Formulas for Foreign Price Verification
Foreign Price Verification formulas are updated by PMPRB staff in December of each year. These formulas are then posted on the website in January and apply to that entire calendar year.
This section outlines the sources and methodology used by Board Staff to derive ex-factory prices from national formulary prices published in the seven countries listed in the Patented Medicines Regulations. These ex-factory prices are used by Board Staff to verify the foreign price information reported by patentees in their Form 2 Block 5.
Note that hospital ex-factory prices are usually not provided in national formularies and cannot be derived like other ex-factory prices (pharmacy, wholesale). As a result, patentees filing a hospital price in their Form 2 Block 5 may be requested to provide substantiation. This also applies to the pharmacy price in Switzerland.
Foreign Price Verification formulas by year:
Recognized Sources
Board Staff gathers and/or derives ex-factory prices in the seven reference countries listed in the Patented Medicines Regulations from the following sources:
France:
VIDAL
21, rue Camille-Desmoulins
Issy-Les-Moulineaux
www.vidalfrance.com
Germany:
LAUER-TAXE
LAUER-FISCHER GmbH
25 Maria Trost, Koblenz
www.lauer-fischer.de
Italy:
L'INFORMATORE FARMACEUTICO
Codifa
EDRA SpA
https://www.codifa.it/
Sweden:
DENTAL AND PHARMACEUTICAL BENEFITS AGENCY (TLV)
20 Fleminggatan, Stockholm
www.tlv.se
Switzerland:
FEDERAL OFFICE OF PUBLIC HEALTH (BAG)
157 Schwarzenburgstrasse, Bern
www.spezialitätenliste.ch
United Kingdom:
MONTHLY INDEX OF MEDICAL SPECIALTIES (MIMS)
Haymarket Medical Ltd.
174 Hammersmith Road, London
www.mims.co.uk
United States of America:
RED BOOK – Wholesale Acquisition Cost (WAC), Direct Price (DP)
Truven Health Analytics
3 Times Square, New York, (New York)
www.truvenhealth.com
US DEPARTMENT OF VETERANS AFFAIRS – Federal Supply Schedule (FSS)
810 Vermont Avenue, NW
Washington, (DC)
www.va.gov/nac/Pharma/List
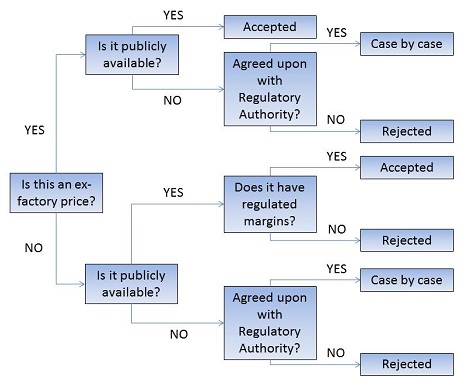
Note: In the event that an ex-factory price is not available from the PMPRB’s list of recognized sources, the PMPRB will determine whether an alternate price source is acceptable. The alternate price source must be both publicly available and publish an ex-factory price, or a retail price when the distribution margins are set by a national regulatory body.
Decision Tree for Determining Foreign Price Sources