2013-14 Report on Plans and Priorities
Patented Medicine Prices Review Board
The Honourable Leona Aglukkag
Minister of Health
Table of Contents
Chairperson's Message
Section I: Organizational Overview
Section II: Analysis of Programs by Strategic Outcome
Section III: Supplementary Information
Section IV: Other Items of Interest
Endnotes
Chairperson's Message
I am pleased to present the 2013-14 Report on Plans and Priorities for the Patented Medicine Prices Review Board (PMPRB).
December 2012 marks the 25th anniversary of the organization. The PMPRB was established in 1987 under amendments to the Patent Act, with the objective of protecting consumers by ensuring that prices of patented medicines are not excessive. Twenty-five years later, the PMPRB continues to contribute to the Canadian healthcare system in the same vital consumer-protection role and by reporting on pharmaceutical trends.
Last year, the PMPRB embarked on a program evaluation to assess the extent to which outcomes are being achieved, and resources are appropriate for effective delivery of its mandate. The evaluation addressed value for money by including clear and valid conclusions about the relevance and performance of the PMPRB's programs. Both the Patented Medicines Price Regulation Program and the Pharmaceutical Trends Program (Regulation and Trends Programs) were found to be appropriate for delivery by a federal agency and well-aligned with both government-wide priorities and the PMPRB's Strategic Outcome. The review found the PMPRB to be successful at achieving its expected outcomes. According to the evaluation, the incremental funding that was received in 2008-2009 was effectively used and has achieved the results for which it was approved. The Board is currently preparing a Management Response and Action Plan to address considerations proposed in the Evaluation Report. These documents are available on the PMPRB website under Accountability.
For the coming year, the PMPRB's priorities are to continue the implementation of the Management Action Plan in response to the Program Evaluation Report by looking for ways to expedite PMPRB processes and further simplify the Guidelines and to decrease regulatory burden and make effective use of Board Staff resources. The latter will be accomplished through a review of the PMPRB's regulatory requirements and its succession planning.
The PMPRB's Monitoring and Evaluation Plan for the Major Changes in the Guidelines has proven to be an excellent platform for continued dialogue with patentees and stakeholders that enables us to be more responsive and allows for timely adjustments to our Guidelines. It is the Board's intention that the Guidelines be responsive to changes in the drug distribution and pricing environment, in an appropriate timeframe. As it moves forward, the PMPRB will continue to improve its programs by monitoring the impact of the Guidelines and clarifying, adjusting and amending them as appropriate. It will also seek opportunities to make its studies and reports available to policy decision-makers in the timeliest manner.
As Chair of the PMPRB, it is my objective to ensure that our framework continues to have a positive impact for consumers while recognizing the value that innovative medicines offer to patients. The PMPRB remains committed to effectively meeting challenges, serving Canadians, and contributing to the health care system.
Section I: Organizational Overview
Raison d'être
The Patented Medicine Prices Review Board (PMPRB) is an independent, quasi-judicial body created by Parliament in 1987. Its mandate is two-fold:
- Regulatory – to ensure that prices charged by patentees for patented medicines sold in Canada are not excessive; and
- Reporting – to report on pharmaceutical trends of all medicines and on R&D spending by pharmaceutical patentees.
In carrying out its mandate, the PMPRB ensures that Canadians are protected from excessive prices for patented medicines sold in Canada and that stakeholders are informed on pharmaceutical trends.
Responsibilities
The PMPRB was created as a result of revisions to the Patent Act (Act) in 1987 (Bill C-22). The Act was further amended in 1993 (Bill C-91). The revisions were intended to balance the extension of patent protection with the need to protect consumers from possible excessive patented drug prices.
As a result of its dual mandate the PMPRB has two responsibilities:
Patented Medicine Price Regulation
The PMPRB is responsible for ensuring the factory-gate prices that patentees charge for prescription and non-prescription patented medicines sold in Canada to wholesalers, hospitals, pharmacies or others, for human and veterinary use, are not excessive. The PMPRB regulates the price of each patented medicine to which Health Canada has assigned a Drug Identification Number (DIN) as part of its review process. The Board's mandate also includes medicines that are available under the Special Access Programme; through a Clinical Trial Application; and Investigational New Drug Products. Over-the-counter (OTC) patented medicines and patented medicines for veterinary use are regulated by the Board on a complaints basis.
In the event that the Board finds, after a public hearing, that the price of a patented medicine is or was excessive in any market, it may order the patentee to reduce the price and take measures to offset any excess revenues it may have received.
Pharmaceutical Trends Reporting
The PMPRB reports annually to Parliament through the Minister of Health on its major activities, its analyses of the prices of patented medicines and of the pharmaceutical trends of all medicines, and on the R&D expenditures as reported by patent-holding drug manufacturers. In addition to its Annual Report, the PMPRB reports through its quarterly NEWSletter and various studies.
Through the National Prescription Drug Utilization Information System (NPDUIS) initiativei, the PMPRB provides critical analyses of drug price, utilization, and cost trends in Canada. This work supports drug plan policy decision-making for participating federal, provincial, and territorial governments.
Strategic Outcome and Program Alignment Architecture (PAA)
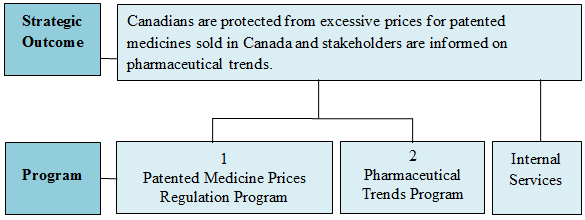
Strategic Outcome and Program Alignment Architecture (PAA)
The following chart illustrates the Strategic Outcome and the Patented Medicine Prices Review Board's three Programs.
Organizational Priorities
| Priority |
Type1 |
Strategic Outcome and/or Program(s)2 |
|
1 Type is defined as follows: previously committed to—committed to in the first or second fiscal year prior to the subject year of the report; ongoing—committed to at least three fiscal years prior to the subject year of the report; and new—newly committed to in the reporting year of the RPP or DPR.
2 The PMPRB has only one Strategic Outcome (SO) and all priorities are linked to that SO. Links to Program(s) are indicated in this column, for each priority.
|
| Continue implementation of the Management Action Plan in response to the PMPRB Program Evaluation Report |
Previously committed to |
Links to Program 1 and Program 2 |
| Description |
|
Why is it a priority?
While the evaluation in 2011-12 affirmed the relevance, performance and efficiency and economy of the Patented Medicine Prices Regulation Program and the Pharmaceutical Trends Program, it identified four considerations for the Board:
- Expedite PMPRB processes – quicker processes would result in quicker results; this could mean among other things, a more efficient use of resources, shorter periods of excessive pricing, quicker access to information on price, sales, and utilization trends which could result in greater use of information produced by the PMPRB.
- Further simplify the Guidelines - stakeholders are concerned about inherent complexities in the Guidelines which they feel impede the predictability of the regulatory process. Further simplification of the Guidelines may make them more predictable and less open to varying interpretation, which may result in greater compliance with the Guidelines.
- Expand plain language use throughout PMPRB publications – some stakeholders noted a need for shorter, less complex, plain language documents; these changes may make PMPRB publications clearer and more useful for all stakeholder groups.
- Expand the target audience for outreach efforts, increasing focus on third party payers and patient advocacy groups. The evaluation revealed that a number of interviewees in the patient advocacy and third party payer categories were unaware of the kind of information/analysis the PMPRB provided and that they were eager to be better informed.
|
|
Plans for meeting the priority
- Expedite PMPRB Processes
- Implement the revised Rules of Practice and Procedure for hearings
- Implement performance targets for selected processes/procedures
- Improve timeliness of reporting
- Improve efficiency of consultation process with key stakeholders named in the Act through the use of more informal processes
- Further simplify the Guidelines
- Continue to assess the application and impact of changes made to the Guidelines through the Monitoring and Evaluation Plan for Major Changes to the Guidelines (GMEP), annually update the Board and post findings on the PMPRB website
- Perform analysis of the CPI Adjustment Methodology to determine if changes could be beneficial
- Monitor the judicial review results for clarification of regulatory issues and reflect results in the Board's Guidelines, as appropriate
- Expand plain language use throughout PMPRB publications
- Continue use of “In Brief” for the Annual Report
- Continue use of recently introduced “Analysis Brief” series to highlight findings and policy implications of analytical studies
- Continue use of Executive Summary in all NPDUIS reports
- Develop a “Guide to Price Regulation” to explain the regulatory framework and price review process
- Expand target audience of outreach efforts
- Continue implementation of non-industry stakeholder engagement policy
- Develop and implement a bi-lateral meeting series with non-industry stakeholders
- Report on the Consumer Complaints Process in the PMPRB's Annual Report
|
Organizational Priorities
| Priority |
Type |
Strategic Outcome and/or Program(s) |
| Decrease regulatory burden and make effective use of Board Staff resources |
Previously committed to |
Links to Program 1 and Program 2 |
|
Why is this a priority?
In January 2011, the Prime Minister launched the Red Tape Reduction Commission, as part of the Economic Action Plan. The Commission was asked to identify irritants to businesses that have clear detrimental effects on growth, competitiveness and innovation, and to recommend ways to address those irritants and reduce the compliance burden on a lasting basis without compromising the environment or the health and safety of Canadians.
Irritants identified by the pharmaceutical industry were ultimately determined to be outside the scope of this initiative, and did not figure in the Commission's report. Nonetheless, the PMPRB will support the spirit of this initiative by examining its price review process to identify possible ways to reduce the regulatory burden on patentees without adversely affecting its mandate to protect consumers.
In addition, as a result of the Budget 2010 Cost Containment Measures, which froze departmental operating budgets at their 2010-11 levels, the PMPRB identified certain positions that would remain vacant. In doing so, certain operational efficiencies were adopted, and others may be necessary, to ensure the most effective use of resources.
|
|
Plans for meeting the priority
- Examine the feasibility of changing to one regulatory filing for existing patented medicines by patentees per year
- Examine the PMPRB's Consumer Price Index (CPI) Adjustment Methodology
- Examine possible changes to the criteria for commencing an investigation
- Consider options for more effective use of Board Staff resources through the Succession Planning and Knowledge Transfer initiative
|
Risk Analysis
The Patented Medicine Prices Review Board (PMPRB) is an independent, quasi-judicial body established by Parliament in 1987 under the Patent Act (Act).
The Board consists of not more than five part-time members, including a Chairperson and a Vice-Chairperson. The Chairperson is designated under the Act as the Chief Executive Officer of the PMPRB, with the authority and responsibility to supervise and direct its work.
The Members of the Board are responsible for the implementation of the applicable provisions of the Act. Together, they establish the guidelines, rules, by-laws and other policies of the Board provided by the Act and consult as necessary with stakeholders including the Minister of Health and representatives of consumer groups, the pharmaceutical industry and provincial and territorial governments and, when needed, conduct hearings.
Appointments to the Board are recommended to the Privy Council Office by the Minister of Health. At present one of the five Board positions is vacant. A shortage in the number of Board Members may impact the timeliness of the hearing process. It is important that the Board have an appropriate skills mix. The Chairperson will continue to provide the Minister of Health with recommendations that are appropriate to the expertise needed on the Board.
Currently a number of the Board's decisions stemming from hearings are under judicial review. While court decisions pose a risk they also provide an opportunity for clarification to patentees and Board Staff with respect to key legislative and regulatory provisions and the application of the Board's related Guidelines. The Board will continue to monitor court decisions relating to these cases.
Spending on health care continues to rise in most industrialized countries according to the Organization for Economic Co-operation and Development (OECD). Most industrialized countries, including Canada, have publicly funded drug programs and systems that regulate drug prices or the profits of drug manufacturers in various ways in an attempt to contain rising drug costs.
According to the Canadian Institute for Health Information (CIHI), drugs account for the second-largest share of total health spending, after hospital expenditures. In recent years, the high price of new patented drug products, in particular biologics with subsequent indications have raised questions about the need for additional price reviews for these new indications. Through its Guidelines Monitoring and Evaluation Plan and through its work with F/P/T drug plan representatives the Board will continue to monitor issues related to its regulatory mandate.
As the federal expert body on questions related to drug prices, the PMPRB, via its Pharmaceutical Trends Program, contributes to informed decision-making by reporting on pharmaceutical trends. Through critical analyses of price, utilization and cost trends conducted under the National Prescription Drug Utilization Information System (NPDUIS) initiative, the PMPRB provides Canada's health system with comprehensive and accurate information on how prescription drugs are being used and on cost drivers. The challenge for the PMPRB is to ensure that its reports provide relevant information and are issued in a timely manner such that they will provide credible pharmaceutical trend information and will contribute to the information needs of a variety of policy decision-makers.
The PMPRB's activities under the NPDUIS initiative were initiated through a Memorandum of Agreement (MOA) between Health Canada and the PMPRB. Health Canada and the PMPRB review this MOA from time to time and may conclude or modify this MOA or any provisions therein in light of future budget or policy requirements.
As a small organization the PMPRB has difficulty attracting and retaining highly specialized subject matter experts. Currently the PMPRB is having difficulty finding individuals with the skills required to conduct quantitative economic analysis. In addition, within the next 12 to 36 months people in key positions in other branches of the organization will be eligible for retirement. These positions often require varied skills that are used intermittently making knowledge transfer difficult and creating challenges within the organization. The opportunity, and the challenge, in 2013-2014 will be to ensure that succession planning supports the evolving organizational needs and operational policy directions. This will include the development of its staff through an assignment and cross-training program to build the required skill sets within the organization. The PMPRB will also work to identify other options for finding the needed skills.
Finally, the Government has introduced a number of government-wide initiatives such as, Shared Services, Service Standards Initiative, Financial Management Transformation and Common Human Resources Business Processes Project. These initiatives pose a challenge to all departments because they impact workload and resources. The PMPRB will continue to work with the central agencies and organizations such as the Small Administrative Agency Network to support these initiatives in a manner that is appropriate for the size of the organization and to benefit from arrangements within the small agency community.
Planning Summary
Financial Resources (Planned Spending — $ thousands)
Total Budgetary Expenditures (Main Estimates)
2013-14 |
Planned Spending3 2013–14 |
Planned Spending 2014–15 |
Planned Spending 2015–16 |
|
3 Planned Spending for 2013-14 includes an estimated carry-forward of approximately $234.5 thousand.
|
| 10,944.1 |
11,178.6 |
10,902.6 |
10,902.6 |
Human Resources (Full-Time Equivalents—FTE)
| 2013–14 |
2014–154 |
2015-16 |
|
4 It was determined that as a result of efficiencies gained through other initiatives the PMPRB was in a position to eliminate one position without significantly impacting its operations.
|
| 74 |
73 |
73 |
Planning Summary Table for Programs ($ thousands)
| Strategic Outcome |
Program |
Actual Spending 2010–11 |
Actual Spending 2011–12 |
Forecast Spending 2012–135 |
Planned Spending |
Alignment to Government of Canada Outcomesii |
| 2013-146 |
2014-15 |
2015-16 |
|
5 The Forecast Spending for 2012-13 projects that $2,204.2 thousand of the Special Purpose Allotment will not be spent to year-end in the Patented Medicine Prices Regulation Program.
6 Planned Spending for 2013-14 includes the full Special Purpose Allotment amount of $2,470.0 thousand.
|
| Canadians are protected from excessive prices for patented medicines sold in Canada and stakeholders are informed on pharmaceutical trends. |
Patented Medicine Prices Regulation Program |
4,232.0 |
7,346.8 |
3,391.1 |
6,781.3 |
6,781.3 |
6,781.3 |
Healthy Canadian. |
| Pharmaceutical Trends Program |
890.4 |
1,010.5 |
899.8 |
1,328.8 |
1,287.4 |
1,287.4 |
Healthy Canadian. |
| Sub –Total |
5,122.4 |
8,357.3 |
4,290.9 |
8,110.1 |
8,068.7 |
8,068.7 |
|
Planning Summary Table for Internal Services ($ thousands)
| Program |
Actual Spending
2010–11 |
Actual Spending
2011–12 |
Forecast Spending
2012–13 |
Planned Spending |
| 2013–147 |
2014–15 |
2015–16 |
| 7 The estimated carry-forward of approximately $234.5 thousand has been included in Planned Spending for Internal Services. |
| Internal Services |
4,348.3 |
3,397.1 |
2,838.5 |
3,068.4 |
2,833.9 |
2,833.9 |
| Sub –Total |
4,348.3 |
3,397.1 |
2,838.5 |
3,068.4 |
2,833.9 |
2,833.9 |
Planning Summary Total ($ thousands)
| Strategic Outcome Programs, and Internal Services |
Actual Spending
2010–11 |
Actual Spending
2011–12 |
Forecast Spending
2012–13 |
Planned Spending |
| 2013–148 |
2014–15 |
2015–16 |
| 8 Total Planned Spending for 2013-14 includes an estimated carry-forward of approximately $234.5 thousand. |
| Total |
9,470.7 |
11,754.4 |
7,129.4 |
11,178.6 |
10,902.6 |
10,902.6 |
Expenditure Profile
Departmental Spending Trend
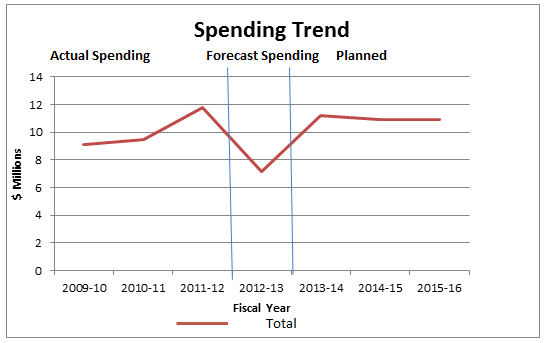
Departmental Spending Trend
The following graph shows the PMPRB's spending trend. It illustrates on a line graph the actual spending in 2009-10, 2010-11 and 2011-12; the forecast spending for 2012-13; and the planned spending for 2013-14, 2014-15 and 2015-16.
To meet workload pressures and continue ongoing initiatives related to the delivery of its mandate, Treasury Board granted the PMPRB authority to increase reference levels in Vote 45 (Program expenditures) by $5.6 million for 2009-10, $6.2 million for 2010-11, and $5.8 million for 2011-12 and future years (including EBP and excluding PWGSC accommodation charges).
The PMPRB has now staffed all of the positions it intends to staff and it is anticipated that actual spending (excluding the Special Purpose Allotment) will closely resemble the funding provided in 2013-14 and ongoing.
Included in the additional funding were 2.2 million in 2009-10, and $2.8 million in 2010-11 and subsequent years, in the Special Purpose Allotment (SPA) reserved for conducting public hearings. This increased total ongoing funding in the SPA to $3.1 million. The SPA can only be used to cover costs related to public hearings.
When hearings are underway, on average 90% of the expenditures from the SPA are allocated to the cost of legal services and expert witnesses. These expenditures are completely dependent on the number of hearings held and the length and complexity of the hearings, and are therefore very difficult to predict. For this reason, any SPA funds not required for hearings are returned to the Consolidated Revenue Fund. In recent years the Board has issued fewer Notices of Hearing, and, as a result, it is anticipated that the SPA will not be fully utilized during this planning period.
As a result of the Budget 2010 Cost Containment Measures, departmental operating budgets were frozen at their 2010-11 levels for 2011-12 and 2012-13.
In addition, Budget 2012 resulted in savings of $1 million, achieved through a $144 thousand reduction of funding for the Pharmaceutical Trends Program and a $630 thousand reduction in funding for the SPA which is included in the funding for the Patented Medicine Prices Review Program. Funding for the Pharmaceutical Trends Program was further reduced by $167 thousand in 2013-14 and $63 thousand in 2014-15 and beyond.
Estimates by Vote
For information on our organizational appropriations, please see page 162 of the 2013–14 Main Estimates iii publication.
Section II: Analysis of Programs by Strategic Outcome
Strategic Outcome
| Strategic Outcome: Canadians are protected from excessive prices for patented medicines sold in Canada and stakeholders are informed on pharmaceutical trends. |
| Performance Indicators |
Targets |
| Canada's prices on average are in line with the seven comparator countries listed in the Regulations. |
Canada's prices on average are at or below the median of international prices. |
Programs
The PMPRB has one Strategic Outcome which is supported by two Programs:
- Patented Medicine Price Regulation Program
- Pharmaceutical Trends Program
Program 1 – Patented Medicine Price Regulation Program
The PMPRB is an independent quasi-judicial body that is responsible for ensuring that the prices that patentees charge for patented medicines sold in Canada are not excessive based on the price review factors in the Patent Act (the Act). To make this determination the Board must consider each of the following factors: prices at which the medicine and other medicines in the same therapeutic class have been sold in Canada and in the seven comparator countries listed in the Patented Medicines Regulations (Regulations); changes in the Consumer Price Index (CPI); and in accordance with the Act, such other factors as may be specified in any regulations made for the purposes of the price review. Under the Act, and as per the Regulations, patentees are required to file price and sales information for each patented medicine sold in Canada, for the duration of the patent(s). Board Staff reviews the introductory and ongoing information filed by patentees, for all patented medicines sold in Canada. When it finds that the price of a patented medicine appears to be excessive, Board Staff will conduct an investigation into the price. An investigation could result in: its closure where it is concluded that the price was non-excessive; a Voluntary Compliance Undertaking (VCU) by the patentee to reduce the price and offset excess revenues obtained as a result of excessive prices through a payment and/or a price reduction of another patented drug product; or a public hearing to determine if the price is excessive, including any remedial order determined by the Board. In the event that the Board Hearing Panel finds, after a public hearing, that a price is or was excessive, it may order the patentee to reduce the price and take measures to offset any excess revenues. This program, by reviewing the prices charged by patentees for patented medicines sold in Canada, protects Canadians and the health care system from excessive prices.
Financial Resources ($ thousands)
Total Budgetary Expenditures
(Main Estimates)
2013-14 |
Planned Spending
2013–14 |
Planned Spending 2014–15 |
Planned Spending 2015–16 |
| 6,781.3 |
6,781.3 |
6,781.3 |
6,781.3 |
Human Resources (FTE)
| 2013–14 |
2014–15 |
2015–16 |
| 44 |
44 |
44 |
| Program Expected Results |
Performance Indicators |
Targets |
| Patentees comply with the Patent Act, the Regulations and the Excessive Price Guidelines (Guidelines) |
Percentage of patented medicines that are priced, as a result of voluntary compliance, within the Guidelines or at a price which does not trigger the investigation criteria. |
95 % of patented medicines are voluntarily priced within the Guidelines or at a price which does not trigger the investigation criteria. |
| Percentage of patented medicines that are subject to a Board Order |
100% of Board Orders are complied with |
Planning Highlights
The PMPRB is responsible for ensuring that the prices that patentees charge, the “factory-gate” price, for patented medicines sold in Canada for human or veterinary use, are not excessive. The PMPRB relies on voluntary compliance whenever possible since it is less time consuming and less costly to all parties. Voluntary compliance by patentees is facilitated by published Guidelines, which are intended to assist patentees in setting prices that are not excessive by providing transparent and predictable information on how a price review is conducted.
Since the implementation of the new Guidelines in January 2010, Board Staff has been monitoring and evaluating the application and impact of the major changes on an ongoing basis. In June 2011, the PMPRB published its Monitoring and Evaluation Plan for the Major Changes in the Guidelines and in December 2011 it provided the first annual assessment under the plan. Impacts may not be immediately apparent due to the timing of the regulatory filings of patentees, the price review process of existing patented medicines which is based on a full calendar year, and the fact that it may take several reporting periods before any discernible trends become evident. However, the Board will monitor emerging issues to identify the need for any future amendments to the Guidelines or to the Monitoring Plan.
The key priorities for the Patented Medicine Price Regulation (Price Regulation) Program over the planning period are:
- Continue to monitor and evaluate the impact of the new Guidelines;
- Expedite PMPRB processes by:
- Implementing the revised Rules of Practice and Procedure for Hearings
- Implementing performance targets for selected processes, where appropriate
- Improving efficiency of the consultation process through the uses of more informal processes
- Further simplify the Guidelines by:
- Performing analysis of the CPI Adjustment Methodology to determine if changes could be beneficial
- Monitoring judicial review results for clarification of regulatory issues and reflect results in the Board's Guidelines, as appropriate
- Expand use of plain language throughout PMPRB publications by:
- Developing a “Guide to Price Regulation” to explain the rationale for the regulatory regime and how it works
- Decrease regulatory burden and make effective use of Board Staff resources by:
- Examining feasibility of changing from bi-annual to annual data filing for existing patented medicines
- Evaluating appropriateness of the current triggers for commencing an investigation
- Consider options for more effectively using Board Staff resources as part the Succession Planning and Knowledge Transfer initiative.
Benefits for Canadians
This program activity contributes to the Government of Canada outcome of Healthy Canadians by ensuring that prices of patented medicines are not excessive. Price reviews, investigations and, when necessary, hearings must be conducted in a transparent, effective and timely fashion so as to protect the interests of consumers and the Canadian health care system.
Program 2 – Pharmaceutical Trends Program
The PMPRB reports annually to Parliament through the Minister of Health on its price review activities, the prices of patented medicines and price trends for all drugs, and R&D expenditures as reported by pharmaceutical patentees. In supporting this requirement, the Pharmaceutical Trends Program provides complete and accurate information on trends in manufacturers' prices of patented medicines sold in Canada and on patentees' research-and-development expenditures to interested stakeholders including: industry (i.e., brand-name, biotech, generic); federal, provincial and territorial (F/P/T) governments; consumer and patient advocacy groups; third party payers; and others. This information also provides assurance to Canadians that the prices of patented medicines are not excessive. In addition, as a result of the establishment of the National Prescription Drug Utilization Information System (NPDUIS) by F/P/T ministers of health, the Minister of Health requested that the PMPRB conduct analysis of price, utilization and cost trends for prescription drugs so that Canada's health system has comprehensive, accurate information on how prescriptions drugs are being used and on the sources of cost increases. The PMPRB publishes specific NPDUIS reports based on the research and reporting priorities identified by the NPDUIS Steering Committee.
Financial Resources ($ thousands)
Total Budgetary Expenditures
(Main Estimates)
2013-14 |
Planned Spending
2013–14 |
Planned Spending
2014–15 |
Planned Spending
2015–16 |
| 1,328.8 |
1,328.8 |
1,287.4 |
1,287.4 |
Human Resources (FTE)
| 2013–14 |
2014–15 |
2015–16 |
| 11 |
10 |
10 |
| Program Expected Results |
Performance Indicators |
Targets |
| Stakeholders are more aware of pharmaceutical trends and cost drivers |
Number of website hits |
5% increase in requests |
| Number of presentations by PMPRB at external meetings |
10 events per year |
Planning Highlights
The PMPRB will continue to provide relevant and timely reporting, provide credible pharmaceutical trend information and contribute to the information needs of a variety of policy decision-makers by:
- Reporting annually to Parliament through the Minister of Health, on its major activities, the PMPRB's price review activities, the prices of patented medicines and price trends for all drugs, and R&D expenditures as reported by pharmaceutical patentees;
- Responding to F/P/T information needs identified by the NPDUIS Steering Committee;
- Expanding the use of plain language throughout PMPRB publications
- Continuing the use of “in Brief” for Annual Report
- Continuing the use of recently introduced “Analysis Brief” series to communicate policy implications of trends analysis findings
- Continuing the use of an Executive Summaries in all NPDUIS reports
- Providing the results of relevant research analysis conducted under the NPDUIS initiative in a timelier manner.
Benefits for Canadians
This program activity contributes to the Government of Canada outcome of Healthy Canadians by providing critical analyses of price, utilization and cost trends so that Canada's health system has more comprehensive and accurate information to inform drug policy decision-making.
Internal Services
Internal Services are groups of related activities and resources that are administered to support the needs of programs and other corporate obligations of an organization. These groups are: Management and Oversight Services; Communications Services; Legal Services; Human Resources Management Services; Financial Management Services; Information Management Services; Information Technology Services; Real Property Services; Material Services; Acquisition Services; and Travel and Other Administrative Services. Internal Services include only those activities and resources that apply across an organization and not to those provided specifically to a program.
Financial Resources ($ thousands)
Total Budgetary Expenditures
(Main Estimates) 2013-14 |
Planned Spending9
2013–14 |
Planned Spending
2014–15 |
Planned Spending
2015–16 |
| 9 The estimated carry-forward of approximately $234.5 thousand has been included in Planned Spending for Internal Services. |
| 2,833.9 |
3,068.4 |
2,833.9 |
2,833.9 |
Human Resources (FTE)
| 2013–14 |
2014–15 |
2015–16 |
| 19 |
19 |
19 |
Section III: Supplementary Information
Financial Highlights
Future-Oriented Financial Statements
The future-oriented financial highlights presented within this RPP are intended to serve as a general overview of the PMPRB's financial position and operations. These future-oriented financial highlights are prepared on an accrual basis to strengthen accountability and improve transparency and financial management.
The PMPRB's Future-Oriented Financial Statementsiv can be found on the PMPRB's website: http://www.pmprb-cepmb.gc.ca under Reports to Parliament.
Future-Oriented Condensed Statement of Operations and Departmental Net Financial Position (unaudited)
For the Year ended March 31, 2013 |
|
% Change |
Forecast
2013-14 |
Estimated Results
2012-13 |
| Total expenses |
50% |
$12,083,054 |
$8,058,202 |
| Total revenues |
(100.0%) |
0 |
(26,004,613) |
| Net cost of operations before government funding and transfers |
1,673% |
$12,083,054 |
$(17,946,411) |
Total Expenses: The PMPRB is projecting $12.1 million in expenses based on 2013-14 Main Estimates and accrued information. This amount does not include supplementary estimates. This difference of $4.0 million from 2012-13 projections does not represent an actual increase in budget allotments, which are the same as set out in the Planning Summary. The $4.0 million difference forecasts spending of the full Special Purpose Allotment (SPA) of $2.5 million and also assumes the full spending of all voted and statutory amounts provided to the PMPRB in 2013-14. For further information on the SPA please refer to the Expenditure Profile.
The expenses by program activity are as follows: Patented Medicine Prices Regulation Program $7.4 million; Pharmaceutical Trends Program $1.5 million; and Internal Services $3.2 million including vacation pay and compensatory leave and Employee future benefits.
Total Revenues: The amount reported as Revenues is made up entirely of non-respendable revenues. Non-respendable revenue does not represent revenues generated by the PMPRB. This money is a result of payments made by patentees to the Government of Canada through Voluntary Compliance Undertakings (VCUs) or Board Orders to offset excess revenues. The Minister may enter into agreements with any province or territory respecting the distribution to that province/territory of amounts received by the Receiver General, less any costs incurred in relation to the collection and distribution of those amounts. The non-respendable revenues are collected on behalf of the Government.
Future-Oriented Condensed Statement of Financial Position (Unaudited)
For the Year ended March 31, 2013 |
|
% Change |
Forecast
2013-14 |
Estimated Results
2012-13 |
| Total net liabilities |
(4%) |
$1,704,981 |
$1,769,934 |
| Total net financial assets |
(0%) |
779,176 |
781,133 |
| Departmental net debt |
(6%) |
$925,805 |
$988,801 |
| Departmental net financial position |
(6%) |
(925,805) |
(988,801) |
Liabilities by Type: Total net liabilities are anticipated to be $1.7M for 2013-14, a net decrease of $65.0K from 2012-13 projections.
The breakdown of liabilities is as follows: Accounts payable and accrued liabilities $3.6M; Vacation pay and compensatory leave $259.0K; and Employee future benefits $691.0K. Accrued liabilities held on behalf of Government are expected to be $2.8M.
Assets by Type: Total assets are anticipated to be $779.2K for 2013-14, a decrease of $2.0K from 2012-13 projections.
Due from Consolidated Revenue Fund is projected to be $624.4K for 2013-14, representing an increase of $11.9K from 2012-13 estimates. Accounts receivable and advances are expected to be $154.8K. Trend analysis indicates that the net change in PMPRB's Accounts receivable and advances will be a decrease of $13.8K.
List of Supplementary Information Tables
All electronic supplementary information tablesv listed in the 2013-14 Reports on Plans and Priorities can be found on the PMPRB website: www.pmprb-cepmb.gc.ca under Reports to Parliament, Report on Plans and Priorities.
- Greening Government Operations;
- Sources of Respendable and Non-Respendable Revenue;
- Upcoming Internal Audits and Evaluations over the next three fiscal years; and
Tax Expenditures and Evaluations Report
The tax system can be used to achieve public policy objectives through the application of special measures such as low tax rates, exemptions, deductions, deferrals and credits. The Department of Finance publishes cost estimates and projections for these measures annually in the Tax Expenditure and Evaluation publicationvi. The tax measures presented in the Tax Expenditures and Evaluations publication are the sole responsibility of the Minister of Finance.
Section IV: Other Items of Interest
Organizational Contact Information
The Patented Medicine Prices Review Board
Box L40
Standard Life Centre
333 Laurier Avenue West
Suite 1400
Ottawa, Ontario K1P 1C1
Telephone: (613) 952-7360
Toll-free no.: 1-877-861-2350
Facsimilie: (613) 952-7626
TTY: (613) 957-4373
Email: pmprb@pmprb-cepmb.gc.ca
Website: www.pmprb-cepmb.gc.ca
Additional Information
PMPRB Annual Report 2011
Quarterly NEWSletter
Patentee's Guide to Reporting
Compendium of Policies, Guidelines and Procedures – Updated June 2012
Patent Act
Patented Medicines Regulations
Endnotes
- For more in depth information on the National Prescription Drug Utilization Information System (NPDUIS) initiative, refer to the PMPRB's website: http://www.pmprb-cepmb.gc.ca under NPDUIS
- Information on departmental alignment to Government of Canada outcomes is available on the Secretariat's website: http://www.tbs-sct.gc.ca/ppg-cpr/frame-cadre-eng.aspx
- For information on our organizational appropriations, see the 2013–14 Main Estimates publication on the Treasury Board of Canada Secretariat website: http://www.tbs-sct.gc.ca/ems-sgd/esp-pbc/me-bpd-eng.asp
- The PMPRB's Future-Oriented Financial Statements can be found on the PMPRB's website, under Reports to Parliament: http://www.pmprb-cepmb.gc.ca/CMFiles/Reports%20to%20Parliament/Future%20Oriented%20Financial%20Statements%20Final%20Signed.pdf
- All electronic supplementary information tables listed in the 2013-14 Report on Plans and Priorities can be found on the PMPRB website: www.pmprb-cepmb.gc.ca.
- The annual Tax Expenditures and Evaluations publication can be found on the Department of Finance website: http://www.fin.gc.ca/purl/taxexp-eng.asp