increasing. The use of this drug is currently monitored and/or limited in some public drug plans (Saskatchewan and the Non-Insured Health Benefits (NIHB) plan). This has prompted NPDUIS to undertake a comprehensive analysis of the use of gabapentin across public drug plans in Canada.
Findings of this analysis are consistent with other studies that suggest that gabapentin is mainly used for the treatment of off-label indications. The analysis also highlights the concomitant use of gabapentin with abuse-prone prescription drugs, such as opioids and benzodiazepines, which may raise some concerns and warrant further study.
Key Findings
The Share of Gabapentin Users Across Select NPDUIS Plans is Increasing
The share of drug plan beneficiaries using gabapentin in 2013 ranged from 2.5% to 5.7%. This represents increase of 0.3% to 2.6% over a 5-year period, depending on the plan. Variations across plans may be due to the disease profile of the beneficiary populations as well as the plan designs, among other things.
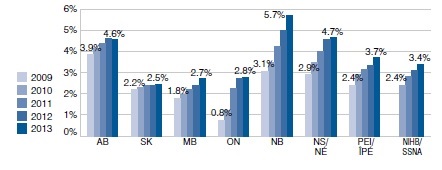
Percentage of active beneficiaries with at least one claim for gabapentin, by year, by public plan
This bar graph gives the percentage of active beneficiaries with at least one claim for gabapentin from 2009 to 2013 in select public plans in Canada. There has been an increase in all jurisdictions. AB - 2009: 3.9%, 2013: 4.6%; SK – 2009: 2.2%, 2013: 2.5%; MB – 2009: 1.8%, 2013: 2.7%; ON – 2009: 0.8%, 2013: 2.8%; NB – 2009: 3.1%, 2013: 5.7%; NS – 2009: 2.9%, 2013: 4.7%; PEI – 2009: 2.4%, 2013: 3.7%; NIHB – 2010: 2.4%, 2013: 3.4%.
Most Gabapentin Use is in the Treatment of Off-label Indications
Despite Health Canada’s sole approved indication as an adjunctive therapy for epilepsy, only 5% to 8% of gabapentin users are believed to use the drug for this condition.
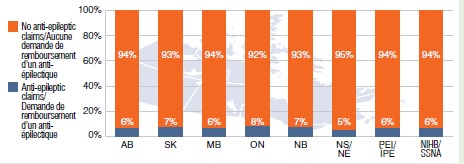
Share of gabapentin users with a claim for an anti-epileptic drug, 2013
This bar graph gives the share of gabapentin users with a claim for an anti-epileptic drug in select public drug plans in 2013. AB: 6%; SK: 7%; MB: 6%; ON: 8%; NB: 7%; NS: 5%; PEI: 6%; NIHB: 6%.
An Important Portion of Gabapentin Users are Treated for Diabetes
Approved indications for gabapentin outside Canada include diabetic neuropathy. The data suggests that (depending on the plan) between 22% and 31% of gabapentin users also claimed drugs used in diabetes.
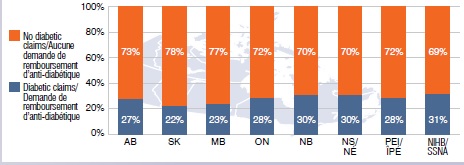
Share of gabapentin users with a claim for a diabetic drug, 2013
This bar graph gives the share of gabapentin users with a claim for a diabetic drug in select public drug plans in 2013. AB: 27%; SK: 22%; MB: 23%; ON: 28%; NB: 30%; NS: 30%; PEI: 28%; NIHB: 31%.
Approximately One Third of New Gabapentin Users Used Opioids* Just Prior to Starting to Use Gabapentin
A large proportion of opioid users continued to use these drugs after starting to use gabapentin (between 67% in SK and 84% for the NIHB). One year later, these users increased the relative potency of their opioid use, as measured in morphine equivalents.
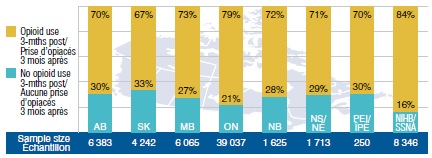
New gabapentin users with opioid use 3-months prior
Two years of study: Q3-2011 to Q2-2013
This bar graph gives the percentage of beneficiaries in select public drug plans that continued to use opioids after being prescribed gabapentin. It measures the percentage of beneficiaries that used an opioid 3 months prior to starting gabapentin and continued to use an opioid in the 3-month post period (study period: Q3-2011 to Q2-2013). Sample sizes are given in parentheses. AB: 70% (6,383); SK: 67% (4,242); MB: 73% (6,065); ON: 79% (39,037); NB: 72% (1,625); NS: 71% (1,713); PEI: 70% (250); NIHB: 84% (8,346).
Up to One Quarter of New Gabapentin Users Used Benzodiazepines* Just Prior to Starting to Use Gabapentin
A large proportion of benzodiazepine users continued to use these drugs after starting to use gabapentin (between 67% in SK and 81% for the NIHB).
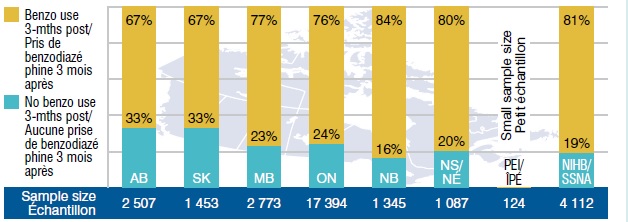
New gabapentin users with benzodiazepine use 3-months prior
Two years of study: Q3-2011 to Q2-2013
This bar graph gives the percentage of beneficiaries in select public drug plans that continued to use benzodiazepine after being prescribed gabapentin. It measures the percentage of beneficiaries that used benzodiazepine 3 months prior to starting gabapentin and continued to use benzodiazepine in the 3-month post period (study period: Q3-2011 to Q2-2013). Sample sizes are given in parentheses. AB: 67% (2,507); SK: 67% (1,453); MB: 77% (2,773); ON: 76% (17,394); NB: 84% (1,345); NS: 80% (1,087); PEI: too small to measure (124); NIHB: 81% (4,112).
*The use of gabapentin concomitantly with opioids and benzodiazepines may raise some concerns given the potential for inappropriate use related to the latter two drug categories.
Data Source: NPDUIS, Canadian Institute for Health Information Database.
NPDUIS is a research initiative that operates independently of the regulatory activities of the PMPRB.