2014–15 Report on Plans and Priorities
Supplementary Information (Tables):
Patented Medicine Prices Review Board
The Honourable Rona Ambrose
Minister of Health
Catalogue No.: H79-3/2014E-PDF
ISSN: 2292-6283
2014–15 ESTIMATES
PART III – Departmental Expenditure Plans: Reports on Plans and Priorities
Purpose
Reports on Plans and Priorities (RPP) are individual expenditure plans for each department and agency. These reports provide increased levels of detail over a three-year period on an organization's main priorities by strategic outcome, program and planned/expected results, including links to related resource requirements presented in the Main Estimates. In conjunction with the Main Estimates, Reports on Plans and Priorities serve to inform members of Parliament on planned expenditures of departments and agencies, and support Parliament's consideration of supply bills. The RPPs are typically tabled soon after the Main Estimates by the President of the Treasury Board.
Estimates Documents
The Estimates are comprised of three parts:
Part I - Government Expenditure Plan - provides an overview of the Government's requirements and changes in estimated expenditures from previous fiscal years.
Part II - Main Estimates - supports the appropriation acts with detailed information on the estimated spending and authorities being sought by each federal organization requesting appropriations.
In accordance with Standing Orders of the House of Commons, Parts I and II must be tabled on or before March 1.
Part III - Departmental Expenditure Plans - consists of two components:
- Report on Plans and Priorities (RPP)
- Departmental Performance Report (DPR)
DPRs are individual department and agency accounts of results achieved against planned performance expectations as set out in respective RPPs.
The DPRs for the most recently completed fiscal year are tabled in the fall by the President of the Treasury Board.
Supplementary Estimates support Appropriation Acts presented later in the fiscal year. Supplementary Estimates present information on spending requirements that were either not sufficiently developed in time for inclusion in the Main Estimates or have subsequently been refined to account for developments in particular programs and services. Supplementary Estimates also provide information on changes to expenditure forecasts of major statutory items as well as on such items as: transfers of funds between votes; debt deletion; loan guarantees; and new or increased grants.
For more information on the Estimates, please consult the Treasury Board Secretariat websitei.
Links to the Estimates
As shown above, RPPs make up part of the Part III of the Estimates documents. Whereas Part II emphasizes the financial aspect of the Estimates, Part III focuses on financial and non-financial performance information, both from a planning and priorities standpoint (RPP), and an achievements and results perspective (DPR).
The Management Resources and Results Structure (MRRS) establishes a structure for display of financial information in the Estimates and reporting to Parliament via RPPs and DPRs. When displaying planned spending, RPPs rely on the Estimates as a basic source of financial information.
Main Estimates expenditure figures are based on the Annual Reference Level Update which is prepared in the fall. In comparison, planned spending found in RPPs includes the Estimates as well as any other amounts that have been approved through a Treasury Board submission up to February 1st (See Definitions section). This readjusting of the financial figures allows for a more up-to-date portrait of planned spending by program.
Changes to the presentation of the Report on Plans and Priorities
Several changes have been made to the presentation of the RPP partially to respond to a number of requests – from the House of Commons Standing Committees on Public Accounts (PAC - Report 15ii), in 2010; and on Government and Operations Estimates (OGGO - Report 7iii), in 2012 – to provide more detailed financial and non-financial performance information about programs within RPPs and DPRs, thus improving the ease of their study to support appropriations approval.
- In Section II, financial, human resources and performance information is now presented at the Program and Sub-program levels for more granularity.
- The report's general format and terminology have been reviewed for clarity and consistency purposes.
- Other efforts aimed at making the report more intuitive and focused on Estimates information were made to strengthen alignment with the Main Estimates.
How to read this document
RPPs are divided into four sections:
Section I: Organizational Expenditure Overview
This Organizational Expenditure Overview allows the reader to get a general glance at the organization. It provides a description of the organization's purpose, as well as basic financial and human resources information. This section opens with the new Organizational Profile, which displays general information about the department, including the names of the minister and the deputy head, the ministerial portfolio, the year the department was established, and the main legislative authorities. This subsection is followed by a new subsection entitled Organizational Context, which includes the Raison d'être, the Responsibilities, the Strategic Outcomes and Program Alignment Architecture, the Organizational Priorities and the Risk Analysis. This section ends with the Planned Expenditures, the Alignment to Government of Canada Outcomes, the Estimates by Votes and the Contribution to the Federal Sustainable Development Strategy. It should be noted that this section does not display any non-financial performance information related to programs (please see Section II).
Section II: Analysis of Program(s) by Strategic Outcome(s)
This Section provides detailed financial and non-financial performance information for strategic outcomes, Programs and sub-programs. This section allows the reader to learn more about programs by reading their respective description and narrative entitled “Planning Highlights”. This narrative speaks to key services or initiatives which support the plans and priorities presented in Section I; it also describes how performance information supports the department's strategic outcome or parent program.
Section III: Supplementary Information
This section provides supporting information related to departmental plans and priorities. In this section, the reader will find future-oriented statement of operations and a link to supplementary information tables regarding transfer payments, as well as information related to the greening government operations, internal audits and evaluations, horizontal initiatives, user fees, major crown and transformational projects, and up-front multi-year funding, where applicable to individual organizations. The reader will also find a link to the Tax Expenditures and Evaluations, produced annually by the Minister of Finance, which provides estimates and projections of the revenue impacts of federal tax measures designed to support the economic and social priorities of the Government of Canada.
Section IV: Organizational Contact Information
In this last section, the reader will have access to organizational contact information.
Definitions
Appropriation
Any authority of Parliament to pay money out of the Consolidated Revenue Fund.
Budgetary Vs. Non-budgetary Expenditures
Budgetary expenditures – operating and capital expenditures; transfer payments to other levels of government, organizations or individuals; and payments to crown corporations.
Non-budgetary expenditures – net outlays and receipts related to loans, investments and advances, which change the composition of the financial assets of the Government of Canada.
Expected Result
An outcome that a program is designed to achieve.
Full-Time Equivalent (FTE)
A measure of the extent to which an employee represents a full person-year charge against a departmental budget. FTEs are calculated as a ratio of assigned hours of work to scheduled hours of work. Scheduled hours of work are set out in collective agreements.
Government of Canada Outcomes
A set of high-level objectives defined for the government as a whole.
Management Resources and Results Structure (MRRS)
A common approach and structure to the collection, management and reporting of financial and non-financial performance information.
An MRRS provides detailed information on all departmental programs (e.g.: program costs, program expected results and their associated targets, how they align to the government's priorities and intended outcomes, etc.) and establishes the same structure for both internal decision making and external accountability.
Planned Spending
For the purpose of the RPP, planned spending refers to those amounts for which a Treasury Board (TB) submission approval has been received by no later than February 1, 2014. This cut-off date differs from the Main Estimates process. Therefore, planned spending may include amounts incremental to planned expenditure levels presented in the 2014–15 Main Estimates.
Program
A group of related resource inputs and activities that are managed to meet specific needs and to achieve intended results, and that are treated as a budgetary unit.
Program Alignment Architecture
A structured inventory of a department's programs, where programs are arranged in a hierarchical manner to depict the logical relationship between each program and the Strategic Outcome(s) to which they contribute.
Spending Areas
Government of Canada categories of expenditures. There are four spending areasiv (social affairs, economic affairs, international affairs and government affairs) each comprised of three to five Government of Canada outcomes.
Strategic Outcome
A long-term and enduring benefit to Canadians that is linked to the department's mandate, vision, and core functions.
Sunset Program
A time-limited program that does not have on-going funding or policy authority. When the program is set to expire, a decision must be made as to whether to continue the program. (In the case of a renewal, the decision specifies the scope, funding level and duration).
Whole-of-Government Framework
A map of the financial and non-financial contributions of federal organizations receiving appropriations that aligns their Programs to a set of high level outcome areas defined for the government as a whole.
Table of Contents
Chairperson's Message
Section I: Organizational Expenditure Overview
Section II: Analysis of Program(s) by Strategic Outcome(s)
Section III: Supplementary Information
Section IV: Organizational Contact Information
Endnotes
Chairperson's Message
I am pleased to present the 2014–15 Report on Plans and Priorities for the Patented Medicine Prices Review Board (PMPRB).
The PMPRB was established in 1987 under the amendments to the Patent Act, with the objective of protecting consumers by ensuring that prices of patented medicines are not excessive. As a member of the Health Portfolio, the PMPRB plays an important role in the broader objective of improving the health of Canadians.
For the coming year, the PMPRB's priorities are: first, to assess the impact of recent and pending changes to foreign and domestic pharmaceutical regulatory systems on the work of the PMPRB; second, to continue implementing the Management Action Plan in response to the PMPRB Program Evaluation Report; third, to decrease regulatory burden and make the most effective use of Board Staff resources; and, finally, to align the PMPRB's internal operating framework with the requirement of central agencies.
As Chairperson of the Board, it is my objective to ensure that our framework continues to put consumer protection first, while recognizing the value that innovative medicines offer to patients. Our pricing framework makes reference to countries such as France, Germany and the United Kingdom. As the drug reimbursement policies in these countries evolve, it is important that we assess the impact these changes may have on the Board's ability to deliver on its core mandate of ensuring that prices of patented medicines in Canada are not excessive.
Moving forward, the PMPRB will continue to improve its programs by monitoring the impact of the Guidelines and clarifying, adjusting and amending them as appropriate. It will also work to ensure that its studies and reports account for the very latest market trends and reflect the immediate needs of both public and private payers who are eager to make informed reimbursement decisions in real time.
In the spirit of the Government's Red Tape Reduction Action Plan, we will continue to take steps towards reducing our administrative burden on patentees. Last year, the Board consulted stakeholders on two priority initiatives in this area. One is to simplify the Consumer Price Index (CPI) Adjustment Methodology. The other is to move from twice to once a year filings under the Patented Medicines Regulations. For 2014, we will work towards having the proposed amendments pre-published in Part I of the Canada Gazette for formal consultation. As for the new CPI-Adjustment Methodology, it is scheduled for implementation in 2015.
The PMPRB remains committed to effectively meeting challenges in these rapidly changing times, serving Canadians, and contributing to the continued sustainability of the health care system.
We look forward to working with our diverse industry and non-industry stakeholder groups and to forging new associations.
Mary Catherine Lindberg
Section I: Organizational Expenditure Overview
Organizational Profile
Minister: The Honourable Rona Ambrose
Deputy Head: Mary Catherine Lindberg, Chairperson
Ministerial portfolio: Health
Year established: 1987
Main legislative authorities: Patent Actv and Patented Medicines Regulationsvi
Other: The Minister of Health is responsible for the pharmaceutical provisions of the Patent Act (Act) set out in sections 79 to 103. The PMPRB is part of the Health Portfolio, which also includes Health Canada, the Public Health Agency of Canada, the Canadian Institutes of Health Research and the Canadian Food Inspection Agency. The Health Portfolio supports the Minister of Health in maintaining and improving the health of Canadians.
Although part of the Health Portfolio, the PMPRB carries out its mandate at arm's length from the Minister. It also operates independently of other bodies such as Health Canada, which authorizes the sale of drugs in Canada after their assessment for safety, efficacy and quality; federal, provincial and territorial public drug plans, which are responsible for listing and reimbursement decisions for their respective plans; and the Common Drug Review, administered by the Canadian Agency for Drugs and Technologies in Health (CADTH), which provides listing recommendations to participating public drug plans based on cost-effectiveness.
Organizational Context
Raison d'être
The PMPRB is an independent, quasi-judicial body created by Parliament in 1987. Its mandate is twofold:
Regulatory – to ensure that prices charged by patentees for patented medicines sold in Canada are not excessive; and
Reporting – to report on pharmaceutical trends of all medicines and on R&D spending by pharmaceutical patentees.
In carrying out its mandate, the PMPRB ensures that Canadians are protected from excessive prices for patented medicines sold in Canada and that stakeholders are informed on pharmaceutical trends.
Responsibilities
The PMPRB was created as a result of amendments to the Patent Act (Act) in 1987 (Bill C-22), and its remedial powers strengthened by further amendments in 1993 (Bill C-91). These amendments were part of policy reforms intended to balance the PMPRB's consumer protection mandate with patent protection measures intended to encourage the research and development efforts of pharmaceutical patentees.
The PMPRB has a dual mandate:
Patented Medicine Prices Regulation
The PMPRB is responsible for ensuring the factory-gate prices that patentees charge for prescription and non-prescription patented medicines sold in Canada to wholesalers, hospitals, pharmacies or others, for human and veterinary use, are not excessive. The PMPRB regulates the price of each patented medicine to which Health Canada has assigned a Drug Identification Number (DIN) as part of its review process. The Board's mandate also includes medicines that are available under the Special Access Programme; through a Clinical Trial Application; and Investigational New Drug Products. The prices of over-the-counter (OTC) patented medicines and patented medicines for veterinary use are regulated by the Board on a complaints basis.
In the event that the Board finds, after a public hearing, that the price of a patented medicine is or was excessive in any market, it may order the patentee to reduce the price and take measures to offset any excess revenues that may have accrued.
Pharmaceutical Trends Reporting
The PMPRB reports annually to Parliament through the Minister of Health on its price review activities, the prices of patented medicines and price trends of all prescription drugs, and on the R&D expenditures reported by pharmaceutical patentees. In addition, as a result of the establishment of the National Prescription Drug Utilization Information System (NPDUIS) by federal/provincial/territorial (F/P/T) ministers of health, the Minister of Health, pursuant to her statutory authority under section 90 of the Patent Act, requested that the PMPRB conduct analysis of price, utilization, and cost trends for patented and non-patented prescription drugs so that Canada's health system has more comprehensive, accurate information on how all prescription drugs are being used and on the sources of cost increases. This function is aimed at providing F/P/T governments and other interested stakeholders with a centralized credible source of information on pharmaceutical trends.
Strategic Outcome and Program Alignment Architecture (PAA)
1. Strategic Outcome: Canadians are protected from excessive prices for patented medicines sold in Canada and stakeholders are informed on pharmaceutical trends.
- 1.1 Program: Patented Medicine Prices Regulation Program
- 1.2 Program: Pharmaceutical Trends Program
Internal Services
Organizational Priorities
| Priority |
Type1 |
Strategic Outcome and/or Program(s)* |
| 1Type is defined as follows: previously committed to—committed to in the first or second fiscal year prior to the subject year of the report; ongoing—committed to at least three fiscal years prior to the subject year of the report; and new—newly committed to in the reporting year of the RPP or DPR. If another type that is specific to the department is introduced, an explanation of its meaning must be provided. |
| Assess the impact of recent and pending changes to foreign and domestic pharmaceutical regulatory systems on the work of the PMPRB |
New |
The PMPRB has only one SO and all risks are linked to that SO. This priority is linked to Programs 1.1 and 1.2. |
| Description |
Why is this a priority?
Canada, like many countries, is facing escalating health care costs. Drugs represent a growing proportion of these costs. Increasingly, public payers are employing drug cost containment measures. Domestically, this has taken the form of confidential product listing agreements negotiated between provincial reimbursement authorities and pharmaceutical manufacturers. Internationally, countries are exploring cost effectiveness strategies like life-cycle and reference-based pricing approaches to pharmaceutical reimbursement. The former trend complicates the Board's ability to ascertain the true price of a drug, and may result in upward pressure on prices in the private market. The latter may negatively impact average foreign-to-Canadian price ratios. Finally, Canada's recent commitment under CETA to amend the Patent Act to extend the terms of pharmaceutical patents by up to two years is expected to reopen the debate over the appropriate balance between IP and consumer protection, at a time when the R&D activity of pharmaceutical patentees in Canada, both in the aggregate and as a ratio of sales, is in marked decline. The Board will closely monitor these developments to ensure the Guidelines and procedures in place continue to protect consumers from excessive prices and reflect the evolving national and international environments. |
What are the plans for meeting this priority?
- Examine foreign to Canadian price trends.
- Study international developments related to drug pricing and reimbursement regimes and their significance to Canada.
- Raise awareness in relevant federal departments (i.e., Industry Canada and Health Canada) on domestic and international pricing and R&D trends
- Engage with industry, public and private drug plan managers, and other stakeholders, on identifying potential improvements to the price review process.
|
| Priority |
Type |
Strategic Outcome and/or Program(s)* |
| Continue implementation of the Management Action Plan in response to the PMPRB Program Evaluation Report |
Previously committed to |
The PMPRB has only one SO and all risks are linked to that SO. This priority is linked to Programs 1.1 and 1.2. |
| Description |
Why is this a priority?
While the evaluation in 2011-12 affirmed the relevance, performance and efficiency and economy of the Patented Medicine Prices Regulation Program and the Pharmaceutical Trends Program, it identified four considerations for the Board:
1. Expedite all PMPRB processes
Quicker processes could mean, among other things, more efficient use of resources, shorter periods of excessive pricing, quicker access to information on price, sales and utilization trends which could result in greater use of information produced by the PMPRB.
2. Further simplify the Guidelines
Some stakeholders are concerned about inherent complexities in the Guidelines which they feel impede the predictability of the regulatory process. Further simplification of the Guidelines may make them more predictable, which may result in greater compliance with the Guidelines.
3. Expand plain language offerings throughout all PMPRB communications
Some stakeholders noted a need for shorter, less complex, plain language documents; these changes may make PMPRB publications clearer and more useful for all stakeholder groups.
4. Expand the target audience for outreach efforts
The evaluation revealed that a number of interviewees in the patient advocacy and third party payer categories were unaware of the kind of information/analysis the PMPRB provided and that they were eager to be better informed.
|
What are the plans for meeting this priority?
1. Expedite all PMPRB processes
- Report results of the service standards for: the scientific review of new patented drug products; the price review of new patented drug products; and the price review of existing patented drug products. Take appropriate action if required.
What are the plans for meeting this priority?
2. Further simplify the Guidelines
- Continue to assess the application and the impact of changes made to the Guidelines through the annual Monitoring and Evaluation Plan for Major Changes to the Guidelines (GMEP), update the Board and post findings on the PMPRB website.
- Monitor the judicial review results for clarification of regulatory issues and reflect results in the Board's Guidelines, as appropriate
3. Expand plain language offerings throughout all PMPRB communications
- Continue use of “In Brief” for the Annual Report
- Continue use of Executive Summary in all NPDUIS reports.
- Continue use of “Research Briefs” to highlight findings and policy implications of analytical studies conducted under the NPDUIS initiative
4. Expand the target audience for outreach efforts
- Liaise with appropriate public and private drug plan officials
- Continue search for opportunities to dialogue and share information on pharmaceutical trends and prices with other government and non-government agencies/organization
- Participate in the Health Portfolio Working Group on Regulatory Reform
|
| Priority |
Type |
Strategic Outcome and/or Program(s)* |
| Decrease regulatory burden and make effective use of Board Staff resources |
Previously committed to |
The PMPRB has only one SO and all risks are linked to that SO. This priority is linked to Programs 1.1 and 1.2. |
| Description |
Why is this a priority?
In support of the Prime Minister Red Tape Reduction Commission launched in January 2011, the PMPRB continues to support the spirit of this initiative by examining its price review process to identify possible ways to reduce the regulatory burden on patentees without adversely affecting its mandate to protect consumers. It is anticipated that reduced regulatory burden will lead to increased compliance and a more effective use of resources.
|
What are the plans for meeting this priority?
- Continue work on changing from two to one regulatory filing of price and sales data for existing patented medicines per year by patentees
- Implementation of the new Consumer Price Index (CPI) Adjustment methodology in 2015
|
| Priority |
Type |
Strategic Outcome and/or Program(s)* |
| Align internal operational framework of the PMPRB with Central Agency requirements. |
New |
The PMPRB has only one SO and all risks are linked to that SO.
|
| Description |
Why is this a priority?
As part of the Government's ongoing focus on containing costs, the Treasury Board Secretariat (TBS) continues to standardize and consolidate the delivery of external and internal services across the government. TBS remains focused on strengthening financial management and enhancing oversight of expenditures by implementing key reforms to the estimates process and advancing initiatives such as the electronic reporting pilot project.
The PMPRB must align its internal operating framework in order to strengthen its financial management and enhance stewardship of its public assets. By strengthening and streamlining how the PMPRB works, it will be more effective and accountable.
|
What are the plans for meeting this priority?
- Examine the PMPRB's financial and human resources forecasting process for ways to improve the effectiveness of forecasts for decision making
- Assess the practices and processes in place to manage security
- Complete the development and implementation of an electronic records and information management system to provide relevant and timely information to support decision making
|
Risk Analysis
| Risk |
Risk Response Strategy |
Link to Program Alignment Architecture |
| Non-compliance with the Board's new Guidelines |
- The PMPRB approved a Guidelines Monitoring and Evaluation Plan (GMEP).
- The Board continues to address challenges in the operationalization of the new Guidelines. The Board has been quick to clarify the interpretation and application of its Guidelines and to adopt approaches to facilitate efficient implementation of the new elements. This process is ongoing.
|
The PMPRB has only one SO and all risks are linked to that SO. |
| The evolution of the nature and distribution of patented medicines into more complex and innovative areas may hinder the effectiveness of the PMPRB's ability to deliver on its mandate |
- The Board is committed in its work to assess and consider potential modifications to its Guidelines so that they remain effective both in facilitating Board Staff's review of patented drug prices and in promoting voluntary compliance on the part of patentees.
- The Board continues to focus on transparency and communications.
- PMPRB funds continue to be committed to holding face-to-face outreach sessions with patentees and other relevant stakeholders, and to improving the accessibility and usefulness of material on the Board's website.
|
The PMPRB has only one SO and all risks are linked to that SO. |
| Changes respecting the pricing and reimbursement of patented medicines in the seven comparator countries listed in the Regulations may require the PMPRB to make adjustments to how international reference pricing is applied. |
- The PMPRB continues to monitor and assess changes respecting the pricing and reimbursement of patented medicines occurring in the seven foreign comparator countries it considers in conducting its price reviews.
- The PMPRB monitors for new publicly available ex-factory prices to improve the accuracy and transparency of the price review process.
|
The PMPRB has only one SO and all risks are linked to that SO. |
| Greater use of Product Listing Agreements by public payers and, in the future, perhaps private payers, could challenge the PMPRB's ability to ascertain the true price of a drug when used for reference purposes. |
- The PMPRB monitors and assesses changes respecting the pricing and reimbursement to public payers for patented medicines.
- The PMPRB monitors for new publicly available ex-factory prices to improve the accuracy and transparency of the price review process.
- The PMPRB builds trust with provincial plan managers through the NPDUIS initiative.
- The PMPRB dialogues with private and public payers on ways to contribute to the sustainability of the health care system.
|
The PMPRB has only one SO and all risks are linked to that SO. |
| Changes to Canadian intellectual property policy, as a result of international trade negotiations and treaties and declining R&D activity. |
- The PMPRB consults with stakeholders on the impact of these developments.
|
The PMPRB has only one SO and all risks are linked to that SO. |
Voluntary compliance by patentees is facilitated by published Guidelines intended to assist companies in setting prices that are not excessive. To date the PMPRB has benefitted from a high rate of compliance with the Guidelines (89.5% for 2009, 91% for 2010, 94.7% for 2011 and 92.5% for 2012). However, fluctuation in the rate of compliance could have a significant impact on the workload of Board Staff.
As in past years, many countries continue to wrestle with the containment of rising health care costs. In examining domestic drug prices, many countries, like Canada, are placing greater reliance on international price information to ensure that prices charged in their countries are not out of line with those being charged elsewhere. Recent changes to foreign and domestic pharmaceutical regulatory systems have focused on cost containment measures. The PMPRB continues to monitor and assess the impact of foreign and domestic changes to pharmaceutical regulatory systems on its price review process, particularly, changes to the systems in the seven foreign comparator countries.
Finally Canada's recent commitment under CETA to amend the Patent Act to extend the terms of pharmaceutical patents by up to two years is expected to reopen the debate over the appropriate balance between IP and consumer protection, at a time when the R&D activity of pharmaceutical patentees in Canada, both in the aggregate and as a ratio of sales, is in marked decline. Because criticism of the government's patent policy is sometimes expanded to include the price regulation program of the PMPRB, its mandate and performance may come under increased scrutiny.
Planned Expenditures
Budgetary Financial Resources (Planned Spending—dollars)
2014-15
Main Estimates |
2014-15
Planned Spending |
2015-16
Planned Spending |
2016-17
Planned Spending |
| 10,927,030 |
10,927,030 |
10,927,030 |
10,927,030 |
Human Resources (Full-time equivalents—FTEs)
| 2014-15 |
2015-16 |
2016-17 |
| 73 |
73 |
73 |
Budgetary Planning Summary for Strategic Outcome and Programs (dollars)
| Strategic Outcome, Programs and Internal Services |
2011-12 Expenditures |
2012-13 Expenditures |
2013-14 Forecast Spending |
2014-15 Main Estimates |
2014-15 Planned Spending |
2015-16 Planned Spending |
2016-17 Planned Spending |
| Strategic Outcome 1: Canadians are protected from excessive prices for patented medicines sold in Canada and stakeholders are informed on pharmaceutical trends. |
| Patented Medicine Prices Regulation Program |
7,346,773 |
3,888,795 |
6,137,834 |
6,827,010 |
6,827,010 |
6,827,010 |
6,827,010 |
| Pharmaceutical Trends Program |
1,010,528 |
983,279 |
1,183,470 |
1,267,557 |
1,267,557 |
1,267,557 |
1,267,557 |
| Internal Services Subtotal |
3,397,074 |
3,184,729 |
2,966,000 |
2,832,463 |
2,832,463 |
2,832,463 |
2,832,463 |
| Total |
11,754,375 |
8,056,803 |
10,287,304 |
10,927,030 |
10,927,030 |
10,927,030 |
10,927,030 |
Expenditures for 2011-12 were significantly higher than expenditures in 2012-13. This variance is due in large part to a Federal Court decisionvii that quashed a Board Order and directed the PMPRB return to the patentee the sum of $2,512,878 paid to the Board as a payment of excess revenues earned, plus appropriate interest and costs totalling $46.9 thousand.
Forecast Spending for 2013–14 is significantly higher than anticipated because of a Federal Court decisionviii that quashed the Board Order of February 2012 and ordered the PMPRB to return to the patentee the sum of $2,801,285 plus appropriate interest and costs totalling $70,628 thousand.
The 2014–15 Main Estimates amount includes funding for a Special Purpose Allotment (SPA) in the amount of $2,470,000. The SPA is for conducting Public Hearings and can only be used to cover costs such as external legal counsel, expert witnesses, etc. Any SPA funds not required for hearings are returned to the Consolidated Revenue Fund (CRF).
Planned spending in 2014–15 and future years is based on the assumption that the PMPRB will spend the full $2.47 million held in the SPA reserved for conducting public hearings. This is done because these expenditures are dependent on the number of hearings, and the length and complexity of the hearings held, which are difficult to predict.
Alignment to Government of Canada Outcomes
014-15 Planned Spending by Whole-of-Government-Framework Spending Areaix (dollars)
| Strategic Outcome |
Program |
Spending Area |
Government of Canada Outcome |
2014-15 Planned Spending |
| 1 Canadians are protected from excessive prices for patented medicines sold in Canada and stakeholders are informed on pharmaceutical trends. |
1.1
Patented Medicine Prices Regulation Program |
Social Affairs |
Healthy Canadians |
6,827,010 |
1.2
Pharmaceutical Trends Program |
Social Affairs |
Healthy Canadians |
1,267,557 |
Total Planned Spending by Spending Area (dollars)
| Spending Area |
Total Planned Spending |
| Economic Affairs |
|
| Social Affairs |
8,094,567 |
| International Affairs |
|
| Government Affairs |
|
Departmental Spending Trend
Departmental Spending Trend Graph
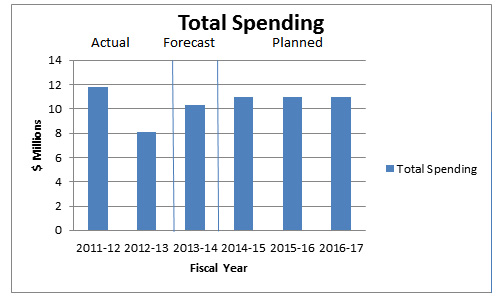
In 2011–12, the PMPRB's Actual Spending is higher than usual because the Board was directed by the Federal Court to return, to a patentee, the sum of $2,512,878, received through an earlier Board Order, plus appropriate interest and specified costs which totalled $46.9 thousand.2
Actual Spending for 2012–13 was significantly less than the previous year as a result of a lapse of approximately $3 million in the SPA3 and $776 thousand of operating funds which was in large part attributable to savings gained through operational efficiencies and streamlining processes.
In 2013–14, the PMPRB's Forecast Spending is higher than originally anticipated because it includes a repayment of $2,801,285 plus appropriate interest to a patentee. In these cases, the PMPRB receives additional authorities to cover the repayment.
Due to challenges related to forecasting the number and complexity of hearings, for purposes of forecasting Planned Spending for future years it is assumed that the entire SPA funding will be spent each fiscal year.
Estimates by Vote
For information on the Patented Medicine Prices Review Board's organizational appropriations, please see the 2014-15 Main Estimates publication.x
Section II: Analysis of Program(s) by Strategic Outcome(s)
Strategic Outcome: Canadians are protected from excessive prices for patented medicines sold in Canada and stakeholders are informed on pharmaceutical trends.
Program 1.1: Patented Medicine Prices Regulation Program
Description:
The PMPRB is an independent quasi-judicial body that is responsible for ensuring that the prices that patentees charge for patented medicines sold in Canada are not excessive based on the price review factors in the Patent Act (Act). To make this determination the Board must consider each of the following factors: prices at which the medicine and other medicines in the same therapeutic class have been sold in Canada and in the seven comparator countries listed in the Patented Medicines Regulations (Regulations); changes in the Consumer Price Index (CPI); and in accordance with the Act, such other factors as may be specified in any regulations made for the purposes of the price review. Under the Act, and as per the Regulations, patentees are required to file price and sales information for each patented medicine sold in Canada, for the duration of the patent(s). Board Staff reviews the introductory and ongoing information filed by patentees, for all patented medicines sold in Canada. When it finds that the price of a patented medicine appears to be excessive, Board Staff will conduct an investigation into the price. An investigation could result in: its closure where it is concluded that the price was non-excessive; a Voluntary Compliance Undertaking (VCU) by the patentee to reduce the price and offset excess revenues obtained as a result of excessive prices through a payment and/or a price reduction of another patented drug product; or a public hearing to determine if the price is excessive, including any remedial order determined by the Board. In the event that the Board Hearing Panel finds, after a public hearing, that a price is or was excessive, it may order the patentee to reduce the price and take measures to offset any excess revenues. This program, by reviewing the prices charged by patentees for patented medicines sold in Canada, protects Canadians and the health care system from excessive prices.
2014-15
Main Estimates |
2014-15
Planned Spending |
2015-16
Planned Spending |
2016-17
Planned Spending |
| 6,827,010 |
6,827,010 |
6,827,010 |
6,827,010 |
Human Resources (Full-time equivalents—FTEs)
| 2014-15 |
2015-16 |
2016-17 |
| 44 |
44 |
44 |
Performance Measurement
| Expected Results |
Performance Indicators |
Targets |
Date to be Achieved |
| Patentees comply with the Patent Act, the Regulations, and the Excessive Price Guidelines (Guidelines) |
Percentage of patented medicines that are priced, as a result of voluntary compliance, within the Guidelines or at a price which does not trigger the investigation criteria |
95% |
March 31 of each year |
| Percentage of compliance with Board Orders related to price and/or jurisdiction and with Voluntary Compliance Undertakings (VCUs) |
100% |
March 31 of each year |
| Canadian prices for patented medicines are on average in line with prices in the seven comparator countries listed in the Regulations |
Canadian prices for new patented medicines are on average at or below the median of international prices |
100% |
March 31 of each year |
| Canadian prices for existing patented medicines are on average at or below the median of international prices |
100% |
March 31 of each year |
Planning Highlights
The PMPRB relies on voluntary compliance whenever possible since it is less time consuming and less costly to all parties. Voluntary compliance by patentees is facilitated by published Guidelines, which are intended to assist patentees in setting prices that are not excessive by providing transparent and predictable information on how a price review is conducted.
Since the implementation of the new Guidelines in January 2010, Board Staff has been monitoring and evaluating the application and impact of the major changes on an ongoing basis through the annual publication of its Monitoring and Evaluation Plan for the Major Changes in the Guidelines.
The key priorities for the Patented Medicine Price Regulation Program over the planning period are to:
- Examine foreign to Canadian price trends.
- Study international developments related to pricing and reimbursement regimes and their significance to Canada.
- Raise awareness in relevant federal departments (i.e., Industry Canada and Health Canada) on domestic and international pricing and R&D trends.
- Engage with industry, public and private drug plan managers, and other stakeholders, on identifying potential improvements to the price review process.
- Continue to monitor and evaluate the impact of the new Guidelines.
- Continue consultation on and implementation of the CPI Adjustment Methodology.
- Continue work to change from two to one regulatory filing of price and sales data for existing patented medicines per year by patentees.
- Continue monitoring judicial review results for clarification of regulatory issues and reflect results in the Board's regulatory framework, as appropriate.
Program 1.2: Pharmaceutical Trends Program
Description:
The PMPRB reports annually to Parliament through the Minister of Health on its price review activities, the prices of patented medicines and price trends for all drugs, and R&D expenditures as reported by pharmaceutical patentees. In supporting this requirement, the pharmaceutical trends program provides complete and accurate information on trends in manufacturers' prices of patented medicines sold in Canada and on patentees' research-and-development expenditures to interested stakeholders including: industry (i.e., brand-name, biotech, generic); federal, provincial and territorial (F/P/T) governments; consumer and patient advocacy groups; third party payers; and others. This information also provides assurance to Canadians that the prices of patented medicines are not excessive. In addition, as a result of the establishment of the National Prescription Drug Utilization Information System (NPDUIS) by F/P/T ministers of health the Federal Minister of Health requested that the PMPRB conduct analysis of price, utilization and cost trends for patented and non-patented prescription drugs so that Canada's health system has more comprehensive, accurate information on how all prescription drugs are being used and on the sources of cost increases. This function is aimed at providing F/P/T governments and other interested stakeholders with a centralized credible source of information on all prescription drug prices.
Budgetary Financial Resources (dollars)
2014-15
Main Estimates |
2014-15
Planned Spending |
2015-16
Planned Spending |
2016-17
Planned Spending |
| 1,267,557 |
1,267,557 |
1,267,557 |
1,267,557 |
Human Resources (Full-time equivalents—FTEs)
| 2014-15 |
2015-16 |
2016-17 |
| 10 |
10 |
10 |
Performance Measurement
| Expected Results |
Performance Indicators |
Targets |
Date to be Achieved |
| Information on pharmaceutical trends and cost drivers is available to stakeholders |
Number of new reports/studies posted on the PMPRB website |
12 reports/studies |
March 31 each year |
| Number of presentations made by the PMPRB to an external audience |
10 information sessions |
March 31 each year |
Planning Highlights
The PMPRB will continue to provide relevant and timely reporting, provide credible pharmaceutical trend information and contribute to the information needs of a variety of policy decision-makers by:
- Reporting annually to Parliament through the Minister of Health, on its major activities, the PMPRB's price review activities, the prices of patented medicines and price trends for all drugs, and R&D expenditures as reported by pharmaceutical patentees.
- Responding to F/P/T information needs identified by the NPDUIS Steering Committee.
- Expanding the use of plain language throughout PMPRB publications.
- Continuing the use of Executive Summaries in all NPDUIS reports
- Providing the results of relevant research analysis conducted under the NPDUIS initiative in a timelier manner.
Internal Services
Description:
Internal Services are groups of related activities and resources that are administered to support the needs of programs and other corporate obligations of an organization. These groups are: Management and Oversight Services; Communications Services; Legal Services4; Human Resources Management Services; Financial Management Services; Information Management Services; Information Technology Services; Real Property Services; Materiel Services; Acquisition Services; and Other Administrative Services. Internal Services include only those activities and resources that apply across an organization and not to those provided specifically to a program.
Budgetary Financial Resources (dollars)
2014-15
Main Estimates |
2014-15
Planned Spending |
2015-16
Planned Spending |
2016-17
Planned Spending |
| 2,832,463 |
2,832,463 |
2,832,463 |
2,832,463 |
Human Resources (Full-time equivalents—FTEs)
| 2014-15 |
2015-16 |
2016-17 |
| 19 |
19 |
19 |
Planning Highlights
The key priorities for Internal Services over the planning period are to:
- Examine the PMPRB's financial and human resources forecasting process for ways to improve the effectiveness of forecasts for decision making;
- Assess the practices and processes in place to manage security; and
- Complete the development and implementation of an electronic records and information management system to provide relevant and timely information to support decision-making
The PMPRB is a participant in the 2013-16 Federal Sustainable Development Strategy and contributes to the Theme IV (Greening Government Operations) targets through the internal services program. The department plans to
- Take action to embed environmental considerations into public procurement, in accordance with the federal Policy on Green Procurement;
- Develop an approach to maintain or improve the sustainability of its workplace operations; and
- Establish SMART targets to reduce the environmental impact of its services to clients.
Additional details on the PMPRB's activities can be found in the Greening Government Operations Supplementary Information Table.
Section III: Supplementary Information
Future-Oriented Statement of Operations
The future-oriented condensed statement of operations presented in this subsection is intended to serve as a general overview of the Patented Medicine Prices Review Board's operations. The forecasted financial information on expenses and revenues are prepared on an accrual accounting basis to strengthen accountability and to improve transparency and financial management.
Because the future-oriented statement of operations is prepared on an accrual accounting basis and the forecast and planned spending amounts presented in other sections of this report are prepared on an expenditure basis, amounts will differ.
A more detailed future-oriented statement of operations and associated notesxi, including a reconciliation of the net costs of operations to the requested authorities, can be found on the Patented Medicine Prices Review Board's website.
Future-Oriented Condensed Statement of Operations
For the Year Ended March 31 (dollars)
| Financial information |
Estimated Results
2013−14 |
Planned Results
2014–15 |
Change |
| 1 The PMPRB collects non-respendable revenue as a result of payments made by patentees to the Government of Canada through Voluntary Compliance Undertakings (VCUs) or Board Orders to offset excess revenues. The Minister may enter into agreements with any province or territory respecting the distribution to that province/territory of amounts received by the Receiver General, less any costs incurred in relation to the collection and distribution of those amounts. As at December 31, 2013, the PMPRB collected $10,356,886 in non-respendable revenue. This amount will be offset by a Court ordered refund to a patentee of a payment of excess revenues; the net amount of non-respendable revenues is $7,555,601. Revenues that are non-respendable are not available to discharge the PMPRB's liabilities. While the Deputy Head is expected to maintain accounting control, she has no authority regarding the disposition of non-respendable revenues. As a result, non-respendable revenues are considered to be earned on behalf of the Government of Canada and are therefore presented in reduction of the entity's gross revenues. |
| Total expenses |
8,930,618 |
12,515,942 |
3,585,324 |
| Total revenues1 |
0 |
0 |
|
| Net cost of operations |
8,930,618 |
12,515,942 |
3,585,324 |
The $3,585,324 change from Estimated Results for 2013–14 to Planned Results for 2014–15 is largely a result of the fact that Planned Results for 2014–15 is based on the assumption that the entire $2,470 thousand held in the SPA will be used while the 2013–14 Planned Results recognizes that there will be a lapse of $2,423.7 thousand of funds in the SPA. The remaining difference is the result of a lapse in salaries and O&M caused by process streamlining, gained efficiencies, and delays in staffing unanticipated vacant positions.
The PMPRB is currently awaiting a Federal Court decision on its jurisdiction with respect to patented generic drug products. A favourable decision for the PMPRB would result in a significant workload increase and potentially the need for additional staff.
List of Supplementary Information Tables
The supplementary information tables listed in the 2014–15 Report on Plans and Prioritiesxii can be found on the Patented Medicine Prices Review Board's website.
- Greening Government Operations;
- Upcoming Internal Audits and Evaluationsover the next three fiscal years;
Tax Expenditures and Evaluations Report
The tax system can be used to achieve public policy objectives through the application of special measures such as low tax rates, exemptions, deductions, deferrals and credits. The Department of Finance publishes cost estimates and projections for these measures annually in the Tax Expenditures and Evaluationsxiii publication. The tax measures presented in the Tax Expenditures and Evaluations publication are the sole responsibility of the Minister of Finance.
Section IV: Organizational Contact Information
The Patented Medicine Prices Review Board
Box L40
Standard Life Centre
333 Laurier Avenue West
Suite 1400
Ottawa, Ontario K1P 1C1
Telephone: (613) 952-7360
Toll-free: 1-877-861-2350
Facsimile: (613) 952-7626
TTY: (613) 957-4373
Email: pmprb@pmprb-cepmb.gc.ca
Website: www.pmprb-cepmb.gc.ca
Endnotes
- Treasury Board Secretariat Estimates Publications and Appropriation Acts, http://www.tbs-sct.gc.ca/ems-sgd/esp-pbc/esp-pbc-eng.asp
- Selected Departmental Performance Reports for 2008-2009 – Department of Industry, Department of Transport. Report of the Standing Committee on Public Accounts, September 2010: http://www.parl.gc.ca/HousePublications/Publication.aspx?Mode=1&Parl=40&Ses=3&Language=E&DocId=4653561&File=0
- Strengthening Parliamentary Scrutiny of Estimates and Supply, Report of the Standing Committee on Government and Operations Estimates, June 2012:
http://www.parl.gc.ca/HousePublications/Publication.aspx?DocId=5690996&Language=E&Mode=1&Parl=41&Ses=1
- For information on the “four spending areas” go to:
http://www.tbs-sct.gc.ca/ppg-cpr/frame-cadre-eng.aspx
- Patent Act: http://laws.justice.gc.ca/en/P-4/SOR-94-688/index.html)
- Patented Medicines Regulations: http://laws.justice.gc.ca/en/P-4/SOR-94-688/index.html
- The Federal Court decision in the matter of Sanofi Pasteur Limited's request for judicial review of the decision of the Patented Medicines Prices Review Board dealing with the remedy granted with respect to the excessive revenues can be found on the PMPRB Website: http://www.pmprb-cepmb.gc.ca/CMFiles/Hearings%20and%20Decisions/
Decisions%20and%20Orders/T-83-10-Quadracel-Pentacel-FC-DECISION-July-12-2011.pdf
- The Federal Court decision on Teva Canada Innovation's request that the court set aside a decision of the Patented Medicines Prices Review Board dated February 23, 2012 can be found at: http://decisions.fct-cf.gc.ca/site/fc-cf/decisions/en/item/62146/index.do
- For information on the Alignment to Government of Canada Outcomes see: Whole-of-Government-Framework: http://www.tbs-sct.gc.ca/ppg-cpr/frame-cadre-eng.aspx
- 2014-15 Main Estimates: http://www.tbs-sct.gc.ca/ems-sgd/esp-pbc/esp-pbc-eng.asp
- A more detailed future-oriented statement of operations and associated notes including a reconciliation of the net costs of operations to the requested authorities, can be found on the Patented Medicine Prices Review Board's website: http://www.pmprb-cepmb.gc.ca/english/View.asp?x=1802
- The supplementary information tables listed in the 2014–15 Report on Plans and Priorities can be found on the Patented Medicine Prices Review Board's website:
www.pmprb-cepmb.gc.ca, under Reports to Parliament, Report on Plans and Priorities
- Government of Canada Tax Expenditures: http://www.fin.gc.ca/purl/taxexp-eng.asp
2 The PMPRB's Actual Spending in 2011–12 increased as a result of receiving additional funds in the amount of $2,559.8 thousand to cover a court awarded refund of a Board Order. Following a hearing of the Board conducted in 2008–09 pursuant to the Patent Act, the Board concluded that the patentee had sold two patented medicines in Canada at excessive prices. The patentee was ordered by the Board to pay the amount of $2,512,878 to the Crown. In 2011–12, the Federal Court quashed the Board Order and directed that the sum of $2,512,878 be returned to the patentee with appropriate interest and specified costs which totalled $46.9 thousand.
3 In 2012–13, the total funds in the SPA were $3,100K. In 2013–14 and beyond the amount in the SPA is $2,470K.
4 Since the PMPRB is an independent quasi-judicial body with a regulatory mandate, the PMPRB reviews the prices patentees charged for each individual patented drug product sold in Canada. Under the Patent Act, if a price is found to be excessive the Board may hold a public hearing and order a price reduction and/or the offset of excess revenues. Organizationally, the PMPRB is structured with an internal Legal Services department which is responsible for providing legal research and advice related to the PMPRB's regulatory mandate and leads the prosecution in the context of a hearing before a Hearing Panel. Consequently, a large portion of the cost of legal services is allocated to the Patented Medicine Price Regulation Program.