Summary of Progress Against Priorities
| Priority |
Type1 |
Strategic Outcome(s) and/or Program Activity(ies) |
| Monitoring and evaluation of the impact of the new Guidelines |
Previously committed to |
The PMPRB has only one Strategic Outcome (SO) and all priorities are linked to that SO. This priority is linked to PA 1. |
- Since the implementation of the new Guidelines on January 1, 2010, the PMPRB has been monitoring and evaluating the application and impact of the changes to the Guidelines on an ongoing basis. In June 2011, the PMPRB published the Monitoring and Evaluation Plan for the Major Changes to the Guidelines (GMEP) on its website. In December 2011, Board Staff presented the first annual assessment under the Plan to the Board. The results of this assessment are included in the GMEP.
- The DIP Methodology Technical Working Group (DIP WG) was reconvened early in 2012 to discuss experiences to date regarding the pilot application of the DIP Methodology. During the pilot application, the DIP Methodology was invoked 40 times and was instrumental in resolving 22 investigations opened prior to 2010 and 12 investigations opened during and after 2010. The DIP WG proposed options to address application-related issues identified during the pilot, and recommended the adoption of the DIP Methodology (Simplified and Regular) on a permanent basis. The Board agreed with the DIP WG.
- Two other Guidelines issues were identified as pending assessment: (1) any market review for existing patented drug products; and (2) offset of excess revenues (Does not Trigger Investigation Criteria; and Thresholds for Opening an Investigation). In the April 2012 NEWSletter, the Board provided clarification on the application of “any market” price reviews. Following consultation with stakeholders, the Board adopted changes to the Guideline on “Offset of Excess Revenues” to allow patentees to offset revenues in a timely manner and the investigation criteria for existing patented drug products were revised to eliminate the 5% trigger.
|
| Priority |
Type |
Strategic Outcome(s) and/or Program Activity(ies) |
| Increasing awareness and understanding of the PMPRB mandate and regulatory framework among a variety of stakeholders |
New |
The PMPRB has only one SO and all priorities are linked to that SO. This priority is linked to PA 1 and 2. |
- The PMPRB provides an update of its upcoming engagement activities in its quarterly NEWSletter.
- To increase patentees' awareness of the PMPRB regulatory framework and their understanding of the Guidelines, PMPRB Staff held a number of Outreach sessions and Webinars in 2011 and 2012:
- Outreach sessions were held in Montreal and Toronto on March 2 and 3, 2011. Seventy-two (72) patentees and consultants attended.
- A webinar on the DIP methodology was conducted on April 20, 2011. Thirty-seven (37) patentees and consultants participated.
- Outreach sessions were held in Montreal and Toronto on February 28 and 29, 2012. Eighty-two patentees/consultants attended.
- A webinar on the DIP methodology was conducted on June 7, 2012. Forty-one (41) patentees/consultants participated.
For all outreach sessions, the feedback from participants was mostly positive; the vast majority of participants indicated that the session answered their questions on the Guidelines and related topics.
- The Chairperson and Executive Director conducted a series of meetings with provincial health care and public drug program officials, and various other stakeholders to discuss their areas of concern and their understanding of the role of the PMPRB. They both also made presentations at a number of conferences and other events throughout the fiscal year.
- In 2011-12 the PMPRB continued work on enhancing the content of its website. On January 31, 2012, the PMPRB launched its user-friendly Web application for New Patented Medicines Reported to the PMPRB. This new application includes a complete list of medicines reported to the PMPRB since 1998 and is searchable by the year reported and by the status of the price review (for 2001 onward).
- Through the National Prescription Drug Utilization Information System (NPDUIS) the PMPRB continues its partnership with the Canadian Institute for Health Information, Health Canada and the provinces and territories. In fiscal year 2011-12, the PMPRB released five new analytical reports.
- In addition, the NPDUIS Steering Committee annual meeting in October 2011 provided an opportunity for the PMPRB to share the results of completed and ongoing research studies, discuss priorities for future research, and engage with members of the Pharmaceutical Policy Research Collaboration (PPRC), a publicly funded network of university-based researchers from across Canada.
|
| Priority |
Type |
Strategic Outcome(s) and/or Program Activity(ies) |
| Succession Planning and knowledge management |
New |
The PMPRB has only one SO and all priorities are linked to that SO. This priority is linked to PA 1, 2 and 3. |
- The PMPRB has adopted its succession planning framework and is in the process of implementing the associated activities. Anticipating that a few key senior management positions will become vacant in the coming 12 to 24 months, management has identified the senior management team positions as key positions. The PMPRB is currently in the process of reviewing the Key Position Profiles, including the competencies and skills sets required for the team.
|
| Priority |
Type |
Strategic Outcome(s) and/or Program Activity(ies) |
| Program evaluation |
Previously committed to |
The PMPRB has only one SO and all priorities are linked to that SO. This priority is linked to PA 1 and 2. |
- In conjunction with the provision of increased resources in 2008-09 and ongoing, the PMPRB committed to an evaluation in 2011-12 to assess the extent to which the increase in resources have helped the PMPRB achieve its objectives.
- The PMPRB's Logic Model, Evaluation Matrix and Performance Measurement Framework provided the structure for evaluation activities used to assess performance against desired outcomes.
- The evaluation included:
- Interviews of 46 key informants;
- A public survey with 74 respondents;
- A document/literature review; and
- Analysis of performance data provided by the PMPRB.
- The Final draft of the Evaluation Report is currently under review, finalization of the Report and a Management Response are expected to be completed by the end of calendar year.
|
Risk Analysis
To fulfil its regulatory mandate the PMPRB conducts price reviews, investigations and, when needed, hearings. The new Excessive Price Guidelines which came into force in January 2010 are intended to promote the continuing effectiveness, fairness, transparency, predictability and relevance of the price review process in today's current and evolving pharmaceutical environment.
The PMPRB has observed changes in the pharmaceutical environment, domestically and internationally, as distribution practices evolve, sales models change, patentees introduce different types of benefit programs, and new types of drugs continue to reach the market. Other countries are adopting price control policies and enacting new legislation. In light of these shifts, the PMPRB has focused on assessing its relevance, effectiveness and strategic direction.
The PMPRB has continued to experience some challenges related to operationalizing the revised Guidelines, including increased complexity of scientific reviews (due to additional therapeutic improvement factors) and price reviews. Board Staff continues to monitor and evaluate the application and impact of the revised Guidelines. In keeping with the Board's commitment to adjust and amend its Guidelines as required, the Board published a clarification on the Guideline on “Any Market” Price Reviews as well as a request for Notice and Comment on the elimination of the 5% investigation trigger in the April 2012 issue of the NEWSletter. Following a thorough review of the comments received, a consolidated updated version of the Compendium of Policies, Guidelines and Procedures was issued in June 2012.
In December 2011, Board Staff presented the first annual assessment of the major Guidelines changes to the Board. The results of this assessment are included in the Guidelines Monitoring and Evaluation Plan (GMEP). The next annual assessment will be presented to the Board in December 2012.
The PMPRB has been working on the development of a new compliance database since 2010-11. In 2011-12 the new database underwent rigorous testing; the July 2011 patentee filings were processed in both the old and new systems. The results generated by running both systems in parallel were less satisfactory than anticipated. As a result it was decided that both systems would also be used for the January 2012 filing. Since then minor adjustments have been made to the new database and Board Staff is now satisfied with the results it generated. The July 2012 patentee filings will be processed in the new system only and the Regulatory Affairs and Outreach Branch has taken over the data input process entirely. Complete adoption and implementation of the new system is expected to translate into efficiencies and possible financial savings over the coming fiscal year.
As a result of the increased resources made available to the PMPRB in 2009-10, the PMPRB was able to eliminate the backlog of investigations and continues to reallocate resources as required to meet challenges arising from changes to the Guidelines.
The Board's Rules of Practice and Procedure (Rules)2 for Hearings were revised to codify the Board's practices and procedures and to take into consideration relevant current practices in other federal administrative tribunals and Courts. The proposed Rules were pre-published in the Canada Gazette, Part 1. The Board will be seeking enactment of the Rules and their publication in Canada Gazette, Part II this Fall.
In 2011-12 the PMPRB conducted an evaluation of its two programs: compliance and enforcement of non-excessive prices for patented drug products (compliance and enforcement program); and pharmaceutical trends analysis. As part of this evaluation 46 stakeholders were interviewed; a stakeholder survey, a document and literature review and an analysis of performance data were conducted. The final Report will be presented to the Board for acceptance in September. With guidance from the Chairperson, Board staff will prepare a management response and action plan which is expected to be released by the end of the calendar year. A tracking system will be put in place to follow up on management action plans arising from the exercise.
Summary of Performance
2011–12 Financial Resources ($ thousands)
| Planned Spending |
Total Authorities* |
Actual Spending* |
|
* The variance between Planned Spending and Total Authorities is a result of additional funding received through an adjustment warrant in the amounts of $2,512,878 to refund amounts credited to revenues in previous years, and $46.9 thousand to cover an interest and costs payment awarded by the Federal Court. In addition the PMPRB received an additional $1 million for severance cash outs and the carry-forward from 2010-11.
Following a hearing of the Board conducted in 2008-09 pursuant to the Patent Act, the Board concluded that the patentee had sold two patented medicines in Canada at excessive prices. The patentee was ordered by the Board to pay the amount of $2,512,878 to the Crown. The patentee complied with the Board Order but applied for judicial review of the Order. The Federal Court quashed the Board Order and directed in its judgment that the sum of $2,512,878 be returned promptly to the patentee with appropriate interest and specified costs.
|
| $11,855.0 |
$15,248.9 |
$11,754.4 |
2011–12 Human Resources (full-time equivalents [FTEs])
| Planned |
Actual* |
Difference* |
|
*Budget 2010 announced that departments would not be funded for the 2010-11 to 2012-13 wage and salary increases resulting from collective agreements. The PMPRB has estimated the impact of this government-wide initiative to be $177,937 in 2011-12. In order to address this resource issue, the PMPRB's determined that as a result of efficiencies gained through other initiatives, it was in a position to not staff some vacant positions without significantly impacting its operations.
|
| 76 |
63 |
13 |
Summary of Performance Tables
Progress Toward Strategic Outcome
| Strategic Outcome: Canadians and their health care system are protected from excessive prices for patented drug products sold in any market in Canada and key stakeholders are informed by pharmaceutical trends analysis. |
| Performance Indicators |
Targets |
2011–12 Performance |
| Canada's prices on average are in line with the seven comparator countries listed in the Regulations. |
Canada's prices on average are at or below the median of international prices. |
The average median international price (MIP)-to-Canadian price ratio stood at 1.05 in 2011. This indicates that the median price of patented drug products in the seven comparator countries was 5% higher than Canadian prices last year. |
Performance Summary, Excluding Internal Services
| Program Activity |
2010-11
Actual
Spending |
2011–12
($ thousands) |
Alignment to Government of Canada Outcomes |
Main
Estimates |
Planned
Spending |
Total
Authorities* |
Actual
Spending** |
|
* The variance between Planned Spending and Total Authorities is a result of additional funding received through an adjustment warrant in the amounts of $2,512,878 to refund amounts credited to revenues in previous years. This money was returned to the patentee.
|
|
** PA 1 – The variance between Total Authorities and Actual Spending is a result of a lapse of $2.4 million in the Special Purpose Allotment (SPA). Funding for PA 1 includes a SPA to conduct Public Hearings, in Vote 35 (Program expenditures) of $3.1 million. The SPA can only be used to cover the costs of public hearings such as, external legal counsel, expert witnesses, etc. Any unspent amount is returned to the Consolidated Revenue Fund (CRF). In 2011-12 expenditures from the SPA were $668 thousand.
|
PA 1
Compliance and enforcement of non-excessive pricing for patented drug products |
4,232.0 |
7,464.8 |
7,464.8 |
10,318.0 |
7,346.7 |
Healthy Canadians |
PA 2
Pharmaceutical trend analysis |
890.4 |
1,438.7 |
1,438.7 |
1,425.0 |
1,010.5 |
Healthy Canadians |
| Total |
5,122.4 |
8,903.5 |
8,903.5 |
11,743.0 |
8,357.3 |
|
Performance Summary for Internal Services
| Program Activity |
2010-11
Actual
Spending |
2011–12
($ thousands) |
Main
Estimates |
Planned
Spending |
Total
Authorities* |
Actual
Spending |
|
* The variance between Planned Spending and Total Authorities is a result of additional funding received through an adjustment warrant in the amounts of $2,512,878 to refund amounts credited to revenues in previous years. This money was returned to the patentee.
|
| Internal Services |
4,348.3 |
2,951.5 |
2,951.5 |
3,505.9 |
3,397.1 |
Strategic Environmental Assessment
During 2011–12, none of the initiatives undertaken by the PMPRB were subject to the Cabinet Directive on the Environmental Assessment of Policy, Plan and Program Proposals 3.
Expenditure Profile
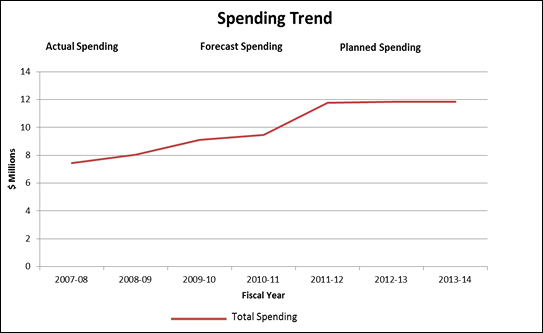
[D]
Estimates by Vote
In September 2008, the PMPRB received $4.7 million (excluding EBP), in addition to its core A-base of $5.8 million to meet workload pressures and continue ongoing initiatives related to the delivery of its mandate. Vote 35 (Program expenditures) was increased by $5.6 million for 2009-10, $6.2 million for 2010-11, and $5.8 million for 2011-12 and future years (including EBP and excluding Public Works and Government Services Canada accommodation charges).
The PMPRB's total funding includes a Special Purpose Allotment (SPA) of $3.1 million which is used to conduct Public Hearings. The SPA can only be used to cover the costs of public hearings such as, external legal counsel, expert witnesses, etc. Any unspent amount is returned to the Consolidated Revenue Fund (CRF).
In 2011-12, the PMPRB is reporting an increase in Total Authorities as a result of receiving additional funds in the amount of $2,559.8 thousand to cover a court awarded refund of a Board Order. Following a hearing of the Board conducted in 2008-09 pursuant to the Patent Act, the Board concluded that the patentee had sold two patented medicines in Canada at excessive prices. The patentee was ordered by the Board to pay the amount of $2,512,878 to the Crown. In 2011-12, the Federal Court quashed the Board Order and directed that the sum of $2,512,878 be returned promptly to the patentee with appropriate interest and specified costs which totalled $46.9 thousand.
The variance between Total Authorities and Actual Spending in 2011-12 is a result of a lapse of $2.4 million in the SPA. In 2011-12 expenditures from the SPA amounted to $668 thousand because there were fewer hearings than in previous years due in part to the submission of VCUs.
The PMPRB had planned a lapse of approximate $400 thousand in order to maximize its Operating budget carry forward. However, as a result of unanticipated vacant positions and an increased number of employees on maternity leave the PMPRB lapsed an additional $400 thousand.
For information on the PMPRB's organizational Votes and/or statutory expenditures, please see the Public Accounts of Canada 2011 (Volume II). An electronic version of the Public Accounts 20114 is available on the Public Works and Government Services Canada's website.
Section II: Analysis of Program Activities by Strategic Outcome
Strategic Outcome
Canadians and their health care system are protected from excessive prices for patented drug products sold in any market in Canada and key stakeholders are informed by pharmaceutical trends analysis.
| Performance Indicator |
Target |
|
Canada's prices on average are in line with the seven comparator countries listed in the Regulations.
|
Canada's prices on average are at or below the median of international prices.
|
On an annual basis, the PMPRB conducts bilateral and multilateral price comparisons for each of the seven comparator countries using market exchange rates for currency conversion.
As in previous years, in 2011, Canadian prices were typically within the range of prices observed among the comparator countries. Results indicate that Canadian prices were roughly in line with Swedish and Swiss prices (5% lower and 3% higher, respectively). Prices in Italy, France and the United Kingdom were appreciably lower than Canadian prices (16%, 16% and 18%, respectively), while those in Germany and the United States were much higher than prices in Canada (20% and 98% higher, respectively).
The median international price (MIP), the median of prices observed among the seven comparator countries, is one of the measures used in multilateral price comparisons. The average MIP-to-Canadian price ratio stood at 1.05 in 2011. This indicates that the median price of patented drug products in the seven comparator countries was 5% higher than Canadian prices last year. Since 2006, the ratio has remained somewhat consistent, ranging between 1.07 and 1.03.
Program Activity 1: Compliance and enforcement of non-excessive pricing of patented drug products
Program Activity Description
The PMPRB is responsible for regulating the non-excessive average prices for patented drug products sold in Canada for human and veterinary use. Through this program activity, the PMPRB reviews the prices that patentees charge for patented drug products, based on the price review factors in the Patent Act, to ensure that these prices are not excessive. In the event that the Board finds, following a public hearing, that a price is excessive in any market, it may order the patentee to reduce the price and take measures to offset any excess revenues it may have received as a result of excessive prices.
2011–12 Financial Resources ($ thousands)
| Planned Spending |
Total Authorities* |
Actual Spending* |
|
*The variance between Total Authorities and Actual Spending is a result of a lapse of $2.4 million in the SPA. Funding for PA 1 includes a SPA to conduct Public Hearings, of $3.1 million. The SPA can only be used to cover the costs of public hearings such as, external legal counsel, expert witnesses, etc. Any unspent amount is returned to the CRF. In 2011-12 expenditures from the SPA were $668 thousand.
|
| 7,464.8 |
10,318.0 |
7,346.8 |
2011–12 Human Resources (full-time equivalents [FTEs])
| Planned |
Actual |
Difference |
| 44.0 |
35.0 |
9.0 |
Program Activity Performance Summary
| Expected Results |
Performance Indicators |
Targets |
Actual Results |
| Prices charged by patentees for patented drug products sold in Canada are not excessive according to the factors of the Patent Act. |
Percentage of patented drug products that are within the Guidelines. |
95% of patented drug products are within Guidelines. |
84.2% of patented drug products are within the Guidelines.
10.5% of patented drug products do not trigger an investigation
|
Performance Summary and Analysis of Program Activity
In 2011-12, Board Staff completed the price reviews of all patented drug products for human use reported as Under Review in the 2010 Annual Report, the 109 new patented drug products5 for human use reported as sold in 2011, and the 1,173 existing patented drug products6.
The Compliance and Enforcement Policy provides for the Board to establish criteria for identifying cases for investigation. The criteria balance the need for pricing flexibility on the part of patentees with the PMPRB's mandate of protecting consumers by ensuring that the prices of patented drug products are not excessive. In accordance with Schedule 5 of the old Compendium – Criteria for Commencing an Investigation7, a price was considered to be within the Guidelines unless it met the criteria for commencing an investigation.
With the introduction of the revised Guidelines, the Policy for When a Price May be Considered Excessive8 was incorporated. In summary, the policy states that if the patentee's price exceeds the Maximum Average Potential Price or National Non-Excessive Average Price, but does not trigger the criteria for commencing an investigation, the price of the patented drug product will be reported on the PMPRB website as “Does Not Trigger Investigation” not “Within the Guidelines” as in the past.
As a result of this change reporting on this performance indicator presents a challenge. Under the revised Guidelines 84.2% of the prices of new and existing patented drug products are within the Guidelines, up 4.4% from 2010-11. In 2011-12, 10.5% of the prices of new and existing drug products were above the Guidelines by an amount too small to trigger the investigation criteria, down 0.7% from 2010-11. Under the old Guidelines, which considered prices which did not trigger the investigation criteria to be within the Guidelines, 94.7% of the prices of new and existing patented drug products in 2011-12 would be considered to be within the Guidelines as compared to 91% in 2010-11 and 89.5% compliance in 2009-10.
Lessons Learned
The performance indicator for this Program Activity was changed for fiscal year 2012-13 to address the wording of the new Guidelines and provide a more accurate measure of patentees' compliance with the Guidelines.
| Performance Indicators |
Target |
| Percentage of patented medicines that are priced, as a result of voluntary compliance, within the guidelines or at a price which does not trigger the investigation criteria |
95% of patented medicines are voluntarily priced within guidelines or at a price which does not trigger the investigation criteria |
| Percentage of patented medicines that are subject to a Board Order |
100% of Board Orders are complied with |
Program Activity 2: Pharmaceutical trends analysis
Program Activity Description
The PMPRB conducts research and analysis on pharmaceutical trends and reports annually to Parliament through the Minister of Health on pharmaceutical trends and research and development spending by pharmaceutical patentees. Through the National Prescription Drug Utilization Information System (NPDUIS), the PMPRB also conducts research and provides critical analyses of price, utilization and cost trends for both patented and non-patented prescription drugs.
2011–12 Financial Resources ($thousands)
| Planned Spending |
Total Authorities* |
Actual Spending* |
|
* The variance between Total Authorities and Actual Spending is a result of difficulty staffing positions and a management decision to leave other positions vacant.
|
| 1,438.7 |
1,425.0 |
1,010.5 |
2011–12 Human Resources (full-time equivalents [FTEs])
| Planned |
Actual |
Difference |
| 13.0 |
7.0 |
6.0 |
Program Activity Performance Summary
| Expected Results |
Performance Indicators |
Targets |
Actual Results |
| Stakeholders are more aware of pharmaceutical trends and cost drivers. |
Number of requests for PMPRB publications |
5% increase in requests over previous year |
See Lessons Learned |
| Number of presentations by PMPRB at external meetings |
10 events per year |
11 events plus numerous meetings with various stakeholders |
Performance Summary and Analysis of Program Activity
All PMPRB publications, including studies, Board decisions and reference documents, are available on the PMPRB's website. The PMPRB has completed the comprehensive review and update of its website architecture and content. Content has been reorganized and streamlined to provide added context and create a more user-friendly environment. The revamped website will enhance all PMPRB external communication activities, increasing general awareness of its role and overall activities, including pharmaceutical trends and information on pharmaceutical cost drivers.
Through the NPDUIS the PMPRB continues its partnership with the Canadian Institute for Health Information, Health Canada and the provinces and territories. In fiscal year 2011-12, the PMPRB released five new analytical reports:
- New Drug Pipeline Monitor – Third Edition (July, 2011)
- Generic Drugs in Canada: International Price Comparisons and Potential Cost Savings (September, 2011)
- Public Drug Plan Dispensing Fees: A Cost-Driver Analysis, 2001/02 to 2007/08 (September, 2011)
- The Impact of Generic Entry on the Utilization of the Ingredient (September, 2011 – Revised May, 2012)
- Wholesale Up-charge Policies of Canada's Public Drug Plans (December, 2011 – Revised January, 2012)
These reports and future reports will contribute to informed decision-making in the pharmaceutical area.
The NPDUIS Steering Committee held its annual meeting in Ottawa in October 2011. The meeting provided an opportunity to share the results of completed and ongoing research studies, discuss priorities for future research, and engage with members of the Pharmaceutical Policy Research Collaboration (PPRC), a publicly funded network of university-based researchers from across Canada.
In 2011-12, in addition to numerous meetings with various stakeholders, the PMPRB participated in 11 external events and addressed a variety of audiences, including the PMPRB's main stakeholders: patentees, provinces, third party payers and patient advocacy groups. The PMPRB continues to use new mediums such as, videoconference and webinar, to reach a greater number of stakeholders. In addition, in October, 2011 the PMPRB released its first Analysis Brief9, Trends in Sales of Patented Drug Products.
Lessons Learned
Stakeholders rely on the PMPRB website to access information with, on average, 13,000 visitors per month. It is likely that requests for printed publications will continue to decrease due to the enhanced accessibility of the website. Consequently, the performance indicators for this Program Activity were changed for fiscal year 2012-13 to better reflect the environment in which the PMPRB currently operates.
| Performance Indicators |
Target |
| Number of website hits |
5% increase in requests over previous year |
| Number of presentations by PMPRB at external meetings |
10 events per year |
| Annual Report completed on time |
100% Completed |
| Completion of analytical studies and reports on pharmaceutical trends and issues |
50% of studies initiated are completed each year |
Program Activity Description
Internal Services are groups of related activities and resources that are administered to support the needs of programs and other corporate obligations of an organization. These groups are: Management and Oversight Services; Communications Services; Legal Services; Human Resources Management Services; Financial Management Services; Information Management Services; Information Technology Services; Real Property Services; Materiel Services; Acquisition Services; and Travel and Other Administrative Services. Internal Services include only those activities and resources that apply across an organization and not to those provided specifically to a program.
2011–12 Financial Resources ($ thousands)
| Planned Spending |
Total Authorities* |
Actual Spending |
|
* The increase in Total Authorities is due to the allocation of the entire 2010-11 carry-forward to Internal Services to complete work on the new mission-critical database.
|
| 2,951.5 |
3,505.9 |
3,397.1 |
2011–12 Human Resources (full-time equivalents [FTEs])
| Planned |
Actual |
Difference* |
|
* Two resources were re-allocated from program activities to support the implementation of the mission-critical database.
|
| 19.0 |
21.0 |
(2.0) |
Section III: Supplementary Information
Financial Highlights
Condensed Statement of Financial Position (Unaudited)
As at March 31, 2012
($ ) |
|
Change
$ |
2011-12 |
2010-11 |
| Total net liabilities |
(239,639) |
2,120,210 |
2,359,851 |
| Total net financial assets |
(269,238) |
615,099 |
884,337 |
| Departmental net debt |
29,599 |
1,505,111 |
1,475,514 |
|
|
|
|
| Departmental net financial position |
(29,599) |
(1,505,111) |
(1,475,514) |
Condensed Statement of Operations and Departmental Net Financial Position (Unaudited)
For the Year Ended March 31, 2012
($ ) |
|
Change
% |
2011-12 |
2010-11 |
|
* Total revenues include non-respendable revenues. The money reported as non-respendable revenue does not represent revenues generated by the PMPRB. This money is a result of payments made by patentees to the Government of Canada through Voluntary Compliance Undertakings (VCUs) or Board Orders to offset excess revenues. The Minister may enter into agreements with any province respecting the distribution to that province of amounts received by the Receiver General, less any costs incurred in relation to the collection and distribution of those amounts. The non-respendable revenues are earned on behalf of the Government. In the 2011-12 the non-respendable revenues were offset by the establishment of a contingent liability to be paid on behalf of the Government in the amount of $2.8 million
|
| Total expenses |
(3.62) |
10,506,665 |
10,900,802 |
| Total revenues* |
(63.93) |
(8,394,187) |
(23,272,642) |
| Net cost of operations before government funding and transfers |
(117.07) |
2,112,478 |
(12,371,840) |
|
|
|
|
| Departmental net financial position |
2.01 |
(1,505,111) |
(1,475,514) |
Financial Statements
The financial highlights presented within this Departmental Performance Report are intended to serve as a general overview of the PMPRB's financial position and operations. The PMPRB's Financial Statements10 can be found on its website.
List of Supplementary Information Tables
Electronic supplementary information tables listed in the 2011–12 Departmental Performance Report can be found on the Treasury Board of Canada Secretariat's website11.
- Greening Government Operations
- Internal Audits and Evaluations
- Sources of Respendable and Non-Respendable Revenue
Section IV: Other Items of Interest
Organizational Contact Information
The Patented Medicine Prices Review Board
Box L40
Standard Life Centre
333 Laurier Avenue West
Suite 1400
Ottawa, Ontario K1P 1C1
Telephone: (613) 952-7360
Toll-free no.: 1-877-861-2350
Facsimilie: (613) 952-7626
TTY: (613) 957-4373
Email: pmprb@pmprb-cepmb.gc.ca
Website: www.pmprb-cepmb.gc.ca
Additional Information
PMPRB Annual Report 2011 - http://www.pmprb-cepmb.gc.ca/english/view.asp?x=91
Quarterly NEWSletter - http://www.pmprb-cepmb.gc.ca/english/View.asp?x=287
Patentee's Guide to Reporting - http://www.pmprb-cepmb.gc.ca/english/view.asp?x=146
Compendium of Guidelines, Policies and Procedures – Updated June 30, 2012 http://www.pmprb-cepmb.gc.ca/english/View.asp?x=1206&mp=808
Patent Act (http://laws.justice.gc.ca/en/P-4/index.html)
Patented Medicines Regulations (http://laws.justice.gc.ca/en/P-4/SOR-94-688/index.html)
Endnotes
1 Type is defined as follows: previously committed to—committed to in the first or second fiscal year prior to the subject year of the report; ongoing—committed to at least three fiscal years prior to the subject year of the report; and new—newly committed to in the reporting year of the Report on Plans and Priorities or the Departmental Performance Report.
2 Information on the Board's proposed revised Rules of Practice and Procedure is available on the PMPRB website: http://www.pmprb-cepmb.gc.ca under Consultations/Notice and Comment, list of recent Notices and Comments.
3 The Cabinet Directive on the Environmental Assessment of Policy, Plan and Program Proposals can be found on the Canadian Environmental Assessment Agency's website: http://www.ceaa.gc.ca/default.asp?lang=En&n=B3186435-1
4 An electronic version of the Public Accounts of Canada is available on the Public Works and Government Services Canada's website: http://www.tpsgc-pwgsc.gc.ca/recgen/txt/72-eng.html
5 For purposes of this report, a new patented drug product in 2011 is defined as any patented drug product first sold in Canada, or previously sold but first patented, between December 1, 2010 and November 30, 2011.
6 For purposes of this report, existing patented drug products include all patented drug products that were first sold in Canada and reported to the PMPRB prior to December 1, 2010.
7 For more in depth information on the Criteria for Commencing an Investigation prior to January 1, 2010, refer to the Compendium of Guidelines, Policies and Procedures March 2008, Schedule 5. The Compendium (up to 2009) is available on the PMPRB's website: http://www.pmprb-cepmb.gc.ca under Publications.
8 For additional information on the Policy for When a Price May be Considered Excessive, refer to the Compendium of Policies, Guidelines and Procedures, - Updated June 2012, Part B – Policies, Para. B.5. The Compendium is available on the PMPRB's website: http://www.pmprb-cepmb.gc.ca under Legislation, Regulations and Guidelines.
9 Analysis Briefs are short summaries of recent research and analysis work produced by the PMPRB. They include such topics as trends of sales and prices of pharmaceuticals in Canada; Canadian pharmaceutical markets compared with international markets; R&D spending; and research report summaries.
10 The PMPRB's Financial Statements can be found on the PMPRB's website: http://www.pmprb-cepmb.gc.ca under Reports to Parliament.
11 Electronic supplementary information tables listed in the 2011–12 Departmental Performance Report can be found on the Treasury Board of Canada Secretariat's website: (http://www.tbs-sct.gc.ca/dpr-rmr/2011-2012/index-eng.asp)