2013-14
Departmental Performance Report
Patented Medicine Prices Review Board
The Honourable Rona Ambrose
Minister of Health
Catalogue number: H79-2/2014F-PDF
ISSN: 2368-1063
Table of Contents
Foreword
Chairperson's Message
Section I: Organizational Expenditure Overview
Section II: Analysis of Program(s) by Strategic Outcome
Section III: Supplementary Information
Section IV: Organizational Contact Information
Appendix: Definitions
Endnotes
Foreword
Departmental Performance Reports are part of the Estimates family of documents. Estimates documents support appropriation acts, which specify the amounts and broad purposes for which funds can be spent by the government. The Estimates document family has three parts.
Part I (Government Expenditure Plan) provides an overview of federal spending.
Part II (Main Estimates) lists the financial resources required by individual departments, agencies and Crown corporations for the upcoming fiscal year.
Part III (Departmental Expenditure Plans) consists of two documents. Reports on Plans and Priorities (RPPs) are expenditure plans for each appropriated department and agency (excluding Crown corporations). They describe departmental priorities, strategic outcomes, programs, expected results and associated resource requirements, covering a three-year period beginning with the year indicated in the title of the report. Departmental Performance Reports (DPRs) are individual department and agency accounts of actual performance, for the most recently completed fiscal year, against the plans, priorities and expected results set out in their respective RPPs. DPRs inform parliamentarians and Canadians of the results achieved by government organizations for Canadians.
Additionally, Supplementary Estimates documents present information on spending requirements that were either not sufficiently developed in time for inclusion in the Main Estimates or were subsequently refined to account for developments in particular programs and services.
The financial information in DPRs is drawn directly from authorities presented in the Main Estimates and the planned spending information in RPPs. The financial information in DPRs is also consistent with information in the Public Accounts of Canada. The Public Accounts of Canada include the Government of Canada Consolidated Statement of Financial Position, the Consolidated Statement of Operations and Accumulated Deficit, the Consolidated Statement of Change in Net Debt, and the Consolidated Statement of Cash Flow, as well as details of financial operations segregated by ministerial portfolio for a given fiscal year. For the DPR, two types of financial information are drawn from the Public Accounts of Canada: authorities available for use by an appropriated organization for the fiscal year, and authorities used for that same fiscal year. The latter corresponds to actual spending as presented in the DPR.
The Treasury Board Policy on Management, Resources and Results Structures further strengthens the alignment of the performance information presented in DPRs, other Estimates documents and the Public Accounts of Canada. The policy establishes the Program Alignment Architecture of appropriated organizations as the structure against which financial and non-financial performance information is provided for Estimates and parliamentary reporting. The same reporting structure applies irrespective of whether the organization is reporting in the Main Estimates, the RPP, the DPR or the Public Accounts of Canada.
A number of changes have been made to DPRs for 2013-14 to better support decisions on appropriations. Where applicable, DPRs now provide financial, human resources and performance information in Section II at the lowest level of the organization’s Program Alignment Architecture.
In addition, the DPR's format and terminology have been revised to provide greater clarity, consistency and a strengthened emphasis on Estimates and Public Accounts information. As well, departmental reporting on the Federal Sustainable Development Strategy has been consolidated into a new supplementary information table posted on departmental websites. This new table brings together all of the components of the Departmental Sustainable Development Strategy formerly presented in DPRs and on departmental websites, including reporting on the Greening of Government Operations and Strategic Environmental Assessments. Section III of the report provides a link to the new table on the organization’s website. Finally, definitions of terminology are now provided in an appendix.
Chairperson's Message
I am pleased to present the 2013–14 Departmental Performance Report for the Patented Medicine Prices Review Board (PMPRB).
The PMPRB’s objective to ensure that Canadians do not pay excessive prices for patented medicines is an important one which contributes to the government-wide goal of healthy Canadians.
Over the past fiscal year the PMPRB has focused on improving its programs by monitoring the impact of the Guidelines’ changes and on publishing studies that account for the latest market trends and reflect the immediate interests of both public and private payers. The PMPRB also pursued its outreach activities, expanded and diversified the exchanges with its stakeholders. In addition, the PMPRB became more active and involved with its federal, provincial and international partners.
In the spirit of the Government’s Red Tape Reduction Action Plan, the Board concluded consultations on two initiatives to reduce regulatory burden. First, the Board has simplified the Consumer Price Index (CPI) Adjustment Methodology. The simplified methodology will be implemented in 2015. Second, the Board proposed amendments to the Patented Medicines Regulationsi to move from twice to once a year filing. Work is underway to pre-publish the proposed regulatory amendments in Part I of the Canada Gazette for formal consultation in 2014-15.
Three Board decisions were subject to judicial review by the Federal Court: ratio-Salbutamol HFA (T-1058-11; T-1825-11); ratiopharm Inc. (now Teva Canada) (T-1252-11); and Sandoz Canada Inc. (T-1616-12). The Court heard these matters in November 2013 and released its decisions on May 27, 2014. The Federal Court allowed the applications for judicial review and referred the matters back to the Board with a direction that it find that ratiopharm Inc. and Sandoz Canada Inc. are not patentees for the purpose of Section 79 of the Patent Act and thus outside the Board’s jurisdiction. The Office of the Attorney General filed the Notices of Appeal in respect of these decisions on June 25, 2014.
Despite new challenges and emerging issues, the PMPRB continues to put the protection of consumer interests first, while recognizing the value that innovative medicines offer to patients.
This is only made possible through the collaborative efforts and dedication of the Board Members and its Staff.
Mary Catherine Lindberg,
Chairperson
Section I: Organizational Expenditure Overview
Organizational Profile
Appropriate Minister: The Honourable Rona Ambrose
Institutional Head: Mary Catherine Lindberg, Chairperson
Ministerial Portfolio: Health
Enabling Instrument(s): Patent Actii and Patented Medicines Regulationsiii
Year of Incorporation / Commencement: 1987
Other: The Minister of Health is responsible for the pharmaceutical provisions of the Patent Act (Act) set out in sections 79 to 103. The Patented Medicine Prices Review Board (PMPRB) is part of the Health Portfolio, which also includes Health Canada, the Public Health Agency of Canada, the Canadian Institutes of Health Research and the Canadian Food Inspection Agency. The Health Portfolio supports the Minister of Health in maintaining and improving the health of Canadians.
Although part of the Health Portfolio, the PMPRB carries out its mandate at arm's length from the Minister. It also operates independently of other bodies such as Health Canada, which authorizes the sale of drugs in Canada after their assessment for safety, efficacy and quality; federal, provincial and territorial (F/P/T) public drug plans, which are responsible for listing and reimbursement decisions for their respective plans; and the Common Drug Review, administered by the Canadian Agency for Drugs and Technologies in Health (CADTH), which provides listing recommendations to participating public drug plans based on cost-effectiveness.
Organizational Context
Raison d’être
The PMPRB is an independent, quasi-judicial body created by Parliament in 1987. Its mandate is two-fold:
- Regulatory – to ensure that prices charged by patentees for patented medicines sold in Canada are not excessive; and
- Reporting – to report on pharmaceutical trends of all medicines and on research and development (R&D) spending by pharmaceutical patentees.
In carrying out its mandate, the PMPRB ensures that Canadians are protected from excessive prices for patented medicines sold in Canada and that stakeholders are informed on pharmaceutical trends.
Responsibilities
The PMPRB was created as a result of amendments to the Patent Act (Act) in 1987 (Bill C-22), and its remedial powers were strengthened by further amendments in 1993 (Bill C-91). These amendments were part of policy reforms intended to balance the PMPRB’s consumer protection mandate with patent protection measures intended to encourage the research and development efforts of pharmaceutical patentees.
The PMPRB has a dual mandate:
Patented Medicine Price Regulation
The PMPRB is responsible for ensuring the factory-gate prices that patentees charge for prescription and non-prescription patented medicines sold in Canada to wholesalers, hospitals, pharmacies or others, for human and veterinary use, are not excessive. The PMPRB regulates the price of each patented medicine to which Health Canada has assigned a Drug Identification Number (DIN) as part of its review process. The Board's mandate also includes medicines that are available under the Special Access Programme; through a Clinical Trial Application; and Investigational New Drug Products. Over-the-counter (OTC) patented medicines and patented medicines for veterinary use are regulated by the Board on a complaints basis.
In the event that the Board finds, after a public hearing, that the price of a patented medicine is or was excessive in any market, it may order the patentee to reduce the price and take measures to offset any excess revenues it may have received.
Pharmaceutical Trends Reporting
The PMPRB reports annually to Parliament through the Minister of Health on its price review activities, the prices of patented medicines and price trends of all prescription drugs, and on the R&D expenditures reported by pharmaceutical patentees. In addition, as a result of the establishment of the National Prescription Drug Utilization Information System (NPDUIS)iv by F/P/T ministers of health in September 2001, the PMPRB conducts critical analysis of price, utilization, and cost trends for patented and non-patented prescription drugs so that Canada’s health system has more comprehensive, accurate information on how all prescription drugs are being used and on the sources of cost increases. This function is aimed at providing F/P/T governments and other interested stakeholders with a centralized credible source of information on pharmaceutical trends.
Strategic Outcome and Program Alignment Architecture
1. Strategic Outcome: Canadians are protected from excessive prices for patented medicines sold in Canada and stakeholders are informed on pharmaceutical trends.
1.1 Program: Patented Medicine Prices Review Program
1.2 Program: Pharmaceutical Trends Program
Internal Services
Organizational Priorities
| Priority |
Type1 |
Strategic Outcome [and/or] Program(s) |
| Continue implementation of the Management Action Plan in response to the PMPRB Program Evaluation Report |
Previously committed to |
Links to Program 1 & Program 2 |
| Summary of Progress |
What progress has been made toward this priority?
The Management Action Plan in response to the PMPRB's Program Evaluation Report was intended to address the four considerations identified to the Board.
Expedite PMPRB processes
Implement performance targets for selected processes/procedures
- The PMPRB's performance results against its service standards are measured over the course of the calendar year because the information filings it receives are based on price and sales information for a calendar year.
- The performance results for the 2013 calendar year for each of the following service standard are posted on its website:
- Service Standard for the scientific review of new patented drug productsv – which establishes the timeframe for sending reports from the Human Drug Advisory Panel (HDAP) to the patentee
- Service Standard for the price review of new patented drug productsvi – which establishes timeframes for the communication of the results of the price review for new patented drug products
- Service Standard for the price review of existing patented drug productsvii – which establishes timeframes for the provision of Form 2 and the communication of the results of the price review for existing patented drug products
Improve timeliness of reporting
The PMPRB has launched an inter-branch working group to enhance its understanding of the drivers behind the trends reported in its Annual Report and the Guidelines Monitoring and Evaluation Plan (GMEP). The work of the Working Group will strengthen the breadth of information available for decision making and facilitate a more proactive monitoring of relevant trends.
In addition, the PMPRB is planning to hold a researchers' forum in August 2014 as part of the NPDUIS initiative. The objective of the researchers' forum is to engage with key academics working in the area of pharmaceutical economic research and evidence based policy. The forum will provide for an exchange of information on upcoming NPDUIS research and the work participating researchers are undertaking. The forum will be used to elevate the profile of the NPDUIS work, and build peer relationships with key opinion leaders in the area. The forum will also feed into identifying future research priorities for NPDUIS.
In 2013-14, the PMPRB, under the NPDUIS initiative released five reports:
Further simplify the Guidelines
Continue to assess the application and impact of changes made to the Guidelines
- In December 2013, Board Staff presented the third annual assessment under the Monitoring and Evaluation Plan for the Major Changes to the Guidelines, 2013 (GMEP 2013)xiii to the Board. The results of the GMEP for the last three years can be found on the PMPRB website under Act and Regulations.
- The Board reviewed comments submitted on the October 2013 Notice and Comment on the CPI-Adjustment Methodology initiative. The Board approved a lagged CPI-Adjustment Methodology, replacing a forecast CPI methodology with actual when calculating the CPI-Adjustment Factor for the forecast period. This New Lagged CPI-Adjustment Methodology will be implemented on January 1, 2015.
Expand plain language use throughout PMPRB publications
All NPDUIS reports released in 2013-14 included an Executive Summary
Expand target audience of outreach efforts
In 2013, the PMPRB continued to enhance its non-industry stakeholder engagement through bi-lateral exchanges with F/P/T health representatives, third-party payers and other groups. In addition, the PMPRB began working on a researchers' forum as part of the NPDUIS initiative. The objective of the researchers' forum is to engage with key academics working in the area of pharmaceutical economic research and evidence based policy. The PMPRB also joined the Pharmaceutical Pricing and Reimbursement Information (PPRI) network. The PPRI network is a World Health Organization initiative for networking and information-sharing on issues of pharmaceutical pricing and reimbursement. The PPRI network consists of more than 60 members, mainly competent authorities and third party payers from a total of 38 countries.
|
| Priority |
Type |
Strategic Outcome [and/or] Program(s) |
| Decrease regulatory burden and make effective use of Board Staff resources |
Previously committed to |
Links to Program 1 & Program 2 |
| Summary of Progress |
What progress has been made toward this priority?
Examine the feasibility of changing to one regulatory filing for existing patented medicines by patentees per year
The PMPRB is moving forward with an initiative to reduce the filing requirement on patentees.
Examine the PMPRB's Consumer Price Index (CPI) Adjustment Methodology
The Board reviewed comments submitted by stakeholders on the October 2013 Notice and Comment on the CPI-Adjustment Methodology initiative. The Board approved a lagged CPI-Adjustment Methodology, replacing a forecast CPI methodology with actual when calculating the CPI-Adjustment Factor for the forecast period. This New Lagged CPI-Adjustment Methodology will be implemented on January 1, 2015.
Consider options for more effective use of Board Staff resources through the Succession Planning and Knowledge Transfer initiative
In 2012-13 the PMPRB hired a consultant to undertake a comprehensive consultation with key staff, to establish forums for employees to share leadership experiences and challenges, as well as to conduct an in-depth analysis of the current organization structure. The report which was finalized in early 2013-14 provided considerations which focused on Staff Development, Succession Planning and possible Organizational Design Changes.
Staff Development
- In 2013-14 the PMPRB provided its employees access to skills development and knowledge transfer by offering a number of developmental assignments (acting assignments and across branch job exchanges).
Succession Planning
- The PMPRB has done an analysis of the Key Position Profile for members of the Senior Management team. A baseline understanding of future challenges resulting from planned and anticipated retirements and possible short and long term vacancies in key positions was obtained.
|
Risk Analysis
Key Risks
| Risk |
Risk Response Strategy |
Link to Program Alignment Architecture |
| A shortage in the number of Board Members may impact the timeliness of the hearing process and the appropriate skills mix |
In July 2014, the fifth Board Member was appointed. The PMPRB now has its full complement Board Members. |
1.1 – Patented Medicine Price Review Program |
| The evolution of patented medicines into more complex and innovative areas may hinder the effectiveness of the PMPRB's ability to deliver its mandate |
The Board continues to assess and consider potential modifications to its Guidelines so that they remain effective both in delivering its mandate and promoting voluntary compliance on the part of patentees. |
1.1 – Patented Medicine Price Review Program |
| Adverse judicial review outcomes |
The PMPRB is currently reviewing the potential impact of two recent Federal Court decisions relating to the Board's jurisdiction over certain generic drugs. |
1.1 – Patented Medicine Price Review Program |
| Outdated and irrelevant reporting |
In 2013/14 the PMPRB has increasingly focused on strategies to improve the efficiency and relevance of its reporting mandate. Release of regular reports will be standardized and announced in advance in the NPDUIS Research Agenda. In order to ensure relevance of NPDUIS reporting, the PMPRB has embarked on a broader engagement strategy to identify research priorities and emerging areas of interest. The PMPRB has increased its outreach efforts with key stakeholders including academics, federal health portfolio partners, government policy and research communities, the pharmaceutical and insurance industry as well as health professionals, patient advocacy and consumer groups. |
1.2 – Pharmaceutical Trends Program |
| Difficulty attracting and retaining highly specialized subject matter experts |
The PMPRB is developing an action plan from the 2013-14 report on succession planning. It is also increasing the remuneration paid to the Human Drug Advisory Panel (HDAP) members to make it more competitive with that paid by other public agencies for similar work. |
1.1 – Patented Medicine Price Review Program
1.2 – Pharmaceutical Trends Program |
The Board consists of not more than five part-time members, including a Chairperson and a Vice-Chairperson. The Chairperson is designated under the Act as the Chief Executive Officer of the PMPRB, with the authority and responsibility to supervise and direct its work.
The Members of the Board are responsible for the implementation of the applicable provisions of the Act. Together, they establish the guidelines, rules, by-laws and other policies of the Board provided by the Act and consult as necessary with stakeholders including the Minister of Health and representatives of consumer groups, the pharmaceutical industry and provincial and territorial governments and, when needed, conduct hearings.
Appointments to the Board are recommended to the Privy Council Office by the Minister of Health. In July 2014, a fifth Board Member was appointed with the result that the PMPRB now has its full complement of Board Members.
In recent years, the high price and utilization of new patented drug products, in particular biologics with new indications have raised questions about the need for subsequent additional price reviews for these products. Through its Guidelines Monitoring and Evaluation Plan and its work with F/P/T drug plan representatives and other stakeholders, the Board will explore these issues in greater depth.
In 2015, the PMPRB will implement a new CPI Adjustment Methodologyxiv that replaces the forecast CPI with actual CPI to calculate the CPI Adjustment Factor for the forecast period.
On May 27, 2014, the Federal Court issued its decisions on the applications for judicial review of two of the Board’s decisions stemming from hearings. In both cases, the Federal Court ruled that the company was not a “patentee” within the meaning of Section 79 of the Act and therefore did not fall under the jurisdiction of the Board. While court decisions pose a risk they also provide an opportunity for clarification to patentees and Board Staff with respect to key legislative and regulatory provisions and the application of the Board’s related Guidelines. The Attorney General filed Notices of Appeal in respect of these decisions on June 25, 2014.
As the federal expert body on questions related to drug prices, the PMPRB, via its Pharmaceutical Trends Program, contributes to informed decision-making by reporting on pharmaceutical trends. In addition, the PMPRB undertakes studies and conducts analysis on a variety of topics related to pharmaceutical pricing and costs. Through critical analyses of price, utilization and cost trends conducted under the NPDUIS initiative, the PMPRB provides Canada’s health system with comprehensive and accurate information on how prescription drugs are being used and on cost drivers. In 2013/14 the PMPRB has increasingly focused on strategies to improve the efficiency and relevance of its reporting mandate. Release of regular reports will be standardized and announced in advance in the NPDUIS Research Agenda. In order to ensure relevance of NPDUIS reporting, the PMPRB has embarked on a broader engagement strategy to identify research priorities and emerging areas of interest. The PMPRB has increased its outreach efforts with key stakeholders including academics, federal health portfolio partners, government policy and research communities, pharmaceutical and insurance industry as well as health professionals, patient advocacy and consumer groups. In 2013-14, the PMPRB released five new studies.
In accordance with the TB Directive on Performance Management, the PMPRB is committed to sustaining a culture of high performance. In March 2014, the PMPRB began establishing an employee performance management program including annual written performance assessments for all employees; mid-year reviews will begin in 2014-15. The PMPRB has also undertaken a review of its Employee Recognition Policy and will revise it as necessary to meet the new government requirements. In addition, the PMPRB is committed to establishing a Talent Management Plan documenting learning opportunities and assignments for the development of an employee’s potential career advancement and increased responsibility. The PMPRB has already introduced initiatives to enhance the development of its employees through internal assignments and cross-training programs to build the required skill set within the organization, as well as it has incorporated succession planning in its business planning activities.
Actual Expenditures
Budgetary Financial Resources (dollars)
2013–14
Main Estimates |
2013–14
Planned Spending |
2013–14
Total Authorities
Available for Use |
2013–14
Actual Spending
(authorities used) |
Difference
(actual minus planned) |
| 10,944,073 |
11,178,573 |
*14,235,359 |
10,540,567 |
(638,006) |
* The variance between Main Estimates and Total Authorities Available for Use is largely due to additional funding received through an adjustment warrant to cover the amount ordered by the Federal Court to refund to a patentee. The Federal Court quashed a Board Order and directed in its judgement that a payment of excess revenues in the sum of $2,801,285 be returned by the PMPRB to the patentee with appropriate interest and specified costs.
The Main Estimates and Total Authorities Available for Use amounts include $2,470,000 in a Special Purpose Allotment (SPA). The SPA can only be used to cover the costs of public hearings such as, external legal counsel, expert witnesses, etc. Any unspent amount is returned to the Consolidated Revenue Fund (CRF). In 2013-14 SPA spending was $60,641. |
Human Resources (Full-Time Equivalents [FTEs])
2013–14
Planned |
2013–14
Actual |
2013–14
Difference
(actual minus planned)
|
| 74.0 |
55.4 |
(18.6) |
| The PMPRB determined that as a result of efficiencies gained through various initiatives, it was in a position to leave some positions vacant without significantly impacting its operations at this time.
|
Budgetary Performance Summary for Strategic Outcome and Program(s) (dollars)
| Strategic Outcome, Program(s) and Internal Services |
2013–14 Main Estimates |
2013–14 Planned Spending |
2014–15 Planned Spending |
2015–16 Planned Spending |
2013–14 Total Authorities Available for Use |
2013–14 Actual Spending (authorities used) |
2012–13 Actual Spending (authorities used) |
2011–12 Actual Spending (authorities used) |
| Strategic Outcome 1: Canadians are protected from excessive prices for patented medicines sold in Canada and stakeholders are informed on pharmaceutical trends.
|
| Patented Medicine Price Review Program |
*6,781,301 |
6,781,301 |
6,827,010 |
6,827,010 |
9,669,981 |
**6,395,602 |
3,888,795 |
7,346,773 |
| Subtotal |
6,781,301 |
6,781,301 |
6,827,010 |
6,827,010 |
9,669,981 |
6,395,602 |
3,888,795 |
7,346,773 |
| Pharmaceutical Trends Program |
1,328,833 |
1,328,833 |
1,267,557 |
1,267,557 |
1,314,847 |
1,146,790 |
983,279 |
1,010,528 |
| Subtotal |
1,328,833 |
1,328,833 |
1,267,557 |
1,267,557 |
1,314,847 |
1,146,790 |
983,279 |
1,010,528 |
| Internal Services Subtotal |
2,833,939 |
3,068,439 |
2,832,463 |
2,832,463 |
3,250,531 |
2,998,175 |
3,184,729 |
3,397,074 |
| Total |
10,944,073 |
11,178,573 |
10,927,030 |
10,927,030 |
14,235,359 |
10,540,567 |
8,056,803 |
11,754,375 |
* The 2013-14 Main Estimates amount allocated to the Patented Medicine Price Review Program includes $2,470,000 funding for the SPA.
** Actual Spending (authorities used) for the Patented Medicine Price Review Program includes the sum of $2,801,285 plus appropriate interest and specified costs the Federal Court directed the PMPRB to return to the patentee when it quashed a Board Order.
The PMPRB determined that as a result of efficiencies gained through various initiatives, it was in a position to leave some positions vacant without significantly impacting its operations at this time.
|
Alignment of Spending With the Whole-of-Government Framework
Alignment of 2013-14 Actual Spending With the Whole-of-Government Frameworkxv (dollars)
| Strategic Outcome |
Program |
Spending Area |
Government of Canada Outcome |
2013–
14 Actual Spending |
| Canadians are protected from excessive prices for patented medicines sold in Canada and stakeholders are informed on pharmaceutical trends. |
1.1 Patented Medicine Prices Regulation Program |
Social Affairs |
Healthy Canadians |
6,395,602 |
| 1.2 Pharmaceutical Trends Program |
Social Affairs |
Healthy Canadians |
1,146,790 |
Total Spending by Spending Area (dollars)
| Spending Area |
Total Planned Spending |
Total Actual Spending |
| Economic Affairs |
|
|
| Social Affairs |
8,110,134 |
7,542,392 |
| International Affairs |
|
|
| Government Affairs |
|
|
Departmental Spending Trend
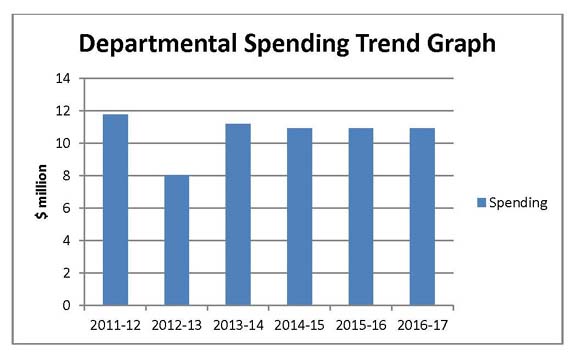
Actual Spending for 2011–12 is significantly higher than spending for fiscal year 2012-13, because the Federal Court quashed a Board decision and ordered the PMPRB to refund $2,559.8 thousand paid to the Crown as a result of a Board Order.
In 2012–13, the PMPRB lapsed approximately $3 million in SPA funding, which is used, as needed, to conduct Public Hearings, as well as $776 thousand of its operating budget. These lapses include the $774 thousand of savings gained through operational efficiencies and streamlining processes. In 2012-13 total SPA funding was $3.1 million. In 2013-14, funding for the SPA was reduced to $2,470 thousand. The SPA can only be used to cover the costs of public hearings, such as external legal counsel, expert witnesses, etc. Any unspent amount is returned to the CRF.
In 2013-14, the Federal Court quashed a Board decision and the PMPRB was ordered to refund the patentee $ 2,801 thousand which was paid to the Crown as a result of a Board Order.
Planned Spending for subsequent years is based on the assumption that the entire SPA funding will be spent each fiscal year.
Estimates by Vote
For information on the Patented Medicine Prices Review Board’s organizational Votes and statutory expenditures, consult the Public Accounts of Canada 2014 on the Public Works and Government Services Canada website.xvi
Section II: Analysis of Program(s) by Strategic Outcome
Strategic Outcome:
Canadians are protected from excessive prices for patented medicines sold in Canada and stakeholders are informed on pharmaceutical trends.
Performance Measurement
| Performance Indicators |
Targets |
Actual Results |
| Canada's prices on average are in line with the seven comparator countries listed in the Regulations. |
Canada's prices on average are at or below the median of international prices. |
Canadian prices are slightly below the average ratio of the median international prices observed among the comparator countries. |
The PMPRB's Annual Report provides detailed statistics comparing the foreign prices of patented medicines to their Canadian prices. For many years, the PMPRB has reported average foreign-to-Canadian price ratios with foreign prices converted to their Canadian dollar equivalents by means of market exchange rates. (Specifically, the 36-month moving averages of market rates the PMPRB normally used in applying its Guidelines.) Focusing on the results with currency conversion at market exchange rates, Canadian prices, on average remain below median international prices (i.e., a ratio of 1.06 to 1). At the same time, with the exception of the U.S.-to-Canada ratios all bilateral average price ratios have fallen substantially in recent years. To put this trend in historical perspective, in 2005, Canadian prices were, on average, approximately equal to or below corresponding prices in all comparators other than Italy. By 2013, Canadian prices were decidedly above prices in the UK, France and Italy, and somewhat higher than prices in Sweden and Switzerland. As in previous years, prices reported for the United States were much higher than prices in Canada or any other comparator country.
Program 1.1: Patented Medicine Price Regulation Program
Description
The PMPRB is an independent quasi-judicial body that is responsible for ensuring that the prices that patentees charge for patented medicines sold in Canada are not excessive based on the price review factors in the Act. To make this determination the Board must consider each of the following factors: prices at which the medicine and other medicines in the same therapeutic class have been sold in Canada and in the seven comparator countries listed in the Regulations; changes in the Consumer Price Index (CPI); and in accordance with the Act, such other factors as may be specified in any regulations made for the purposes of the price review. Under the Act, and as per the Regulations, patentees are required to file price and sales information for each patented medicine sold in Canada, for the duration of the patent(s). Board Staff reviews the introductory and ongoing information filed by patentees, for all patented medicines sold in Canada. When it finds that the price of a patented medicine appears to be excessive, Board Staff will conduct an investigation into the price. An investigation could result in: its closure where it is concluded that the price was non-excessive; a Voluntary Compliance Undertaking (VCU) by the patentee to reduce the price and offset excess revenues obtained as a result of excessive prices through a payment and/or a price reduction of another patented drug product; or a public hearing to determine if the price is excessive, including any remedial order determined by the Board. In the event that the Board Hearing Panel finds, after a public hearing, that a price is or was excessive, it may order the patentee to reduce the price and take measures to offset any excess revenues. This program, by reviewing the prices charged by patentees for patented medicines sold in Canada, protects Canadians and the health care system from excessive prices.
Budgetary Financial Resources (dollars)
| 2013–14 Main Estimates |
2013–14 Planned Spending |
2013–14 Total Authorities Available for Use |
2013–14 Actual Spending (authorities used) |
2013–14 Difference(actual minus planned) |
| 6,781,301 |
6,781,301 |
9,669,981 |
6,395,602 |
(385,699) |
Human Resources (Full-Time Equivalents [FTEs])
| 2013–14 Planned |
2013–14 Actual |
2013–14 Difference(actual minus planned) |
| 44.0 |
25.9 |
(18.1) |
| The PMPRB determined that as a result of efficiencies gained through various initiatives, it was in a position to leave some positions vacant without significantly impacting its operations at this time. |
Performance Results
| Expected Results |
Performance Indicators |
Targets |
Actual Results |
| Patentees comply with the Patent Act, the Regulations and the Excessive Price Guidelines (Guidelines) |
Percentage of patented medicines that are priced, as a result of voluntary compliance, within the Guidelines or at a price which does not trigger the investigation criteria. |
95% of patented medicines are voluntarily priced within the Guidelines or at a price which does not trigger the investigation criteria. |
94.0% of all patented medicines reported to the PMPRB are voluntarily priced within the Guidelines or at a price which does not trigger the investigation criteria. |
| Percentage of patented medicines that are subject to a Board Order |
100% of Board Orders are complied with |
100% of Board Orders were complied with |
Performance Analysis and Lessons Learned
Patentees are to ensure that the prices of their patented drug products are within the Board’s Guidelines during all periods in which the drug products are under the PMPRB's jurisdiction.
Under the Guidelines, patentees are given an opportunity to submit a VCU when Board Staff concludes, following an investigation, that the price for a patented drug product sold in Canada appears to have exceeded the Guidelines. A VCU can also be submitted by a patentee after a Notice of Hearing is issued. A VCU is a written undertaking by a patentee to adjust its price to conform to the Board’s Guidelines.
In 2013, Board Staff completed price reviews of 1,341 of the 1,343 new and existing patented medicines sold in Canada for human use reported to the PMPRB. As at March 31, 2014, two patented medicines were still under review. Of the total patented medicines reported to the PMPRB, 1,098 were priced within the Guidelines and 164 were priced at a price which did not trigger the investigation criteria – 94.0% of the patented medicines were voluntarily priced within the Guidelines or at a price which did not trigger the investigation criteria. This is slightly higher than the 92.5% reported last year in the DPR.
Since the implementation of the new Guidelines in January 2010, the percentage of patented medicines voluntarily priced within in the Guidelines or at a price which does not trigger the investigation criteria has been over 90%: 91.1% in 2010; 94.6% in 2011; 92.5% in 2012; and, 94.0% in 2013.
In its 2012 Annual Report, the PMPRB began reporting the number of VCUs accepted by the Chairperson in the Status of Price Reviews table.
When the number of VCUs is included as part of the calculation of the percentage of all patented medicines reported to the PMPRB that are voluntarily priced within the Guidelines or at a price which does not trigger the investigation criteria the percentage of voluntary compliance moves from 92.5% to 94.9% in 2012, and from 94.0% to 94.9% for 2013
Program 1.2: Pharmaceutical Trends Program
Description
The PMPRB reports annually to Parliament through the Minister of Health on its price review activities, the prices of patented medicines and price trends for all drugs, and R&D expenditures as reported by pharmaceutical patentees. In supporting this requirement, the Pharmaceutical Trends Program provides complete and accurate information on trends in manufacturers’ prices of patented medicines sold in Canada and on patentees’ research-and-development expenditures to interested stakeholders including: industry (i.e., brand-name, biotech, generic); F/P/T governments; consumer and patient advocacy groups; third party payers; and others. This information also provides assurance to Canadians that the prices of patented medicines are not excessive. In addition, as a result of the establishment of the NPDUIS by F/P/T ministers of health, the Minister of Health requested that the PMPRB conduct analysis of price, utilization and cost trends for prescription drugs so that Canada’s health system has comprehensive, accurate information on how prescriptions drugs are being used and on the sources of cost increases. The PMPRB publishes specific NPDUIS reports based on the research and reporting priorities identified by the NPDUIS Steering Committee.
Budgetary Financial Resources (dollars)
| 2013–14 Main Estimates |
2013–14 Planned Spending |
2013–14 Total Authorities Available for Use |
2013–14 Actual Spending (authorities used) |
2013–14 Difference (actual minus planned) |
| 1,328,833 |
1,328,833 |
1,314,847 |
1,146,790 |
(182,043) |
Human Resources (FTEs)
| 2013–14 Planned |
2013–14 Actual |
2013–14 Difference (actual minus planned) |
| 11.0 |
7.2 |
(3.8) |
| The PMPRB determined that as a result of efficiencies gained through various initiatives, it was in a position to leave some positions vacant without significantly impacting its operations at this time. |
Performance Results
| Expected Results |
Performance Indicators |
Targets |
Actual Results |
| Stakeholders are more aware of pharmaceutical trends and cost drivers |
Number of website hits |
5% increase in requests/hits |
Not achieved |
| Number of presentations by PMPRB at external meetings |
10 events per year |
Participated in 26 external events and made presentations at 8 |
Performance Analysis and Lessons Learned
Patentees are required under the Regulations to submit detailed information on their sales of patented drug products, including quantities sold and net revenues received for each product by class of customer in each province/territory. The PMPRB uses this information to analyze trends in sales, prices and utilization of patented drug products. (Additional information on key statistical results from this analysis can be found in the PMPRB’s 2013 Annual Report which is posted on its website.)
In addition, the Act mandates the PMPRB to monitor and report on pharmaceutical research and development (R&D) spending, while giving the PMPRB no regulatory authority to consider the amount or type of patentees’ research spending in the context of its pricing regulations. (Additional information relating to key statistics on the current state of pharmaceutical research investment in Canada can be found in the PMPRB’s 2013 Annual Report which is posted on its website.)
In 2013-14, the PMPRB did not achieve its target of a 5% increase in the number website hits. However, it has seen a steady increase (59% from April 2013 to March 2014) in the number of followers to its Twitter.
All PMPRB publications, including studies, Board decisions and reference documents, are available on the PMPRB website.
In 2013-14, the PMPRB organized numerous meetings with stakeholders in order to consult on its research projects and promote the results of its studies, including the Canadian Generic Pharmaceutical Association, the Canadian Pharmacist Association, the Canadian Agency for Drugs and Technologies in Health (CADTH), the Canadian Diabetes Association and the Canadian Life and Health Insurance Association. In addition, the PMPRB participated in 26 external events and presented on various topics at 8 of these events. The events addressed a variety of audiences, including the PMPRB’s main stakeholders: patentees, provinces, third-party payers and patient advocacy groups.
The PMPRB continues to use new mediums, such as videoconference and webinar, to reach a greater number of stakeholders. In April 2013, the PMPRB hosted 6 webinars for the dissemination of the results of its NPDUIS study, The Use of Blood Glucose Test Strips in Select Public Drug Plans, 2008.
The National Prescription Drug Utilization Information System (NPDUIS) is a research initiative established by F/P/T Ministers of Health in September 2001. Its purpose is to provide policy makers and public drug plan managers with critical analyses of price, utilization and cost trends, so that Canada’s health care system has more comprehensive and accurate information on how prescription drugs are being used and on sources of cost increases.
The NPDUIS Advisory Committee, composed of representatives from public drug plans in British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, New Brunswick, Nova Scotia, Prince Edward Island, Newfoundland and Labrador, Yukon, and Health Canada, advises the PMPRB on its research agenda and on individual studies. The Committee also includes observers from Canadian Institute for Health Information (CIHI) and Canadian Agency for Drugs and Technologies in Health (CADTH).
The PMPRB published five NPDUIS reports in 2013-14:
- New Drug Pipeline Monitor – 5th Edition (December 2013)
- The Drivers of Prescription Drug Expenditures – A Methodological Report (December 2013)
- Analytical Snapshot: International Generic Price Comparison, Early 2011 (August 2013)
- The Use of Blood Glucose Test Strips in Select Public Drug Plans, 2008 (April 2013)
- New Drug Pipeline Monitor – 4th Edition (April 2013)
Other NPDUIS studies are currently under development and are summarized in the NPDUIS Research Agendaxvii.
Internal Services
Description
Internal Services are groups of related activities and resources that are administered to support the needs of programs and other corporate obligations of an organization. These groups are: Management and Oversight Services; Communications Services; Legal Services; Human Resources Management Services; Financial Management Services; Information Management Services; Information Technology Services; Real Property Services; Materiel Services; Acquisition Services; and Other Administrative Services. Internal Services include only those activities and resources that apply across an organization and not to those provided specifically to a program.
Budgetary Financial Resources (dollars)
| 2013–14 Main Estimates |
2013–14 Planned Spending |
2013–14 Total Authorities Available for Use |
2013–14 Actual Spending (authorities used) |
2013–14 Difference (actual minus planned) |
| 2,833,939 |
3,068,439 |
3,250,531 |
2,998,175 |
(70,264) |
Human Resources (FTEs)
| 2013–14 Planned |
2013–14 Actual |
2013–14 Difference(actual minus planned) |
| 19.0 |
22.3 |
*3.3 |
| * Three resources were temporarily re-allocated from program activities to support the implementation of an electronic document management system, to support HR monitoring activities, and to support administrative activities. |
Performance Analysis and Lessons Learned
In 2013-14, the PMPRB published its Code of Conduct which is a statement of the fundamental principles and related standards of conduct applicable to all its employees. By committing to these values and adhering to the expected behaviours, the PMPRB strengthens the ethical culture of the public sector, and contributes to public confidence in the integrity of all public institutions.
In addition, the PMPRB revised and received Ministerial approval of its Delegation of Financial Signing Authorities instrument. The primary purpose of this instrument is to document the PMPRB’s delegated financial and procurement authorities and to satisfy the Minister’s legal requirement that the delegations be formally made in writing. The PMPRB also made significant progress on its electronic document and records management system (named Records and Information Management System – RIMS). In support of this initiative it also offered all employees training sessions on “Duty to Delete” and the “Care and Custody” of information holdings. Furthermore, all employees of the PMPRB are required to take the on-line course Recordkeeping for Public Servants offered by the Canada School of the Public Service.
Section III: Supplementary Information
Financial Statements Highlights
Patented Medicine Prices Review Board
Condensed Statement of Operations and Departmental Net Financial Position (unaudited)
For the Year Ended March 31, 2014
(dollars)
| |
2013–14 Planned Results |
2013–14 Actual |
2012–13 Actual |
Difference (2013–14 actual minus 2013–14 planned) |
Difference (2013–14 actual minus 2012–13 actual) |
| Total expenses |
*12,083,054 |
8,749,006 |
8,338,846 |
(3,334,048) |
410,160 |
| Total revenues |
**0 |
213 |
0 |
213 |
213 |
| Net cost of operations before government funding and transfers |
12,083,054 |
8,748,793 |
8,338,846 |
(3,334,261) |
409,947 |
| Departmental net financial position |
(925,805) |
(467,168) |
(636,797) |
458,637 |
169,629 |
* Planned spending in 2013-14 is based on the assumption that the PMPRB will spend the full $2.47 million held in the SPA reserved for conducting public hearings. This is done because these expenditures are dependent on the number of hearings, and the length and complexity of the hearings held, which are difficult to predict.
** Revenues that are non-respendable are not available to discharge the Board's liabilities. Non-respendable revenues are earned on behalf of the Government of Canada. The PMPRB collects non-respendable revenues as a result of payments made by patentees to the Government of Canada through Voluntary Compliance Undertakings (VCUs) or Board Orders to offset excess revenues. In 2013-14, the PMPRB collected non-respendable revenues in the amount of $ 10,605.1 thousand. In 2012-13, the non-respendable revenues were $19,670.4 thousand.
|
Patented Medicine Prices Review Board
Condensed Statement of Financial Position (unaudited)
As at March 31, 2014
(dollars)
| |
2013–14 |
2012–13 |
Difference (2013–14 minus 2012–13 planned) |
| Total net liabilities |
1,112,115 |
1,228,638 |
(116,523) |
| Total net financial assets |
571,604 |
525,891 |
45,713 |
| Departmental net debt |
540,511 |
702,747 |
(162,236) |
| Total non-financial assets |
73,343 |
65,950 |
7,393 |
| Departmental net financial position |
467,168 |
636,797 |
(169,629) |
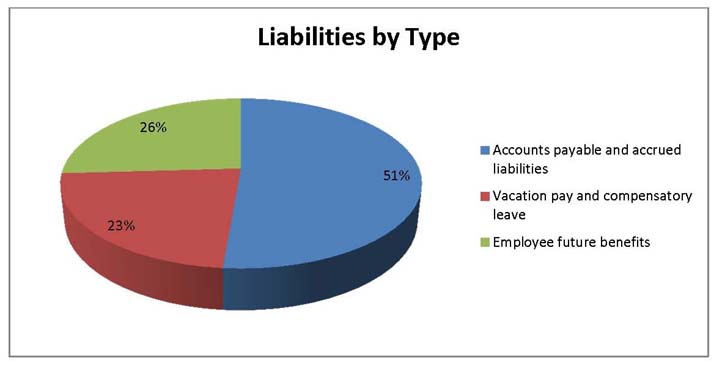
Total Liabilities
Total liabilities were $1,112,115 as at the end of 2013-14, a decrease of $116,523 from the previous year. The decrease in liabilities was as follows:
- Accounts payable and accrued liabilities increased by $32, 444.
- Vacation pay and compensatory leave decreased by $7,340.
- Employee future benefits decreased by $141,627.
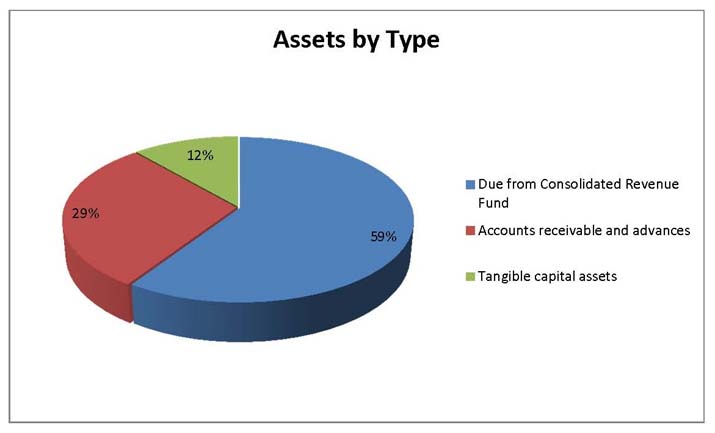
Total Assets
Total assets were $644,947 at the end of 2013-14, an increase of $53,106 from the previous year. The variances in assets were as follows:
- Increase in Due from the Consolidated Revenue Fund of $29,214.
- Increase in Accounts receivable and advances of $16,499.
- Increase in Tangible capital assets of $7,393.
Financial Statements
The financial highlights presented within this Departmental Performance Report are intended to serve as a general overview of the PMPRB’s financial position and operations. The PMPRB’s Financial Statementsxviii can be found on its website.
Supplementary Information Tables
The supplementary information tablesxix listed in the 2013–14 Departmental Performance Report can be found on the Patented Medicine Price’s Review Board’s website.
Tax Expenditures and Evaluations
The tax system can be used to achieve public policy objectives through the application of special measures such as low tax rates, exemptions, deductions, deferrals and credits. The Department of Finance Canada publishes cost estimates and projections for these measures annually in the Tax Expenditures and Evaluationsxx publication. The tax measures presented in the Tax Expenditures and Evaluations publication are the sole responsibility of the Minister of Finance.
Section IV: Organizational Contact Information
The Patented Medicine Prices Review Board
Box L40
Standard Life Centre
333 Laurier Avenue West
Suite 1400
Ottawa, Ontario K1P 1C1
Telephone: (613) 952-7360
Toll-free no.: 1-877-861-2350
Facsimile: (613) 952-7626
TTY: (613) 957-4373
Email: pmprb@pmprb-cepmb.gc.ca
Website: www.pmprb-cepmb.gc.ca
Appendix: Definitions
appropriation: Any authority of Parliament to pay money out of the Consolidated Revenue Fund.
budgetary expenditures: Include operating and capital expenditures; transfer payments to other levels of government, organizations or individuals; and payments to Crown corporations.
Departmental Performance Report: Reports on an appropriated organization’s actual accomplishments against the plans, priorities and expected results set out in the corresponding Reports on Plans and Priorities. These reports are tabled in Parliament in the fall.
full-time equivalent: Is a measure of the extent to which an employee represents a full person-year charge against a departmental budget. Full-time equivalents are calculated as a ratio of assigned hours of work to scheduled hours of work. Scheduled hours of work are set out in collective agreements.
Government of Canada outcomes: A set of 16 high-level objectives defined for the government as a whole, grouped in four spending areas: economic affairs, social affairs, international affairs and government affairs.
Management, Resources and Results Structure: A comprehensive framework that consists of an organization’s inventory of programs, resources, results, performance indicators and governance information. Programs and results are depicted in their hierarchical relationship to each other and to the Strategic Outcome(s) to which they contribute. The Management, Resources and Results Structure is developed from the Program Alignment Architecture.
non-budgetary expenditures: Include net outlays and receipts related to loans, investments and advances, which change the composition of the financial assets of the Government of Canada.
performance: What an organization did with its resources to achieve its results, how well those results compare to what the organization intended to achieve and how well lessons learned have been identified.
performance indicator: A qualitative or quantitative means of measuring an output or outcome, with the intention of gauging the performance of an organization, program, policy or initiative respecting expected results.
performance reporting: The process of communicating evidence-based performance information. Performance reporting supports decision making, accountability and transparency.
planned spending: For Reports on Plans and Priorities (RPPs) and Departmental Performance Reports (DPRs), planned spending refers to those amounts that receive Treasury Board approval by February 1. Therefore, planned spending may include amounts incremental to planned expenditures presented in the Main Estimates.
A department is expected to be aware of the authorities that it has sought and received. The determination of planned spending is a departmental responsibility, and departments must be able to defend the expenditure and accrual numbers presented in their RPPs and DPRs.
plans: The articulation of strategic choices, which provides information on how an organization intends to achieve its priorities and associated results. Generally a plan will explain the logic behind the strategies chosen and tend to focus on actions that lead up to the expected result.
priorities: Plans or projects that an organization has chosen to focus and report on during the planning period. Priorities represent the things that are most important or what must be done first to support the achievement of the desired Strategic Outcome(s).
program: A group of related resource inputs and activities that are managed to meet specific needs and to achieve intended results and that are treated as a budgetary unit.
results: An external consequence attributed, in part, to an organization, policy, program or initiative. Results are not within the control of a single organization, policy, program or initiative; instead they are within the area of the organization’s influence.
Program Alignment Architecture: A structured inventory of an organization’s programs depicting the hierarchical relationship between programs and the Strategic Outcome(s) to which they contribute.
Report on Plans and Priorities: Provides information on the plans and expected performance of appropriated organizations over a three-year period. These reports are tabled in Parliament each spring.
Strategic Outcome: A long-term and enduring benefit to Canadians that is linked to the organization’s mandate, vision and core functions.
sunset program: A time-limited program that does not have an ongoing funding and policy authority. When the program is set to expire, a decision must be made whether to continue the program. In the case of a renewal, the decision specifies the scope, funding level and duration.
target: A measurable performance or success level that an organization, program or initiative plans to achieve within a specified time period. Targets can be either quantitative or qualitative.
whole-of-government framework: Maps the financial contributions of federal organizations receiving appropriations by aligning their Programs to a set of 16 government-wide, high-level outcome areas, grouped under four spending areas.
Endnotes
i. Additional information on the amendments to the Patented Medicines Regulations can be found on the PMPRB website: http://www.pmprb-cepmb.gc.ca/view.asp?ccid=786
ii. Patent Act: http://laws-lois.justice.gc.ca/eng/acts/P-4/index.html
iii. Patented Medicines Regulations: http://laws.justice.gc.ca/en/P-4/SOR-94-688/index.html
iv. Additional information on the National Prescription Drug Utilization Information System can be found on the PMPRB website: http://www.pmprb-cepmb.gc.ca/en/npduis/about-npduis
v. Service standard for the scientific review of new patented drug products: http://www.pmprb-cepmb.gc.ca/view.asp?ccid=499
vi. Service standard for the price review of new patented drug products: http://www.pmprb-cepmb.gc.ca/view.asp?ccid=500
vii. Service standard for the price review of existing patented drug products: http://www.pmprb-cepmb.gc.ca/view.asp?ccid=501
viii. New Drug Pipeline Monitor, April 2013: http://www.pmprb-cepmb.gc.ca/view.asp?ccid=942
ix. The Use of Glucose Test Strips in Select Public Drug Plans, 2008, April 2013: http://www.pmprb-cepmb.gc.ca/view.asp?ccid=941
x. Analytical Snapshot: International Generic Price Comparison, Early 2011, August 2013: http://www.pmprb-cepmb.gc.ca/view.asp?ccid=487
xi. The Drivers of Prescription Drug Expenditure – A Methodological Report, December 2013: http://www.pmprb-cepmb.gc.ca/view.asp?ccid=887
xii. New Drug Pipeline Monitor, December 2013: http://www.pmprb-cepmb.gc.ca/view.asp?ccid=483
xiii. Monitoring and Evaluation Plan for Major Changes to the Guidelines, 2013: http://www.pmprb-cepmb.gc.ca/view.asp?ccid=493
xiv. Additional information on the CPI Adjustment Methodology consultations and changes can be found on the PMPRB website: http://www.pmprb-cepmb.gc.ca/view.asp?ccid=783
xv. Whole-of-government framework, http://www.tbs-sct.gc.ca/ppg-cpr/frame-cadre-eng.aspx
xvi. Public Accounts of Canada 2014, http://www.tpsgc-pwgsc.gc.ca/recgen/cpc-pac/index-eng.html
xvii. The NPDUIS Research Agenda can be found on the PMPRB website: http://www.pmprb-cepmb.gc.ca/en/npduis/research-agenda
xviii. The Financial Statements can be found on the PMPRB’s website: http://www.pmprb-cepmb.gc.ca/view.asp?ccid=1111
xix. The supplementary information tables listed in the 2013–14 Departmental Performance Report can be found on the Patented Medicine Price’s Review Board’s website: http://www.pmprb-cepmb.gc.ca/view.asp?ccid=890
xx. Tax Expenditures and Evaluations publication, http://www.fin.gc.ca/purl/taxexp-eng.asp