Application de la dose quotidienne définie par l’Organisation mondiale de la santé dans les analyses de l’utilisation et des coûts des médicaments au Canada

ISBN : 978-1-100-96268-9
No cat. : H82-8/2010F-PDF
Décembre 2010
La version PDF
Résumé
Les études sur l'utilisation et les coûts des médicaments servent à orienter et à appuyer les décisions stratégiques. Ces décisions permettent non seulement d'assurer une utilisation plus adéquate et plus efficace des produits pharmaceutiques, mais aussi de déterminer la capacité financière et la viabilité du système de santé canadien. Au Canada, diverses bases de données administratives fournissent les données brutes utilisées dans ces études grâce aux volumes d'ordonnances, aux quantités physiques de médicaments et, parfois, au nombre de jours de traitement déclarés. Toutefois, ces mesures présentent des limites sur le plan des analyses.
La DDD (Defined Daily Dose), la dose quotidienne définie par l'Organisation mondiale de la santé (OMS), est une norme de mesure largement appliquée à l'échelle internationale qui permet de convertir les quantités physiques des médicaments (gélules, fioles, inhalateurs, etc.) en une unité de mesure standard. La mesure de la DDD ainsi que le système de classification anatomique thérapeutique chimique (ATC) des médicaments forment un système qui, s'il est appliqué correctement, peut être un outil très efficace pour l'analyse des modes d'utilisation des médicaments et l'assurance de la qualité des données sur l'utilisation des médicaments et les résultats en matière de santé.
La présente étude vise à informer les chercheurs des avantages et des limites de l'application de la méthodologie de la DDD dans leurs analyses de l'utilisation et des coûts des médicaments. Elle est particulièrement axée sur l'application et l'interprétation de la méthodologie de la DDD dans le cadre des bases de données administratives canadiennes.
La DDD par rapport à la dose quotidienne enregistrée au Canada
La DDD est une unité fixe de mesure technique, définie comme la dose d'entretien moyenne supposée de médicament par jour, administrée en vue de son indication principale chez l'adulte.
Même si la DDD devrait être en général conforme à la dose quotidienne enregistrée
(DQE)1 au Canada, des différences existent parfois pour certains médicaments, segments de la population canadienne ou pour certaines périodes. Bien que l'objet du présent document ne soit pas d'analyser l'ampleur de ces différences, il mentionne des exemples pour souligner les avantages et les limites liés à l'utilisation de la méthodologie de la DDD dans le contexte canadien.
Bien qu'elles soient réelles et importantes, les conséquences de ces différences sur l'interprétation des données provenant des analyses de l'utilisation et les coûts des médicaments au Canada sont parfois difficiles à déterminer. L'incompatibilité entre la DQE et la DDD entraîne inéluctablement des différences. Par conséquent, les résultats provenant de la méthodologie de la DDD doivent être interprétés en prenant compte de ce facteur.
Les différences significatives entre la DDD et la DQE ne doivent pas être interprétées comme une utilisation abusive de médicaments ou une mauvaise DDD prescrite, car elles peuvent être causées par d'autres facteurs.
Vous trouverez ci-dessous un résumé des recommandations du rapport concernant l'application de la méthodologie de la DDD dans les analyses de l'utilisation et des coûts des médicaments au Canada.
Recommandations sur l'utilisation de la méthodologie de la DDD dans l'interprétation des données sur l'utilisation des médicaments au Canada :
| Degré d'importance de la différence entre la DDD et la dose quotidienne relevée chez une population |
Application de la méthodologie de la DDD dans les analyses de l'utilisation des médicaments |
| Minime |
 Peut servir à interpréter le volume d'utilisation des médicaments exprimé en DDD Peut servir à interpréter le volume d'utilisation des médicaments exprimé en DDD |
 Peut servir à déduire toute variation ou écart en pourcentage dans le volume d'utilisation des médicaments exprimé en DDD Peut servir à déduire toute variation ou écart en pourcentage dans le volume d'utilisation des médicaments exprimé en DDD |
 Peut servir à déduire toute morbidité en fonction des médicaments utilisés dans le traitement des maladies chroniques et des maladies aiguës à l'aide d'une thérapie standard connue Peut servir à déduire toute morbidité en fonction des médicaments utilisés dans le traitement des maladies chroniques et des maladies aiguës à l'aide d'une thérapie standard connue |
| Importante |
 Ne doit pas être employé pour l'interprétation du volume d'utilisation des médicaments exprimé en DDD Ne doit pas être employé pour l'interprétation du volume d'utilisation des médicaments exprimé en DDD |
 Peut servir à déduire toute variation ou écart en pourcentage dans le volume d'utilisation des médicaments exprimé en DDD Peut servir à déduire toute variation ou écart en pourcentage dans le volume d'utilisation des médicaments exprimé en DDD |
 Ne doit pas être utilisé pour déterminer la morbidité Ne doit pas être utilisé pour déterminer la morbidité |
Recommandations concernant l'utilisation de la méthodologie de la DDD dans les analyses des coûts :
| Degré d'importance de la différence entre la DDD et la dose quotidienne relevée chez une population |
Application de la méthodologie de la DDD dans les analyses des coûts |
| Minime |
 Donne une idée du coût quotidien d'utilisation d'un médicament dans une préparation particulière Donne une idée du coût quotidien d'utilisation d'un médicament dans une préparation particulière |
 Donne une idée de la différence de coût entre deux préparations d'un même médicament Donne une idée de la différence de coût entre deux préparations d'un même médicament |
| Importante |
 Ne doit pas être utilisé dans les analyses des coûts Ne doit pas être utilisé dans les analyses des coûts |
Avantages et limites de la méthodologie de la DDD
Les avantages et les limites de l'application de la méthodologie de la DDD dans les analyses des renseignements sur l'utilisation et les coûts des médicaments provenant des bases de données administratives canadiennes peuvent être résumés de la façon suivante :
Méthodologie de la DDD
| Avantages |
Limites |
| Mesure de comparaison de l'exposition au médicament |
N'est pas actuellement intégrée aux bases de données administratives canadiennes |
| Norme prescrite par l'OMS et liée au système ATC |
Ne devrait pas, en général, servir à interpréter l'utilisation au Canada |
| Facilement accessible, peu coûteuse et conviviale |
Ne devrait pas, en général, être appliquée aux analyses de coûts |
| Intégration aux autres bases de données |
Ne devrait pas, en général, être appliquée aux décisions stratégiques |
Recommandations générales
Voici les recommandations générales pour une meilleure utilisation de la méthodologie de la DDD formulées dans le présent rapport :
- Intégration de la DDD à la Base de données sur les produits pharmaceutiques de Santé Canada pour atténuer les problèmes liés à l'intégration de cette méthode aux bases de données administratives canadiennes.
- Promotion d'une norme de déclaration de la dose quotidienne prescrite ou délivrée dans les données administratives pour mieux comprendre les modes d'utilisation des médicaments.
- Prise en compte des avantages et des limites de la méthodologie de la DDD pour améliorer les résultats des analyses.
1 Dans le présent document, la dose quotidienne enregistrée (DQE) représente la dose prescrite ou délivrée enregistrée dans la base de données administrative.
Table des matières
- 1 Introduction
- 2 La DDD dans le contexte canadien
- 3 L'utilisation des statines dans les bases de données administratives canadiennes
- 4 La méthodologie de la DDD : interprétation des données sur l’utilisation des médicaments
- 5 La méthodologie de la DDD : conséquences sur les analyses des coûts
- 6 La méthodologie de la DDD : avantages et limites
- 7 Conclusion et recommandations
- 8 Références
- Annexe 1 : Intégration de la DDD aux bases de données administratives canadiennes
- Annexe 2 : Analyse comparative des DQE des inhibiteurs de la HMG-CoA réductase dans les régimes provinciaux d’assurance-médicaments de l’Ontario, du Nouveau-Brunswick et de la Nouvelle-Écosse
- Annexe 3 : Nombre de jours d’approvisionnement dans la base de données du Programme de médicaments de l’Ontario : évaluation de la qualité
- Annexe 4 : Modifications récentes apportées aux DDD
- Annexe 5 : Sigles et définitions
1 Introduction
Au Canada, on utilise très souvent les bases de données administratives pour analyser les renseignements sur l’utilisation et les coûts des médicaments. La prolifération des documents électroniques au cours des dix dernières années a favorisé la constitution d’immenses fonds de données, telles que les données de livraisons au départ-usine, les données pharmaceutiques dans les collectivités et les hôpitaux, les données des demandes de remboursement au régime d’assurance-médicaments public et privé, les dossiers sur les erreurs de médicament et les rapports sur les effets indésirables des médicaments.
Le choix d’une norme adéquate de mesure des données sur l’utilisation des médicaments, qu’elle soit exprimée en nombre d’ordonnances, en quantités physiques ou en durée du traitement, est un facteur essentiel des études sur l’utilisation des médicaments. Selon la portée et l’objet de l’étude réalisée, certaines mesures sont mieux adaptées que les autres.
Nombre d’ordonnances : Il permet de mesurer de façon globale l’utilisation des médicaments, mais en négligeant des aspects importants de cette utilisation, tels que la durée du traitement et la quantité de médicaments.
Quantités physiques de médicaments : Également connues sous le nom d’ « unités », elles représentent une mesure détaillée de l’utilisation des médicaments. Les produits pharmaceutiques sont composés d’un large éventail de types de préparations (comprimés, fioles, inhalateurs, etc.), de dosages avec différentes unités de mesure (milligrammes, millilitres, millimoles, etc.) et d’ordres de grandeur (p. ex. dizaines ou centaines de milligrammes). Compte tenu du manque d’uniformité dans les unités de mesure, la quantité physique ne permet pas toujours de mesurer correctement l’utilisation des médicaments dans un groupe.
Durée du traitement : Certaines bases de données administratives (p. ex. celles des régimes d’assurance-médicaments ou des pharmacies) indiquent le nombre réel de jours où les médicaments sont délivrés aux patients (p. ex. « jours d’approvisionnement »),permettant ainsi de mesurer directement l’utilisation des médicaments. Toutefois, cette mesure n’est pas toujours disponible, et même si elle l’est, sa fiabilité doit être évaluée avant toute déclaration de données.
Les restrictions liées à l’application des mesures conventionnelles de l’utilisation des médicaments ont incité divers organismes et groupes de recherche à instaurer des normes de mesure pour les unités. Le système ATC/DDD (anatomique thérapeutique chimique / dose quotidienne définie) de l’Organisation mondiale de la santé (OMS) est devenu la norme acceptée et utilisée par les chercheurs des quatre coins du globe.
La méthodologie ATC/DDD de l’OMS permet de convertir des quantités physiques de médicaments (gélules, fioles, inhalateurs, etc.) en une unité de mesure standard (la dose quotidienne définie ou « DDD »). Elle permet aux chercheurs d’évaluer les tendances de l’utilisation des médicaments et d’effectuer des comparaisons entre des groupes de population.
Dans le cadre du Système national d’information sur l’utilisation des médicaments prescrits (SNIUMP), le Conseil d’examen du prix des médicaments brevetés (CEPMB) s’est servi de la méthodologie ATC/DDD de l’OMS pour effectuer diverses analyses de l’utilisation et des coûts des médicaments. Ces analyses ont permis d’avoir une meilleure idée sur l’application éventuelle de cette norme dans le contexte canadien.
La présente étude vise à indiquer aux chercheurs qui effectuent des analyses de l’utilisation et des coûts des médicaments les avantages et les limites de la méthodologie de la DDD ainsi que les meilleurs procédés pour l’application et l’interprétation des résultats lorsqu’ils utilisent des bases de données administratives canadiennes.
Aperçu du système ATC/DDD de l’OMS
Le système ATC/DDD de l’OMS est composé du système de classification anatomique thérapeutique chimique (ATC) et de la DDD, qui est une unité de mesure technique. La norme ATC/DDD est un outil pratique qui permet de comparer les données sur l’utilisation des médicaments à l’échelon international, national et local.
La DDD est définie comme la dose d’entretien moyenne supposée de médicament par jour, administrée en vue de son indication principale chez l’adulte. Les données sur l’utilisation des médicaments présentées dans les DDD ne donnent qu’une estimation approximative de l’utilisation et non les chiffres réels.
La déclaration des données sur l’utilisation des médicaments exprimées en DDD se fait généralement en contrôlant les différences dans la taille d’une population (p. ex. pour 1 000 personnes), ce qui permet de mesurer l’exposition au médicament et l’élément thérapeutique (Hallas, 2001) dans une population définie. Cette méthode permet de comparer les résultats de différents groupes de populations et de plusieurs périodes. Les types de mesures suivants sont couramment utilisés (Sjoqvist et Brikett, 2004) :
DDD pour 1000 habitants par jour
Ce type de mesure est généralement employé pour les médicaments utilisés dans le traitement des maladies chroniques. Un résultat de 10 DDD pour 1 000 habitants par an est interprété de la façon suivante : dans un groupe représentatif de 1 000 habitants, 10 DDD de médicaments sont utilisées en moyenne par jour de l’année analysée.
DDD par habitant et par an
Ce type de mesure est généralement employé pour les médicaments généralement utilisés dans le traitement d’urgence. Un résultat de 10 DDD par habitant et par an équivaut donc à une moyenne de 10 jours de traitement avec un type de médicament précis par habitant et par an.
DDD pour 100 jours-lits
Ce type de mesure est appliqué dans les analyses des données sur l’utilisation des médicaments en milieu hospitalier. Un résultat de 10 DDD pour 100 jours-lit correspond à 10 % des patients internés recevant le médicament en moyenne par jour.
2 La DDD dans le contexte canadien
La DDD est une unité fixe de mesure technique qui ne correspond pas forcément à la dose quotidienne enregistrée dans les bases de données administratives canadiennes. Nous expliquerons dans cette section les éventuels facteurs à l’origine de ces différences.
La DDD est définie comme la dose d’entretien moyenne supposée, par jour, d’un médicament utilisé dans son indication principale chez l’adulte.
Supposée
La DDD se fonde sur un examen des renseignements disponibles, notamment les doses utilisées dans différents pays européens. Toutefois, la DDD ne correspond pas toujours aux modes d’utilisation des médicaments au Canada en raison des différences sur le marché, telles que les doses commercialisées, la démographie, les indications approuvées, la prévalence des maladies, les lignes directrices de pratique clinique, les politiques de remboursement, les pratiques de prescription et, pour certains médicaments, le poids corporel moyen chez la population.
Le Centre collaborateur de l’OMS indique que les doses pour le patient ou les groupes de patients sont souvent différentes de la DDD. De nombreuses bases de données administratives canadiennes comportent les renseignements de segments précis de la population, tels que les aînés, les bénéficiaires de l’aide sociale, les populations autochtones, les habitants de certaines zones ou provinces, et les patients qui reçoivent des soins dans des centres spéciaux ou qui travaillent pour des employeurs particuliers. Étant donné que la DDD s’appuie sur les modes relevés chez la population générale, elle ne correspond pas toujours aux véritables modes d’utilisation des médicaments chez les groupes sélectionnés.
Selon l’OMS, l’un des principaux objectifs du Centre et du Groupe de travail est de maintenir la stabilité des codes ATC et de la norme DDD. Cette stabilité permettra d’étudier les tendances de l’utilisation des médicaments sans être confronté aux difficultés liées au changement constant du système. Les changements de la norme DDD doivent être réduits au minimum, voire évités si les difficultés qu’ils engendrent chez les consommateurs sont plus importantes que leurs avantages. Le marché pharmaceutique est un environnement dynamique où la dose quotidienne relevée chez une population change souvent.
Moyenne
La moyenne est basée sur deux ou plusieurs doses couramment utilisées. Par conséquent, la DDD indique parfois une dose rarement, voire jamais, prescrite. Les doses commercialisées au Canada peuvent différer de celles commercialisées en Europe.
Dose d’entretien
Dose d’entretien La dose d’entretien est généralement privilégiée dans l’établissement de la DDD. En principe, l’utilisation des médicaments englobe à la fois les doses d’entretien et de départ qui peuvent être différentes pour certains médicaments.
Indication principale
Les autres indications ou utilisations éventuelles non indiquées sont exclues de la DDD prescrite.
Adulte
Pour les médicaments prescrits aux adultes et aux enfants, seule la dose pour adulte est utilisée dans la DDD prescrite. Par conséquent, la valeur de la DDD ne correspond pas toujours aux modes d’utilisation des médicaments relevés chez des groupes d’enfants ou des groupes de population mixte qui comprennent les enfants. Les doses pour enfants sont indiquées dans les médicaments qui leur sont exclusivement prescrits.
Par conséquent, même si en général la DDD devrait être conforme à la dose quotidienne relevée chez une population, il est possible que ce ne soit pas le cas pour certains médicaments, segments de la population canadienne ou périodes. L’incompatibilité entre les deux mesures entraîne inéluctablement des différences. Par conséquent, les résultats provenant de la méthodologie de la DDD doivent être interprétés en prenant compte de ce facteur.
Les différences significatives entre la DDD et la dose quotidienne enregistrée relevées chez la population canadienne ne doivent pas être interprétées comme une utilisation abusive de médicaments ou une mauvaise DDD prescrite, car elles peuvent être causées par plusieurs facteurs légitimes.
3 L'utilisation des statines dans les bases de données administratives canadiennes
Des études antérieures ont montré des exemples de différences significatives entre la DDD et la dose quotidienne prescrite dans certains groupes de médicaments et de populations (voir Muller et coll., 2006; Dalton et coll., 2007). Le but de notre étude n'est pas d'évaluer si la DDD correspond ou non aux modes l'utilisation relevés dans les bases de données administratives canadiennes. Des exemples d'anomalies importantes sont toutefois présentés dans cette section ainsi que leurs conséquences sur l'interprétation des données sur l'utilisation et les coûts des médicaments au Canada.
Par souci de cohérence et de simplicité, les exemples portent sur un groupe particulier de médicaments, les inhibiteurs de la HMG-CoA2 réductase, communément appelés statines. L'utilisation de cette catégorie de médicaments est observée dans le Programme de médicaments de l'Ontario (PMO), l'un des régimes publics d'assurance-médicaments les plus importants au Canada. Les exemples portent exclusivement sur ce programme pour deux raisons : d'une part, la disponibilité des données pour la période souhaitée (dix ans) et, d'autre part, les éléments de données (p. ex. renseignements sur les jours d'approvisionnement). Néanmoins, comme le montre l'analyse présentée à l'annexe 2, des modes d'utilisation analogues ont été relevés dans d'autres régimes publics d'assurance-médicaments, par exemple, au Nouveau-Brunswick et en Nouvelle-Écosse.
La base de données administrative provinciale utilisée contient des données relatives aux demandes de remboursement, à l'utilisation des médicaments et aux dépenses remboursées en vertu des régimes d'assurance-médicaments publics. Les renseignements communiqués sont ceux délivrés par les pharmacies canadiennes. Par conséquent, l'analyse porte sur la dose quotidienne enregistrée (DQE) moyenne3 relevée dans les données des régimes d'assurance-médicaments. Cette mesure est comparée à la DDD.
Les DDD pour les statines ont demeuré constantes au cours de la période de dix ans de la présente étude (exercices 1997-1998 à 2006-2007)4 et en 2008. Nous avons donc utilisé la version de janvier 2008 du système ATC/DDD de l'OMS pour le présent rapport. En janvier 2009, d'importantes modifications à la DDD sont entrées en vigueur pour cinq des six statines, afin de mieux respecter la dose quotidienne enregistrée. L'annexe 4 présente une comparaison entre les anciennes et les nouvelles valeurs de DDD extraites de la base de données du PMO.
La case 1 présente l'exemple d'une étude de cas où il existe des différences significatives entre la DDD et la DQE.
Exemple (Case 1)
Utilisation des inhibiteurs de la HMG-CoA réductase (statines)5 dans le PMO, de 1997-1998 à 2006-2007
Les données du PMO montrent une tendance à la hausse de la dose quotidienne enregistrée (DQE) moyenne pour la plupart des statines au cours de la période de dix ans se terminant en 2006-2007. Cette augmentation est probablement due en partie à des facteurs tels que les changements dans les lignes directrices de pratique clinique, les monographies de produit approuvées par Santé Canada, ainsi que les nouvelles doses et les doses plus élevées des médicaments actuels (p. ex. un comprimé de 80 mg pour l'atorvastatine et la simvastatine). Par exemple, la dose de départ de la simvastatine dans le traitement de la coronaropathie initialement recommandée par les monographies de produit approuvées par Santé Canada était de 20 mg/jour mais a été augmentée à 40 mg/jour en 2005 (APC, 1997-2007).
Les lignes directrices canadiennes de pratique clinique établies au cours des dix dernières années exigeaient une réduction progressive des taux cibles de lipides chez les patients à haut risque (risque sur 10 ans de coronaropathie de ³ 20 %). En 2003, les lignes directrices recommandaient un taux de cholestérol à lipoprotéines de faible densité de £ 2,5 mmol/L (Genest et coll., 2003) pour les patients à haut risque. Il s'agit d'un niveau de contrôle plus strict pour le groupe de patients présentant un risque sur 10 ans de coronaropathie dans la plage de 20 à 30 % (les lignes directrices de 2000 recommandaient un taux cible de cholestérol à lipoprotéines de faible densité de £ 3,0 mmol/L (Voir Fodor et coll., 2000)). En 2006, l'objectif principal visé dans le traitement du cholestérol à lipoprotéines de faible densité chez les patients à haut risque était de réduire le niveau à £ 2,0 mmol/L (McPherson et coll. 2006). Ces taux cibles accrus de cholestérol à lipoprotéines de faible densité nécessitent souvent des doses plus élevées de statines, entre autres.
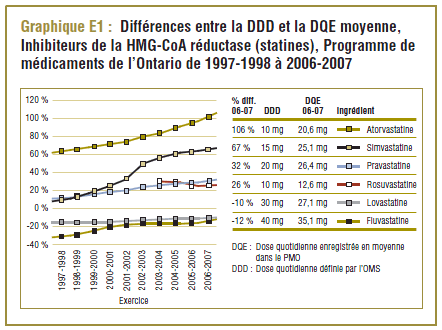
Compte tenu des transformations notables que le marché des statines a connues au cours des dix dernières années de l'étude, une unité standard de mesure stable telle que la DDD ne reproduit pas toujours les véritables modes d'utilisation. Le graphique 1 illustre les tendances des écarts en pourcentage entre la DDD et la DQE moyenne dans le PMO au cours de la période de 1997-1998 à 2006-2007.
Il convient de noter qu'on ne s'attend jamais à ce que la DDD corresponde tout à fait à la dose quotidienne relevée chez une population. Les différences devraient être toutefois minimes. Pour la plupart, les DQE des statines étaient quelque peu comparables aux DDD, c'est-à-dire dans la plage de différence absolue de (±) 8 à 32 % (pour les médicaments tels que la pravastatine, la rosuvastatine, la lovastatine et la fluvastatine). La DQE de la simvastatine se comparait initialement le mieux à la DDD (8 % de différence). Toutefois, la différence est maintenant importante (67 % en 2006-2007). Même si l'exposition accrue au médicament expliquait à l'origine la différence entre les deux valeurs, les différences significatives entre les DDD et les DQE de la simvastatine peuvent avoir une incidence sur les récentes analyses.
L'atorvastatine, qui est devenue le médicament le plus vendu sure le marché des statines, affichait la différence la plus significative entre la DDD et la DQE. Déjà en 1997-1998, la DQE (16,2 mg) était de 62 % plus élevée que la DDD (10 mg) de l'atorvastatine. Dix ans plus tard, la DQE (20,6 mg) était plus du double de la DDD.
En outre, l'analyse dans les sections suivantes est axée sur cette catégorie et examine les conséquences des petites et grandes différences des DQE et des DDD sur l'interprétation des données concernant l'utilisation et les coûts des médicaments au Canada.
Il convient de noter que ces tendances ne concernent pas uniquement le Programme de médicaments de l'Ontario. Comme le montre l'annexe 2, des résultats analogues ont été relevés dans d'autres groupes, tels que les régimes publics d'assurance-médicaments du Nouveau-Brunswick et de la Nouvelle-Écosse.
2 hydroxyméthylglutaryl-coenzyme A.
3 Dans le présent document, la dose quotidienne enregistrée (DQE) représente la dose prescrite ou délivrée enregistrée dans la base de données administrative.
4 Un exercice est défini ici comme une période de 12 mois, à compter du 1er avril de la première année et se terminant le 31 mars de l'année suivante.
5 Les données sur l'utilisation de la cérivastatine ne sont pas indiquées, car elle a été faible et temporaire à cause du retrait du produit du marché.
4 La méthodologie de la DDD : interprétation des données sur l’utilisation des médicaments
La méthodologie de la DDD peut être utilisée efficacement dans de nombreux domaines de l’analyse des données sur l’utilisation des médicaments. Il peut s’agir de simples analyses des tendances des taux d’exposition au médicament ou de comparaisons et analyses complexes des taux d’exposition à différents médicaments ou groupes de médicaments parmi diverses populations. La mesure de la DDD ainsi que le système de classification ATC constituent un système qui, s’il est appliqué correctement, peut être un outil très efficace pour déterminer les modes d’utilisation des médicaments et améliorer la qualité de ces données ainsi que les résultats en matière de santé (OMS 2004a).
Néanmoins, [Traduction] « quand il y a une différence connue entre la dose quotidienne prescrite et la DDD, il est important d’en tenir compte dans l’interprétation des chiffres sur l’utilisation des médicaments. »
Centre collaborateur de l’OMS pour la méthodologie sur l’établissement des statistiques concernant les produits médicamenteux (OMS 2004b)
Cette section est particulièrement axée sur l’utilisation adéquate de la mesure de la DDD dans l’analyse des données issues de bases de données administratives canadiennes. Elle traite plus exactement des domaines d’étude auxquels la méthodologie de la DDD convient le mieux, les domaines où la méthodologie doit être appliquée et interprétée avec prudence, et les domaines où elle ne doit pas être utilisée.
Les exemples aux cases 2 et 3 montrent les limites de l’application de la méthodologie de la DDD dans l’interprétation des données sur l’utilisation des médicaments au Canada, lorsqu’il y a des différences significatives entre la DDD et la dose quotidienne enregistrée. L’exemple à la case 2 illustre un simple cas de l’utilisation d’un médicament et d’une préparation avec la DDD correspondante. L’exemple à la case 3 montre les conséquences moins évidentes de l’analyse de plusieurs médicaments et préparations. Bien qu’elles soient réelles et importantes, ces dernières sont parfois difficiles à déterminer, surtout dans les analyses générales où les données sur l’utilisation de nombreux médicaments sont regroupées.
Exemple (Case 2)
Utilisation de l’atorvastatine dans le PMO
Les jours d’approvisionnement déclarés dans les données du PMO indiquent que l’atorvastatine est généralement délivrée une fois par jour quelle que soit la concentration (10 mg, 20 mg, 40 mg ou 80 mg), ce qui correspond à la monographie du produit approuvée par Santé Canada. Toutefois, comme nous l’avons indiqué dans la section précédente, la dose quotidienne enregistrée (DQE) moyenne a été deux fois plus élevée (106 %) que la DDD dans le PMO en 2006-2007.
Comme l’indique le tableau E2.1, cette différence se traduit par un écart entre le nombre total de DQE et celui des DDD calculées (135,1 millions par rapport à 278,2 millions). Ce nombre est une valeur théorique et ne devrait pas être interprété seul.
Tableau E2.1 : Utilisation de l’atorvastanine, DDD 10 mg, Programme de médicaments de l’Ontario, 2006-2007
| Concentration |
Unités |
Nombre de DQE |
Nombre de DDD |
| 10 mg |
61,7 M |
61,2 M |
61,7 M |
| 20 mg |
49,6 M |
48,6 M |
99,2 M |
| 40 mg |
22,5 M |
21,9 M |
89,9 M |
| 80 mg |
3,4 M |
3,4 M |
27,4 M |
| Total |
137,2 M |
135,1 M |
278,2 M |
| Pour 1 000 bénéficiaires du PMO par jour |
163 |
335 |
DQE – Dose quotidienne délivrée en moyenne dans le PMO
DDD – Dose quotidienne définie par l’OMS
Nota : Sous réserve d’erreurs d’arrondi pour les millions
Le nombre total de DDD calculé est généralement converti en DDD pour 1 000 personnes par jour. En ce qui concerne le PMO, les personnes peuvent être définies comme les bénéficiaires ou ceux qui ont reçu un remboursement du programme. Par conséquent, le nombre de DDD pour 1 000 bénéficiaires par jour de l’atorvastatine était de 335 dans le PMO en 2006-2007. Ces données peuvent être interprétées de la façon suivante : l’exposition à l’atorvastatine chez un groupe représentatif de 1000 bénéficiaires du PMO est de 335 DDD par jour.
Étant donné que l’utilisation de l’atorvastatine dans le PMO ne correspond pas à la norme de DDD, il ne faut pas croire que ces 335 bénéficiaires sur 1 000 l’utilisent en moyenne par jour. Le nombre de DQE par 1 000 bénéficiaires du PMO par jour est en fait 163. Le taux de morbidité est parfois déduit en fonction de la DDD pour 1 000 personnes par jour. Toutefois, tel qu’illustré, la morbidité ne doit être déduite que lorsque la différence entre la DDD et la DQE est minime.
Néanmoins, l’exposition au médicament exprimée en DDD est un élément précieux dans les analyses comparatives. Par exemple, comme l’indique letableau E2.2, le taux d’exposition à l’atorvastatine (nombre de DDD par 1 000 bénéficiaires du PMO par jour) a augmenté de 13,2 % au cours de la période de 2005-2006 à 2006-2007.
Outre l’augmentation de 8,6 % du nombre de DQE pour 1 000 bénéficiaires par jour, la hausse de 13,2 % du taux d’exposition au médicament révèle également une utilisation accrue des concentrations plus élevées de l’atorvastatine.
Tableau E2.2 : Utilisation de l’atorvastatine, Programme de médicaments de l’Ontario, 2005-2006 et 2006-2007
| Exercice |
Nombre d’unités |
Nombre d’unités |
Nombre de DDD |
Pour 1 000 bénéficiaires du PMO par jour |
| Nombre de DQE |
Nombre de DDD |
| 2005-06 |
123,4 M |
121,3 M |
239,5 M |
150 |
296 |
| 2006-07 |
137,2 M |
135,1 M |
278,2 M |
163 |
335 |
| Variation (%) |
11,2 % |
11,4 % |
16,1 % |
8,6 % |
13,2 % |
DQE – Dose quotidienne enregistrée en moyenne dans le PMO
DDD – Dose quotidienne définie par l’OMS
Veuillez noter que lorsqu’une seule préparation d’un médicament est analysée avec la DDD correspondante, l’interprétation des données sur l’exposition au médicament déterminées avec la méthodologie de la DDD est la même que celle de la simple collecte des données sur la quantité de médicaments (milligrammes, millilitres, etc.) prescrite ou délivrée. La méthodologie de la DDD est la technique la plus utile dans l’analyse des groupes de médicaments et des préparations où les quantités physiques de ces médicaments ne sont pas cumulatives. Ces cas concerneraient plusieurs DDD (voir l’exemple à la case 3).
Exemple (Case 3)
Utilisation des inhibiteurs de la HMG-CoA réductase (statines) dans le PMO en 1997-1998 par rapport à 2006-2007
Ce groupe de médicaments est caractérisé par une augmentation remarquable de l’utilisation et des changements dans les principaux produits au cours de la période de dix ans se terminant en 2006-2007. Comme l’indique le tableau E3, trois produits dominent le marché avec des parts à peu près comparables (exprimées en unités/comprimés) en 1997-1998. Il s’agit de la simvastatine (33 %), la pravastatine (30 %) et la lovastatine (26 %). L’atorvastatine nouvellement lancée occupait au début une petite part du marché (7 %), mais en 2006-2007, elle est devenue le produit dominant, avec plus de la moitié du marché (59 %), suivie de loin par la simvastatine (18 %) et la rosuvastatine (14 %).
Tableau E3 : Changements dans la répartition des unités, inhibiteurs de la HMG-CoA réductase (statines), Programme de médicaments de l’Ontario, 1997-1998 et 2006-2007
| Ingrédient |
Répartition des unités |
% difference entre la DDD et la DQE pour 2006-2007 |
| 1997-1998 |
2006-2007 |
| Atorvastatine |
7 % |
59 % |
106 % |
| Simvastatine |
33 % |
18 % |
67 % |
| Rosuvastatine |
— |
14 % |
32 % |
| Pravastatine |
30 % |
7 % |
26 % |
| Lovastatine |
26 % |
2 % |
-10 % |
| Fluvastatine |
5 % |
1 % |
-12 % |
DQE – Dose quotidienne enregistrée en moyenne dans le PMO
DDD – Dose quotidienne définie par l’OMS
Le graphique E3 présente les taux de croissance sur dix ans dans l’utilisation des statines par les bénéficiaires du PMO. Les résultats sont ceux déclarés pour 1 000 bénéficiaires par jour. L’utilisation des médicaments est exprimée avec divers types de mesure de l’exposition : unité (pilule), traitement et exposition au médicament. L’augmentation de 283 % du taux d’exposition unitaire indique pratiquement que près de quatre fois plus de pilules de statines étaient prises par les 1 000 bénéficiaires du PMO en 2006-2007 par rapport à 1997-1998. Ces données sont un peu comparables à celles de l’augmentation de 324 % enregistrée pour les jours d’exposition au traitement.
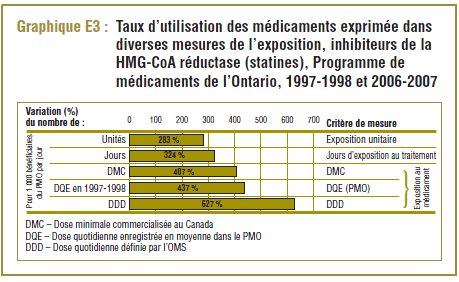
L’exposition au médicament est une notion qui peut être saisie de différentes manières, dont la norme est la DDD. Pour cet exemple, les deux options de mesure du niveau d’exposition au médicament suivantes ont été prises en compte :
- La DMC : représente la dose minimale commercialisée au Canada pour chacune des statines;
- La DQE de 1997-1998 : représente la dose quotidienne enregistrée en moyenne au titre du PMO au début de la période d’étude.
L’augmentation du taux d’exposition au médicament en fonction de ces deux mesures, même si elle peut être comparée avec d’autres taux (407 % et 437 %, respectivement), est remarquablement inférieure à celle déterminée avec la norme de la DDD (627 %). Cette situation s’explique par les changements dans l’utilisation, qui est passée des médicaments avec des plus faibles différences entre la DDD et la DQE (p. ex. la pravastatine, la lovastatine et la simvastatine) à des médicaments dont ces différences sont beaucoup plus élevées (p. ex. l’atorvastatine) – (voir le tableau E3).
Les résultats de l’exposition au médicament et les conclusions varient en fonction de l’unité de mesure utilisée. Selon les normes de la DQE du PMO, l’exposition au médicament pour les statines dans ce programme a augmenté d’un facteur de 5,37 pendant la période de dix ans, tandis qu’elle a augmenté d’un facteur de 7,27 par rapport aux normes de la DDD.
5 La méthodologie de la DDD : conséquences sur les analyses des coûts
L’application de la méthodologie de la DDD aux analyses des coûts est très limitée, car la DDD est une technique de mesure de l’utilisation des médicaments. Contrairement aux analyses sur l’utilisation des médicaments, auxquelles la méthodologie de la DDD peut fournir de précieux renseignements sur l’exposition aux médicaments même lorsqu’il ya des différences entre la DDD et la dose quotidienne enregistrée, dans les analyses des coûts, même de petites différences entre ces deux mesures peuvent générer des conclusions erronées.
[Traduction]« La DDD, si elle est utilisée avec prudence, peut être utilisée pour comparer, par exemple, les coûts de deux préparations du même médicament. Toutefois, il n’est généralement pas valable d’utiliser ce paramètre pour comparer les coûts de différents médicaments ou groupes de médicaments. »
Centre collaborateur de l’OMS pour la méthodologie sur l’établissement des statistiques concernant les produits médicamenteux (OMS 2008)
« La DDD est une technique de mesure de l’utilisation des médicaments et n’est pas forcément conçue pour reproduire des doses équivalentes du point de vue thérapeutique de différents médicaments qui sont très difficiles à établir, surtout pour le niveau de précision généralement requis dans les décisions sur les prix. »
Centre collaborateur de l’OMS pour la méthodologie sur l’établissement des statistiques concernant les produits médicamenteux (OMS 2008)
Par conséquent, l’application de la méthodologie de la DDD peut avoir une incidence sur de nombreuses analyses des coûts et générer ainsi des résultats erronés dans les analyses de coût par DDD, les analyses sur la décomposition des coûts, les études pharmacoéconomiques et les analyses des répercussions sur le budget.
Les exemples aux cases 4 à 6 illustrent les limites de l’application de la méthodologie de la DDD dans les analyses des coûts. Veuillez noter que le coût indiqué dans ces exemples inclut celui des ingrédients et des marges brutes des grossistes et des pharmacies.
Exemple (Case 4)
Coût moyen de l’utilisation de la simvastatine dans le PMO de 1999-2000 par rapport à 2000-2001
De 1999-2000 à 2000-2001, la DDD pour la simvastatine (15 mg) était comparable à la dose quotidienne enregistrée (DQE) moyenne dans le PMO qui était de l’ordre de 17,6 à 18,6 mg. Le coût par DDD peut ainsi donner une idée approximative du coût moyen d’utilisation du médicament par jour (graphique E4), soit entre 1,70 $ et 1,80 $ approximativement. Ce résultat correspond quelque peu au coût moyen par unité et au coût moyen par DQE.
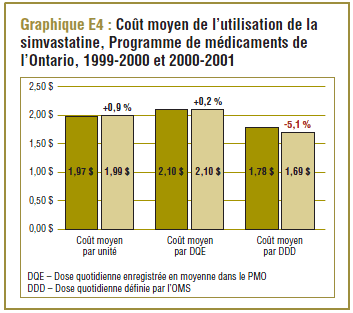
Étant donné que les résultats sur le coût par DDD devraient être interprétés au sens large, en général, les variations de ces résultats entre les périodes ou les populations ne devraient pas être interprétées. Par exemple, la baisse de 5,1 % du coût par DDD relevée au cours de la période de un an ne doit pas être interprétée comme une réduction du coût de la prise de simvastatine, parce qu’elle est due à l’utilisation accrue de dosages plus élevés (qui sont moins coûteux par DDD). Comme le montrent à la fois le coût moyen par unité (normalisé pour des changements dans la répartition des doses unitaires utilisées) et le coût par DQE, le coût d’utilisation du médicament a légèrement augmenté pendant la période analysée.
Exemple (Case 5)
Évolution du coût moyen de l’atorvastatine dans le PMO en 1997-1998 par rapport à 2006-2007
Comme nous l’avons indiqué précédemment, il y a une grande différence dans le cas de l’atorvastatine entre la DDD de 10 mg et la dose quotidienne enregistrée (DQE) moyenne dans le PMO, qui a connu une progression constante, passant de 16,2 mg à 20,6 mg au cours de la période de dix ans.
Comme l’indique le graphique E5, le coût moyen par DDD pour l’atorvastatine avait chuté à moins de 1,00 $ en 2006-2007, ce qui représente environ la moitié du coût moyen par unité ou DQE. Cette baisse est due à la répartition du coût des doses plus élevées de médicaments (20 mg, 40 mg et 80 mg) en plusieurs DDD. Il ne fait aucun doute qu’ici le coût moyen par DDD ne doit pas être interprété.
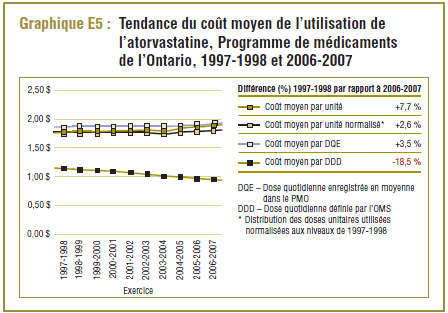
En outre, le coût de l’utilisation de l’atorvastatine a augmenté au cours de la période de dix ans, comme l’indique le pourcentage de la différence du coût moyen par unité (+7,7 %), le coût moyen par unité normalisé pour les changements dans la répartition des doses unitaires utilisées (+2,6 %) et le coût par DQE (+3,5 %). D’autre part, le coût par DDD a reculé de 18,5 %, ce qui laisse supposer une baisse remarquable des coûts de l’atorvastatine alors que ce n’est pas le cas. Ce résultat paradoxal s’explique par une utilisation accrue des doses plus élevées de médicaments (20 mg, 40 mg et 80 mg) au cours de la période de l’étude, qui si on se réfère à la DDD sont moins chères que les doses les plus faibles (10 mg), ce qui réduit ainsi le coût moyen.
Exemple (Case 6)
Analyse comparative des coûts de l’atorvastatine par rapport à la rosuvastatine dans le PMO, 2006-2007
Les données de 2006-2007 du PMO indiquent que le coût d’utilisation de la rosuvastatine a été nettement inférieur à celui de l’atorvastatine lorsqu’il est mesuré soit par unité (-24,1 %), soit par DQE (-26,0 %) (voir le tableau E6).
Toutefois, le coût par DDD donne à entendre une autre interprétation, notamment que le coût d’utilisation de la rosuvastatine a été nettement plus élevé (+21,2 %) que celui de l’atorvastatine. Ce résultat paradoxal s’explique par le fait que la DDD et la DQE se comparent mieux dans le cas de la rosuvastatine que de l’atorvastatine (graphique E1).
Tableau E6 : Coût de l’atorvastatine par rapport au coût de la rosuvastatine, Programme de médicaments de l’Ontario, 2006-2007
| |
Coût moyen par unité |
Coût moyen par DQE |
Coût moyen par DDD |
| Atorvastatine |
1,90 $ |
1,93 $ |
0,94 $ |
| Rosuvastatine |
1,44 $ |
1,43 $ |
1,14 $ |
| Différence (%) |
-24,1 % |
-26,0 % |
+21,2 % |
DQE – Dose quotidienne enregistrée en moyenne dans le PMO
DDD – Dose quotidienne définie par l’OMS
Analyses du coût par DDD
Des études ont été effectuées sur la présentation et la comparaison du coût par DDD pour les médicaments, les groupes de médicaments ou les populations (voir Goel et coll., 1996; Metge et coll., 2003). Si la DDD correspond à la DQE, le coût par DDD peut donner une idée approximative des coûts de traitement. Toutefois, il faut faire preuve de prudence dans l’interprétation des résultats si le degré de correspondance n’est pas connu.
La méthodologie de la DDD peut être utilisée pour effectuer des prévisions, par exemple, sur le coût par dose dans le traitement des données manquantes ou erronées dans un ensemble de données (Lummis et coll., 2008). Toutefois, cette application n’est valide que si la DDD correspond à la DQE ou si les différences éventuelles entre les deux mesures n’ont pas de répercussions sur les résultats.
Analyses sur la décomposition des coûts
Dans le passé, le CEPMB a effectué des analyses sur la décomposition des coûts en fonction de la méthodologie de la DDD et décelé ainsi d’importantes restrictions (CEPMB, 2006).
Les analyses sur la décomposition des coûts sont communément appelées analyses des éléments de coûts, car elles permettent de répartir les divers coûts des médicaments en facteurs déterminants (effets ou détermineurs). Elles sont généralement menées sur l’utilisation globale, mais elles peuvent aussi porter sur un groupe de médicaments (p. ex. catégories thérapeutiques).
En fonction des données disponibles, les analyses des éléments de coûts permettent généralement d’effectuer une évaluation quantitative de l’effet de volume, l’effet de prix, l’effet démographique et l’effet thérapeutique. Chaque effet est dérivé d’une formule mathématique complexe selon laquelle les principaux ingrédients sont le prix et la quantité de médicaments utilisés, ainsi que leurs poids correspondants.
Lorsque la méthodologie de la DDD est appliquée aux analyses des éléments de coûts, les deux principales composantes sont le coût moyen de la DDD et le nombre total de DDD et leur répartition dans les médicaments. En appliquant la méthodologie de la DDD au cadre de décomposition des coûts, les deux principaux effets suivants sont touchés :
Effet de volume – L’effet de volume permet d’évaluer quantitativement le niveau d’augmentation des coûts totaux des médicaments en raison des changements dans le volume de l’utilisation des médicaments. Comme nous l’avons indiqué précédemment, les différences significatives entre la DDD et la DQE doivent être prises en compte dans l’interprétation du volume d’utilisation de médicaments. Elles peuvent apparaître dans les analyses globales, telles que les analyses des coûts portant sur un grand nombre de médicaments.
Effet de la combinaison thérapeutique – L’effet de la combinaison thérapeutique permet d’évaluer quantitativement le niveau d’augmentation des coûts totaux des médicaments en raison des changements dans le niveau d’utilisation des médicaments à moindre coût ou à des coûts plus élevés. Lorsque la méthodologie de la DDD est appliquée à ce cadre, le coût de chaque médicament est déterminé en fonction de la DDD et une comparaison du coût par DDD est effectuée pour tous les médicaments.
Examinons le cas illustré à l’exemple de la case 6 ainsi que l’analyse des éléments de coûts du PMO de 2005-2006 à 2006-2007, période au cours de laquelle il y a eu un changement dans l’utilisation avec le passage de l’atorvastatine à la rosuvastatine. Le coût de la rosuvastatine (exprimé en unités ou DQE) est inférieur à celui de l’atorvastatine. Par conséquent, l’effet thérapeutique devrait avoir des répercussions négatives sur les coûts des médicaments car l’adoption d’un médicament à moindre coût permettrait de réaliser des économies. Toutefois, si la méthodologie de la DDD est appliquée dans cette analyse, le coût par DDD de la rosuvastatine (exprimé en DDD) semble être plus élevé que celui de l’atorvastatine. Ainsi, l’abandon de l’atorvastatine au profit de la rosuvastatine risque d’engendrer un effet thérapeutique avec des répercussions positives sur les coûts des médicaments, alors que cela ne devrait pas être le cas. En effet, ces données seront interprétées comme un facteur d’augmentation de coût.
Tel qu’indiqué par l’OMS, et aussi tel que le montre l’analyse présentée dans cette section, l’utilisation de la mesure de la DDD pour comparer les coûts des différents médicaments génère le plus souvent des résultats non valides ou sujets à caution. Par conséquent, à moins que la DDD ne corresponde tout à fait à la DQE, l’utilisation de la méthodologie de la DDD ne convient pas aux analyses sur la décomposition des coûts.
Études pharmacoéconomiques
Ces études servent à orienter les décisions sur la répartition optimale des ressources en soins de santé, et ce, d’une façon normalisée et scientifiquement fondée. Elles permettent d’évaluer le coût et les effets (valeur monétaire, efficacité ou amélioration de la qualité de vie) des produits pharmaceutiques. Il existe plusieurs types d’évaluations pharmacoéconomiques : analyses du coût de la maladie, du coût-bénéfice, du coûtefficacité, du coût-utilité et de la réduction des coûts.
L’OMS indique que la classification d’une substance dans le système ATC/DDD ne signifie pas une confirmation de l’efficacité ou de l’efficacité relative des médicaments et de groupes de médicaments. conséquent, la DDD n’est pas une mesure qui convient à l’utilisation dans les analyses de coût des maladies, de coût-bénéfice, de coût-efficacité et de coût-utilité, car elles sont fondées sur des données concernant l’équivalence des résultats secondaires ou définitives (Clarke et Gary, 1995).
Même dans la plus simple étude pharmacoéconomique, la réduction des coûts - dans les secteurs où les résultats sont supposés être les mêmes, la comparaison des coûts de traitement étant fondée sur une mesure théorique de l’utilisation des médicaments (la DDD) – ne peut offrir qu’une valeur limitée dans l’orientation des décisions stratégiques (comme le montre l’exemple à la case 5).
Analyse des répercussions sur le budget
Ces analyses sont utilisées par les régimes d’assurance-médicaments publics et privés au Canada afin de prévoir et d’appréhender les éventuelles répercussions financières liées à l’introduction d’un nouveau produit pharmaceutique ou d’une indication approuvée dans un système de remboursement des médicaments aux ressources financières limitées.
La méthode d’évaluation des répercussions sur le budget est assez complexe, mais l’idée principale est de comparer le coût du traitement pour une nouvelle indication pharmaceutique ou déjà approuvée avec ses médicaments de comparaison.
Le CEPMB dans ses Lignes directrices pour l’analyse de l’incidence du prix d’un médicament sur les budgets des régimes d’assurance-médicaments au Canada (CEPMB, 2007) souligne l’importance de la fiabilité des hypothèses et des paramètres dans l’évaluation des répercussions sur le budget. L’hypothèse de coût de traitement doit permettre de reproduire la réalité du régime de façon aussi exacte que possible. Comme le montre cette section, le coût déterminé de la DDD présente d’importantes limites. La DDD est une unité de mesure d’ordre technique et non un outil de mesure des données sur le traitement.
Par conséquent, la méthodologie de la DDD ne doit pas être utilisée dans les analyses de répercussions sur le budget, sauf si la DDD exprime de façon juste la DQE.
6 La méthodologie de la DDD : avantages et limites
Les avantages et les limites de l’application de la méthodologie de la DDD dans le cadre des bases de données administratives canadiennes ont été abordés au préalable (Sketris et coll., 2004). Cette section donne un aperçu détaillé de ces avantages et limites à la lumière de l’analyse fournie à la section 2. Une analyse en profondeur est fournie à l’annexe 1.
Principaux avantages
La DDD est une mesure de comparaison de l’exposition au médicament
Le principal avantage de la méthodologie de la DDD est qu’elle permet non seulement de convertir les quantités physiques de médicaments en une unité de mesure standard, mais aussi de regrouper les données sur l’utilisation dans des groupes de médicaments, ce qui serait impossible avec les autres méthodes. La DDD est une mesure de l’exposition au médicament qui permet de saisir le niveau de traitement dans la population analysée. Elle permet aux chercheurs d’évaluer les tendances de l’utilisation des médicaments et d’effectuer des comparaisons entre des groupes de populations.
Comme nous l’avons précédemment indiqué, la prudence devra être de mise dans l’interprétation des résultats issus de cette méthodologie, si les différences entre la DDD et la DQE sont importantes.
Elle est établie par l’OMS et liée au système ATC
La DDD est établie par le Centre collaborateur de l’OMS pour la méthodologie sur l’établissement des statistiques concernant les produits médicamenteux en collaboration avec le Groupe de travail international de l’OMS pour la méthodologie sur l’établissement des statistiques concernant les produits médicamenteux. L’OMS est un organisme de renommée mondiale et la méthodologie de la DDD est acceptée et appliquée à grande échelle.
En tant que volet du système ATC/DDD de l’OMS, la méthodologie de la DDD est directement liée à un système de classification thérapeutique accepté et utilisé à grande échelle. Le système ATC/DDD de l’OMS est mis à jour chaque année au mois de janvier et les données sur les nouveaux médicaments, les examens et les modifications ajoutées.
Le système est facilement accessible, peu coûteux et facile à utiliser
Le système ATC/DDD de l’OMS est peu coûteux et les données peuvent être obtenues en quelques jours. Les codes ATC sont alphanumériques et classés par catégorie et sont de ce fait faciles à utiliser et à trouver, pour la plupart.
Le système ATC/DDD permet une intégration avec d’autres bases de données
Le système ATC/DDD de l’OMS classe les médicaments et fournit des DDD à un niveau plus modulaire (ingrédients et voie d’administration), ce qui permet l’intégration d’un large éventail de bases de données administratives canadiennes.
Principales limites
La DDD ne correspond pas forcément à l’utilisation au Canada
La DDD est une hypothèse concernant l’utilisation des médicaments, qui, pour beaucoup de raisons valables, ne correspond pas forcément aux modes d’utilisation relevés dans les bases de données administratives canadiennes. Lorsqu’il y a une différence connue entre la DQE et la DDD, il est important d’en tenir compte dans l’interprétation des chiffres sur l’utilisation des médicaments.
Les possibilités d’application de la DDD dans les analyses des coûts sont limitées
L’application de la méthodologie de la DDD dans les analyses de coût est très limitée, car la DDD est une mesure technique de l’utilisation des médicaments. Si la DDD correspond à la DQE, le coût déterminé de la DDD permet d’avoir une estimation approximative du coût quotidien de la prise quotidienne d’une préparation d’un seul médicament ou la différence de coût entre deux préparations du même médicament. Toutefois, cette mesure peut générer des résultats invalides lorsque l’on compare les coûts de différents médicaments ou groupes de médicaments.
La DDD a une utilisation limitée dans la prise de décisions stratégiques
Le système ATC/DDD de l’OMS a été créé comme un outil de recherche sur l’utilisation des médicaments afin d’améliorer la qualité de ces données. L’OMS indique, toutefois, que le système ATC/DDD ne convient pas pour orienter les décisions concernant le remboursement, les prix et la substitution thérapeutique.
[Traduction] « La DDD est une mesure technique de l’utilisation des médicaments et n’est pas forcément conçue pour reproduire des doses équivalentes du point de vue thérapeutique de différents médicaments qui sont très difficiles à établir, surtout pour le niveau de précision généralement requis pour les décisions sur les prix. »
Centre collaborateur de l’OMS pour la méthodologie sur l’établissement des statistiques concernant les produits médicamenteux (OMS 2004c)
Au moment de la prise de décisions stratégiques, les cas suivants d’utilisation erronée du système ATC/DDD de l’OMS doivent être évités.
- Comparaisons des prix au niveau des DDD;
- Utilisation de la DDD pour déterminer l’équivalence thérapeutique;
La DDD ne doit pas être utilisée pour déterminer l’équivalence thérapeutique, car les deux notions ont des significations et des objectifs différents. La DDD est une mesure technique de l’utilisation moyenne des médicaments, tandis que l’équivalence thérapeutique désigne des doses de médicaments différents qui permettent le même contrôle d’un symptôme ou d’une maladie. L’équivalence thérapeutique est déterminée en fonction d’une analyse rigoureuse des résultats des essais cliniques. Même si deux médicaments peuvent avoir la même DDD, ils ne devraient pas être interprétés comme des équivalents thérapeutiques puisque des doses équivalentes sur le plan thérapeutique peuvent effectivement varier.
- Décisions concernant le remboursement, décisions relatives au prix de référence pour le groupe thérapeutique et autres décisions concernant le prix;
En général, ces décisions stratégiques sont fondées sur l’équivalence thérapeutique, les études pharmacoéconomiques et les comparaisons des coûts des différents médicaments en fonction des DQE réelles.
- Certaines analyses des coûts menées à l’appui des décisions stratégiques.
L’intégration de la DDD aux bases de données administratives canadiennes
Cette restriction est de nature technique et peut être allégée grâce à un processus d’intégration adéquat. Toutefois, le processus peut être non seulement assez complexe, mais exiger beaucoup de temps et de ressources (cela nécessite également d’énormes ressources informatiques ainsi que de l’expertise d’ordre clinique et politique). En outre, le processus ne permet pas toujours de prescrire une DDD complète.
Certains médicaments n’ont parfois pas de DDD prescrite. Par conséquent, si la méthodologie de la DDD est utilisée pour des analyses complètes sur les bases de données administratives canadiennes, il est possible que certaines données éventuellement importantes sur l’utilisation des médicaments ne puissent pas être saisies. Pour ces études, il est important que cette omission éventuelle soit indiquée dans les résultats.
L’annexe 1 présente en détail les trois principaux défis à relever afin d’intégrer la DDD aux bases de données administratives canadiennes : (1) gestion des DDD non disponibles; (2) intégration des DDD disponibles; (3) uniformisation des processus de déclaration des unités.
Autres points à prendre en compte
Stabilité du système ATC/DDD
Même si l’objectif de l’OMS est de maintenir la stabilité du système ATC/DDD, des changements se produisent tout de même. Les études dans lesquelles la méthodologie de la DDD est appliquée ne font pas toujours allusion aux valeurs des DDD ni à la version du système ATC/DDD (voir Ronning et coll., 2000). Il est donc difficile de comparer les résultats entre les études, puisque la DDD prescrite peut différer d’une version à l’autre. Les études dans lesquelles la méthodologie de la DDD est appliquée doivent indiquer correctement la version du système ATC/DDD utilisée (Ronning, 2001).
Liens dans la base de données de Santé Canada sur les produits pharmaceutiques
Santé Canada a intégré le système de classification ATC à sa Base de données sur les produits pharmaceutiques (BDPP) en associant directement le système DIN (numéro d’identification du médicament) au système ATC. Par contre, les valeurs des DDD ne sont pas actuellement liées à la BDPP.
7 Conclusion et recommandations
Conclusion
Le système ATC/DDD de l’OMS est un outil précieux qui permet de mener des recherches sur l’utilisation des médicaments au Canada, notamment des analyses comparatives sur les tendances, régionales, provinciales et internationales. Les chercheurs doivent être conscients des avantages et des limites de la méthodologie de la DDD et trouver le juste milieu dans leurs activités de recherche.
Recommandations
L’application de la méthodologie de la DDD dans les études sur l’utilisation des médicaments au Canada peut être améliorée à l’aide des mesures suivantes :
Intégration de la DDD
L’intégration du système de classification ATC de l’OMS à la Base de données sur les produits pharmaceutiques de Santé Canada contribue de façon précieuse aux études sur l’utilisation des médicaments au Canada. L’ajout des renseignements sur les DDD à cette base de données permettrait aux chercheurs de bénéficier pleinement du système de classification ATC/DDD de l’OMS, et de ce fait d’alléger en partie les restrictions liées à l’intégration des données des DDD aux bases de données administratives canadiennes.
Uniformisation des processus de déclaration de renseignements dans les bases de données administratives canadiennes
Les autres restrictions liées à l’intégration des données des DDD aux bases de données administratives canadiennes peuvent être allégées au moment de la saisie des données en favorisant l’uniformisation des processus de déclaration de renseignements concernant l’utilisation de médicaments et plus particulièrement pour ce qui est de leur identification et des renseignements sur la quantité, entre autres.
En outre, l’augmentation des capacités de déclaration afin d’intégrer des renseignements sur la durée réelle du traitement (p. ex. le nombre de jours de prescription ou de délivrance des médicaments) permettrait aux chercheurs et aux décideurs de disposer d’une norme de mesure directe et précieuse des doses quotidiennes au Canada. Elle peut être utilisée dans l’analyse des tendances et les études internationales, ainsi que dans les comparaisons avec la norme de mesure des DDD.
Possibilités d’application et interprétation adéquate de la méthodologie de la DDD
Comme nous l’avons souligné dans le présent rapport, il existe d’importantes restrictions liées à l’application de la méthodologie de la DDD dans les analyses sur l’utilisation des médicaments et des coûts. Ces restrictions doivent être soigneusement prises en compte dans le contexte et la portée de chaque projet de recherche avant de recourir à cette méthode.
Les analyses sur l’utilisation et les coûts des médicaments au Canada effectuées à l’aide de la méthodologie de la DDD doivent indiquer clairement toutes les restrictions et expliquer leur incidence sur l’interprétation des résultats. En outre, les études doivent mentionner la version du système ATC/DDD de l’OMS qui a été utilisée.
8 Références
APC. 1997-2007. Compendium des produits et spécialités pharmaceutiques, édition de 1997-2007, Association des pharmaciens du Canada.
CEPMB. 2006. Analyse de la dose thérapeutique quotidienne dans Rapport sommaire sur les tendances des prix des médicaments, édition de juin, pp. 34–114. Conseil d’examen du prix des médicaments brevetés, Ottawa. Version en date du 2 septembre 2008, http://www.pmprb-cepmb.gc.ca/CMFiles/PTOR_June_0638MBI-6232006-5829.pdf
CEPMB. 2007. Lignes directrices pour l’analyse de l’incidence du prix d’un médicament sur les budgets des régimes d’assurance-médicaments au Canada. Conseil d’examen du prix des médicaments brevetés, Ottawa. Version en date du 2 septembre 2008, http://www.pmprb-cepmb.gc.ca/CMFiles/BIA-may0738LVV-5282007-5906.pdf
Clarke, K. W. et D. Gary. 1995. The defined daily dose as a tool in pharmacoeconomics. Advantages and limitations [La dose quotidienne définie : Un outil en pharmacoéconomie. Avantages et limites]. PharmacoEconomics, vol. 7, no 4, p. 280-283.
Cooke, C., L. Nissen, I. Sketris et S. E. Tett. 2005. Quantifying the use of statin antilipemic drugs: comparison of and contrasts between Nova Scotia, Canada, and Queensland, Australia [Évaluation quantitative de l’utilisation des statines (hypolipémiants) : Comparaison des disparités entre la province de la Nouvelle-Écosse au Canada et l’État du Queensland en Australie]. Clinical Therapeutics, vol. 27, no 4.
Dalton, B., D. Sabuda et J. Conly. 2007. Trends in antimicrobial consumption may be affected by units of measure [Les unités de mesure peuvent avoir une incidence sur les tendances de la consommation d’antimicrobiens]. Chicago Journals – Clinical Infectious Diseases, vol. 45, p. 399-400.
Fodor, JG, JJ. Frohlich, JJG. Genest et PR. McPherson. (Groupe de travail sur l’hypercholestérolémie et les autres dyslipidémies). 2000. Recommendations for the management and treatment of dyslipidemia: Report on the working group of hypercholesterolemia and other dyslipidemia [Recommandations concernant la gestion et le traitement de la dyslipidémie : Rapport sur le groupe de travail de l’hypercholestérolémie et les autres dyslipidémies]. Canadian Medical Association Journal, vol. 162, no 10, p. 1441-1447.
Genest, J., J. Frohlich, G. Fodor et R. McPherson, (Groupe de travail sur l’hypercholestérolémie et les autres dyslipidémies). 2003. Recommendations for the management of dyslipidemia and the prevention of cardiovascular disease: update [Recommandations pour la gestion de la dyslipidémie et la prévention des maladies cardiovasculaires : mise à jour]. Canadian Medical Association Journal, vol. 169, no 9, p. 921–924.
Goel, V., J. Williams, G. Anderson, P. Blackstein-Hirsch et D. Naylor. 1996. Patterns of use of specific drugs in the elderly [Les modes d’utilisation de certains médicaments chez les personnes âgées] dans Patterns of health care in Ontario [Tendances de soins de santé en Ontario]. ICES Practice Atlas, 2e édition. Version en date du 2 septembre 2008, http://www.ices.on.ca/file/Practice2-ch12.pdf.
Hallas, J. 2001. Pharmacoepidemiology –current opportunities and challenges [Pharmaco-épidémiologie – Perspectives et défis actuels]. Norwegian Journal of Epidemiology, vol. 11, no 1, p. 7–12.
Hartzema, AG., HH. Tilson et KA. Chan. 2008. Drug utilization research in Pharmacoepidemiology and therapeutic risk management [Recherche sur l’utilisation des médicaments – La pharmaco-épidémiologie et la gestion du risque thérapeutique], chapitre 7. Harvey Whitney Books Company.
Levy, AR., BJ. O’Brien, C. Sellors, P. Grootendorst et D. Willison. 2003. Coding accuracy of administrative drug claims in the Ontario Drug Benefit database [Exactitude des données des demandes de remboursements de médicaments saisies dans la base de données du Programme des médicaments de l’Ontario]. Journal canadien de pharmacologie clinique, vol. 10, no 2.
Lummis, HL, IS. Sketris, GJ. Gubitz MR. Joffres et GJ. Flowerdew. 2008. Medication persistence rates and factors associated with persistence in patients following stroke: a cohort study [Taux et facteurs de pérennité des médicaments liés à la persistance chez les patients après un AVC : une étude des cohortes]. BMC Neurology, vol. 8, no 25.
McPherson, R., J. Frohlich, G. Fodor et J. Genest. 2006. Canadian Cardiovascular Society position statement – Recommendations for the diagnostic and treatment of dyslipidemia and prevention of cardiovascular disease [Énoncé de position de la Société canadienne de cardiologie – Recommandations concernant le diagnostic et le traitement de la dyslipidémie et la prévention des maladies cardio-vasculaires]. Journal canadien de cardiologie, vol. 22, no 11, p. 913-927.
Merlo, J., A. Wessling et A. Melander. 1996. Comparison of dose standard units for drug utilization studies [Comparaison des unités de dose standard pour les études sur l’utilisation des médicaments]. European Journal of Clinical Pharmacology, vol. 50, p. 27-30.
Metge, C., A. Korzyrskyj, M. Dahl, M. Yogendran et N. Roos. 2003. Pharmaceuticals: Focusing on appropriate utilization [Produits pharmaceutiques : mettre l’accent sur une utilisation adéquate]. Centre manitobain des politiques en matière de santé à Winnipeg. Version en date du 2 septembre 2008, http://mchp-appserv.cpe.umanitoba.ca/reference/pharma.pdf
Muller, A., DL. Monnet, D. Talon, T. Heron et X. Bertrand. 2006. Discrepancies between prescribed daily dose and WHO defined daily dose of antibacterials at a university hospital [Écarts entre la dose quotidienne prescrite et la dose quotidienne définie par l’OMS pour les antibiotiques dans un hôpital universitaire]. British Journal of Clinical Pharmacology, vol. 61, no 5, p. 585-591.
OMS, 2004a. Guidelines for ATC classification and DDD assignment [Lignes directrices sur le système de classification ATC et les DDD prescrites], 7e édition. Centre collaborateur de l’Organisation mondiale de la santé pour la méthodologie sur l’établissement des statistiques concernant les produits médicamenteux, p. 34.
OMS, 2004b. Guidelines for ATC classification and DDD assignment [Lignes directrices sur le système de la classification ATC et les DDD prescrites], 7e édition. Centre collaborateur de l’Organisation mondiale de la santé pour la méthodologie sur l’établissement des statistiques concernant les produits médicamenteux, p. 31.
OMS, 2004c. Guidelines for ATC classification and DDD assignment [Lignes directrices sur le système de la classification ATC et les DDD prescrites], 7e édition. Centre collaborateur de l’Organisation mondiale de la santé pour la méthodologie sur l’établissement des statistiques concernant les produits médicamenteux, p. 33.
OMS, 2008. Uses and misuses [Utilisation adéquate et utilisation inadéquate]. Centre collaborateur de l’Organisation mondiale de la santé pour la méthodologie sur l’établissement des statistiques concernant les produits médicamenteux. Version en date du 2 septembre 2008, http://www.whocc.no/atcddd/use_misuse.html.
Ronning, M. 2001. Coding and classification in drug statistics – From national to global application [Codification et classification dans les statistiques sur l’utilisation des médicaments – D’une application nationale vers une application mondiale]. Norwegian Journal of Epidemiology, vol. 11, no 1, p. 37-40.
Ronning, M, HS. Blix, BT. Harbo et H. Strom. 2000. Different versions of the anatomical therapeutic chemical classification system and the defined daily dose – are drug utilization data comparable [Différentes versions du système de classification anatomique thérapeutique chimique et de la dose quotidienne définie – Les données d’utilisation des médicaments sont-elles comparables?] European Journal of Clinical Pharmacology, vol. 56, p. 723-727.
Sjoqvist, F. et D. Brikett. 2004. Drug Utilization in The IUPHAR Compendium of Basic Principles for Pharmacological Research in Humans [Utilisation des médicaments – Compendium de l’Union internationale de pharmacologie sur les principes fondamentaux de la recherche pharmacologique chez l’homme], chapitre 10. International Union of Basic and Clinical Pharmacology, p. 76-84.
Sketris, IS., CJ. Metge, JL. Ross, ME. Maccara, DG. Comeau, GC. Kephart et JL Blakburn. 2004. The Use of the World Health Organization Anatomic Therapeutic Chemical/Defined Daily Dose Methodology in Canada [Utilisation du système de classification anatomique thérapeutique chimique et de la méthodologie de la dose quotidienne définie de l’Organisation mondiale de la santé au Canada]. Drug Information Journal, vol. 38, p. 15-27.
Smith, AJ, I. Sketris, C. Cooke, D. Gardner, S. Kisely et S.E. Tett. 2008a. A comparison of benzodiazepines and related drug use in Nova Scotia and Australia [Une comparaison des benzodiazépines et de l’utilisation connexe de médicaments en Nouvelle-Écosse et en Australie]. Revue canadienne de psychiatrie, vol. 53, no 8.
Smith, AJ, I. Sketris, C. Cooke, D. Gardner, S. Kisely et S.E. Tett. 2008b. A comparison of antidepressant drug use in Nova Scotia and Australia [Une comparaison des données sur l’utilisation d’antidépresseurs en Nouvelle-Écosse et en Australie]. Pharmacoepidemiology and Drug Safety, vol. 17, p. 697-706.
Wessling, A. et G. Boethius. 1990. Measurement of drug use in a defined population, Evaluation of the Defined Daily Dose (DDD) methodology [Mesure de l’utilisation des médicaments dans une population définie, évaluation de la méthodologie de la dose quotidienne définie par l’OMS]. European Journal of Clinical Pharmacology, vol. 39, p. 207-210.
Annexe 1 : Intégration de la DDD aux bases de données administratives canadiennes
L’intégration de la DDD aux bases de données administratives canadiennes fait face à trois défis importants :
A. DDD non disponibles;
B. Intégration des données sur les DDD disponibles;
C. Uniformisation des processus de déclaration des unités.
DDD non disponibles
Les DDD ne sont prescrites que pour les médicaments qui ont un code ATC, ce que tous les médicaments n’ont pas. En outre, il existe des médicaments qui ont un code ATC qui leur est attribué, mais pas une DDD.
Médicaments sans code ATC
Le Centre collaborateur de l’OMS établit de nouvelles entrées dans le système de classification ATC en fonction des demandes des utilisateurs du système (p. ex. les fabricants, les organismes de réglementation, les chercheurs). Les médicaments qui n’ont pas de code ATC sont des médicaments pour lesquels une demande n’a pas été reçue. Le système n’est donc pas exhaustif.
La Base de données sur les produits pharmaceutiques (BDPP) de Santé Canada contient, entre autres, des renseignements sur les médicaments approuvés pour utilisation au Canada qui ont un numéro d’identification du médicament (DIN) attribué ainsi que le code ATC correspondant pour chaque DIN.
Environ un quart des DIN pour usage humain recensés dans la BDPP de Santé Canada n’ont pas de code ATC attribué par le Centre collaborateur de l’OMS. Toutefois, la grande majorité de ces médicaments ne sont pas actuellement utilisés au Canada, et le reste représente une part insignifiante de l’utilisation des médicaments au Canada.
Certaines bases de données administratives doivent intégrer parfois les DIN de Santé Canada. Une fois cette opération terminée, l’intégration du système de classification ATC se fait par une liaison simple. L’utilisation des médicaments doit ensuite concerner en grande partie les médicaments ayant un code ATC.
Les bases de données administratives comportent parfois les renseignements déclarés sur l’utilisation de produits pharmaceutiques qui n’ont pas de DIN de Santé Canada. Il s’agit notamment de produits et de dispositifs de pharmacie (bandelettes de test glycémiques, seringues, etc.). Ces produits reçoivent parfois ce qu’on appelle un pseudo-DIN.
Médicaments avec un code ATC mais sans DDD
L’OMS établit seulement une DDD pour les médicaments ayant déjà un code ATC. Toutefois, les DDD ne sont pas établies pour les préparations topiques, les sérums, les vaccins, les agents antinéoplasiques, les extraits d’allergènes, les produits d’anesthésie générale et locale, les produits de contraste ainsi que certains produits mixtes. Par conséquent, il existe des médicaments avec un code ATC qui n’ont pas de DDD.
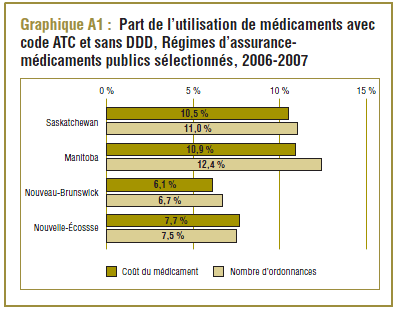
La majorité (65 %) des ingrédients ayant un code ATC n’ont pas de DDD prescrite. Néanmoins, l’analyse effectuée dans la base de données du SNIUMP relativement à certains régimes d’assurance-médicaments publics donne à penser que ces médicaments représentent généralement une part relativement faible de l’utilisation. Comme le montre le graphique A1, un peu plus de 10 % du coût des médicaments ainsi que de nombreuses ordonnances prescrites en Saskatchewan et au Manitoba pour l’exercice 2006-2007 concernent des médicaments qui n’ont pas de DDD. Au Nouveau-Brunswick et en Nouvelle-Écosse, les valeurs correspondantes sont comprises entre 6,1 et 7,7 %.
Principaux médicaments utilisés sans DDD
Produits mixtes :
Adrénergiques et autres médicaments pour les syndrômes obstructifs
Antagonistes des récepteurs de l’angiotensine II (ARA II) et Inhibiteurs de l’enzyme de conversion (IEC) et diurétiques
Progestatifs et estrogènes
Peginterféron alfa-2b et ribavirine
Timolol
Antiviraux pour le VIH
Codéine
Inhibiteur de protéines kinases etc.
La case ci-après présente la liste des principaux médicaments ayant un ATC, mais sans DDD dans les données analysées.
Bref, des études exhaustives menées sur les renseignements concernant l’utilisation des médicaments dans les bases de données administratives à l’aide de la méthodologie de la DDD excluent d’emblée les médicaments qui n’ont pas de DDD prescrites. La nature de l’exclusion doit être indiquée au moment de la présentation des résultats, étant donné que les conclusions tirées d’un sous-ensemble peuvent être différentes de celles tirées d’un ensemble complet de données.
Intégration des données sur les DDD disponibles
Les médicaments ayant un code ATC et une DDD prescrite peuvent être intégrées aux bases de données administratives canadiennes. Toutefois, lorsque deux sources de données sont intégrées, il peut y avoir des différences dans les données déclarées qui doivent être uniformisées.
Le tableau A1 présente un extrait de la version 2008 du système ATC/DDD de l’OMS concernant l’ingrédient estradiol. Le système ATC/DDD de l’OMS contient six champs d’information :
- Codes ATC – Cinq niveaux
- Noms ATC – Noms correspondant aux codes ATC
- Unité « U » – Unité de mesure de la quantité exprimée en g, mcg, mg, mL, mmol, etc.
- VAdm – Voie d’administration
- DDD – Dose quotidienne définie par l’OMS
- Remarques – Toute information pertinente permettant d’interpréter les données
Tableau A1 : Estradiol, version 2008 du système ATC/DDD de l’OMS
| Niveau ATC |
Ingrédient |
U |
VAdm |
DDD |
Remarques |
| G03CA03 |
Estradiol |
mg |
N |
0,3 |
|
| G03CA03 |
Estradiol |
mg |
O |
2 |
|
| G03CA03 |
Estradiol |
mg |
P |
1 |
Dépôt (courte durée) |
| G03CA03 |
Estradiol |
mg |
P |
0,3 |
Dépôt (longue durée) |
| G03CA03 |
Estradiol |
mg |
R |
5 |
|
| G03CA03 |
Estradiol |
mcg |
TD |
50 |
Timbre correspond à la quantité délivrée par 24 heures |
Des descriptions semblables ainsi que les données sur l’utilisation des médicaments sont généralement déclarées dans les bases de données administratives canadiennes. Toutefois, leur mode de déclaration peut varier de façon significative et présenter des différences dans les descriptions du nom de l’ingrédient, de la voie d’administration et de la description de l’unité de mesure. De vastes ressources (essentiellement d’ordre clinique et informatique) doivent être déployées pour uniformiser de façon exhaustive ces types de renseignements et intégrer entièrement les bases de données administratives canadiennes au système ATC/DDD de l’OMS. Santé Canada a déjà commencé ce processus en associant ses DIN aux codes correspondants du système de classification ATC.
Même si l’intégration du système de classification ATC nécessite en général une uniformisation et un regroupement des données sur les ingrédients (à quelques exceptions près), celle du système de DDD est plus complexe. Elle exige une uniformisation à plus grande échelle et une association des données relatives au format ainsi que l’unité de mesure pour la quantité. Une simple intégration du système de classification ATC ne veut pas dire que la DDD est également intégrée. La raison en est qu’il y a des cas où un seul ingrédient et le code ATC (voir le tableau A1) peut comporter plusieurs DDD. Les renseignements des DDD n’ont pas encore été intégrés dans une base de données publique au Canada.
Les chercheurs qui appliquent la méthodologie de la DDD doivent procéder à cette uniformisation. Lorsque la portée du projet est limitée à un groupe de médicaments, l’intégration des données des DDD peut se faire facilement pour chaque médicament. Toutefois, pour les études approfondies qui portent sur l’utilisation globale des médicaments et nécessitent l’intégration complète des DDD de milliers de médicaments, il faudra une programmation de liens automatiques ainsi qu’une fonction factorielle approfondie associée à chaque médicament dans l’expertise clinique.
Uniformisation des processus de déclaration des unités
Deux aspects doivent être abordés pour l’uniformisation des processus de déclaration des unités dans les bases de données administratives canadiennes :
Processus d’uniformisation des unités
La méthodologie de la DDD s’appuie sur les unités (quantités physiques de médicaments), tel qu’il est indiqué dans les données sur l’utilisation des médicaments. Toutefois, en raison de la variété des moyens d’expression des unités et de l’absence de processus de déclaration automatique, ce champ d’information a tendance à contenir des déclarations incohérentes.
Par exemple, la dose d’étanercept utilisée à une concentration de 50 mg/mL sous forme de fiole peut être saisie comme 1 (fiole) ou 50 (mg). Ainsi, les données sur l’utilisation des médicaments peuvent contenir une combinaison de ces deux méthodes de déclaration. Avant de convertir le nombre d’unités en DDD pour l’étanercept (la DDD pour l’étanercept est de 7 mg), les unités déclarées doivent être les mêmes. Cela signifie convertir le chiffre 1 (une fiole) déclaré à 50 (mg). Cette méthode permet ainsi de déclarer toutes les données sur l’utilisation de médicaments avec la même unité de mesure.
L’uniformisation des unités est parfois un processus qui exige beaucoup de temps et de ressources, en particulier pour les études sur l’utilisation des médicaments avec de nombreuses indications à observer. Toutefois, l’assurance de la qualité des données sur les unités est un élément essentiel de l’application de la méthodologie de la DDD.
Étant donné que les écarts dans les déclarations des unités se produisent au niveau des indications à observer (p. ex. l’ordonnance ou la demande de remboursement), l’uniformisation des unités doit se faire à ce niveau, et non au niveau global. Toutefois, si les données disponibles sont regroupées et non uniformisées au préalable, l’analyse devrait être restreinte aux données des groupes de médicaments qui risquent de ne pas pouvoir être déclarées de façon uniforme, telles que les médicaments solides par voie orale à l’exception des médicaments qui ne peuvent être délivrés que pour un certain nombre de pilules (p. ex. contraceptifs oraux; 28 pilules par rapport à une feuille).
Processus de conversion des unités de mesure
Les données administratives, dont l’uniformité des unités est assurée, peuvent nécessiter une uniformisation supplémentaire si l’unité de mesure déclarée ne correspond pas à celle utilisée dans le système ATC/DDD de l’OMS. Par exemple, une DDD en grammes (g), soit 100 mg = 0,1 g peut être attribuée à des médicaments dont les données sur l’utilisation sont exprimées en milligrammes (mg). Les unités de mesure qui ne correspondent pas doivent être relevées et uniformisées.
Annexe 2 : Analyse comparative des DQE des inhibiteurs de la HMG-CoA réductase dans les régimes provinciaux d’assurance-médicaments de l’Ontario, du Nouveau-Brunswick et de la Nouvelle-Écosse
Les exemples fournis dans cette étude portent sur l’utilisation des inhibiteurs de la HMG-CoA réductase dans le PMO. L’analyse comparative suivante confirme que les modes d’utilisation de médicaments déclarés ne sont pas limités au PMO, mais relevés également dans d’autres régimes provinciaux d’assurance-médicaments, tels que ceux du Nouveau-Brunswick et de la Nouvelle-Écosse.
Comme l’indiquent les graphiques A2.1 à A2.6, chacune des statines a des DQE moyennes comparables dans les trois provinces au cours de la période de 1997-1998 à 2006-2007. La DQE est déterminée en fonction de renseignements tels que le nombre de jours d’approvisionnement et la dose du médicament délivrée indiquée dans les données du régime d’assurance-médicaments.
L’analyse comparative est limitée à ces trois régimes provinciaux d’assurance-médicaments en raison des renseignements disponibles au cours de la période de l’étude.
Graphiques A2.1–A2.6 : Données de l’Ontario, du Nouveau-Brunswick et de la Nouvelle-Écosse au cours de la période de 1997-1998 à 2006-2007
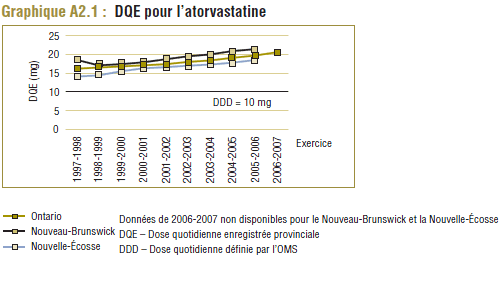
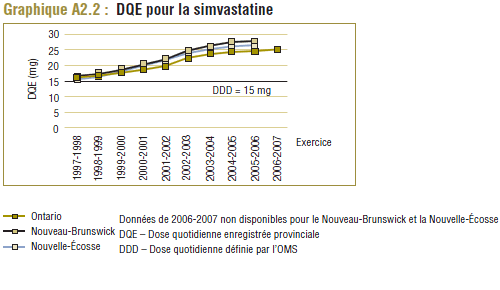
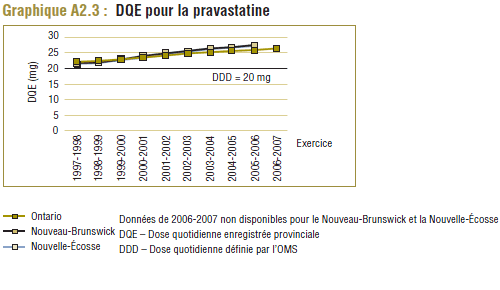
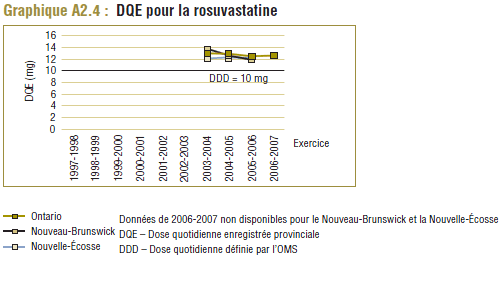
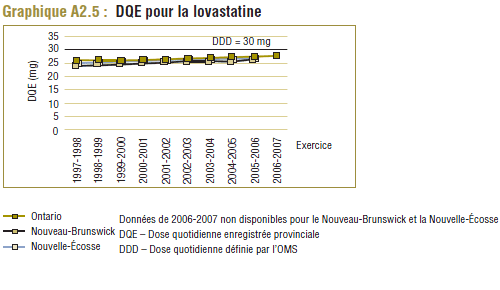
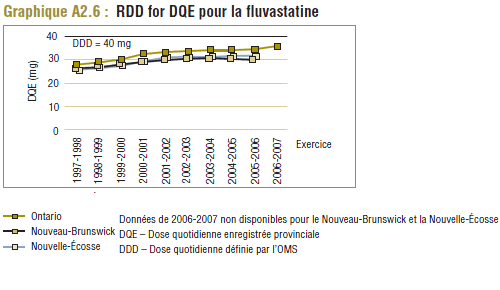
Annexe 3 : Nombre de jours d’approvisionnement dans la base de données du Programme de médicaments de l’Ontario : évaluation de la qualité
Les exemples fournis dans cette étude dressent une analyse et une comparaison des DQE moyennes pour les inhibiteurs de la HMG-CoA réductase (statines) dans le PMO. La mesure de l’utilisation des médicaments est déterminée en fonction du nombre de jours d’approvisionnement déclarés dans les données administratives du PMO. Une évaluation de la qualité de cette information est effectuée dans la présente annexe.
Même si elle ne concerne pas les statines, ni le nombre de jours d’approvisionnement en particulier, une évaluation de l’exactitude des données administratives sur les demandes de remboursement de médicaments dans la base de données du PMO a été effectuée (Levy et coll., 2003). Selon l’évaluation, les pharmaciens ont pratiquement toujours délivré les médicaments prescrits et les renseignements étaient fiables et transmis convenablement à la base de données des demandes de remboursement du PMO. Par conséquent, les conclusions tirées par les chercheurs utilisant ces renseignements ne sont pas susceptibles d’être compromises par la fiabilité des données saisies.
Puisque les statines sont des médicaments solides administrés par voie orale avec en général des doses recommandées une fois par jour, selon les monographies de produit approuvées par Santé Canada, la saisie des données devrait être simple.
L’accès aux données du PMO se fait au niveau global des DIN. Par conséquent, cette évaluation est limitée6 à une analyse des tendances dans le nombre moyen d’unités (comprimés) par jour d’approvisionnement pour chacune des doses unitaires des médicaments analysés.
Les résultats illustrés dans les graphiques A3.1 à A3.6 indiquent que le nombre moyen d’unités par jour exécuté pour toutes les statines et les doses unitaires (dosages) analysées sont légèrement supérieures à 1, ce qui donne à penser que les statines sont généralement délivrées une fois par jour, quelles que soient les concentrations (5 mg, 10 mg, 20 mg, 40 mg ou 80 mg), ce qui est conforme aux monographies de produit approuvées par Santé Canada.
En outre, d’après l’analyse présentée à l’annexe 2, les résultats concernant le nombre de jours d’approvisionnement en Ontario sont comparables à ceux des régimes publics d’assurance-médicaments du Nouveau-Brunswick et de la Nouvelle-Écosse. Cela donne à penser qu’il y a non seulement une uniformité dans les déclarations, mais aussi que les données sur le nombre de jours d’approvisionnement sont fiables.
Il importe de souligner que les renseignements déclarés dans la base de données du PMO sont ceux inscrits par les pharmacies en fonction des médicaments délivrés. Toutefois, on ignore si le médicament a été pris par le bénéficiaire, tel qu’il est indiqué dans les données administratives.
Graphiques A3.1–A3.6 : Nombre moyen d’unités par jour d’approvisionnement dans le Programme de médicaments de l’Ontario, de 1997-1998 à 2006-2007
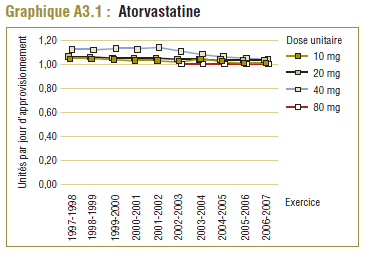
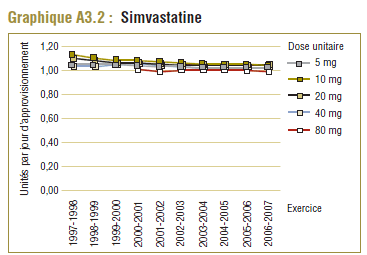
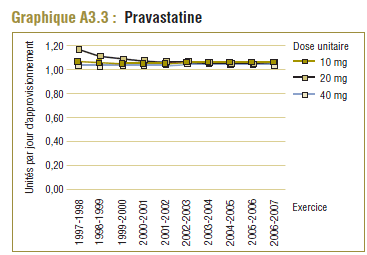
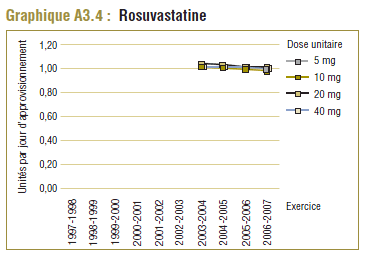
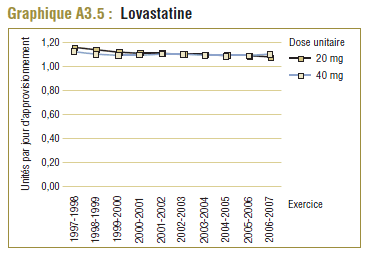
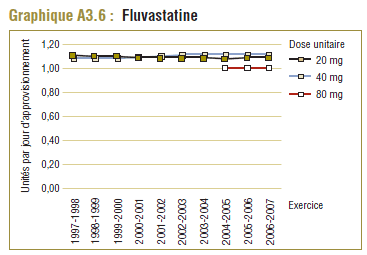
6 Une évaluation globale de la qualité des données sur le nombre de jours d’approvisionnement peut être effectuée au niveau des renseignements sur les demandes de remboursement en déterminant si le calendrier (date de délivrance) de l’ordonnance subséquente pour un patient correspond au calendrier (date de délivrance) et à la durée (nombre de jours d’approvisionnement) des ordonnances antérieures.
Annexe 4 : Modifications récentes apportées aux DDD
La DDD pour les statines est demeurée inchangée au cours de la période de dix ans de la présente étude (exercices 1997-1998 à 2006-2007) et en 2008. Par conséquent, c’est la version de janvier 2008 du système ATC/DDD de l’OMS qui a été utilisée. Depuis janvier 2009, d’importantes modifications ont été apportées à la DDD pour cinq des six statines afin de mieux reproduire l’actuelle dose quotidienne enregistrée. La présente annexe fournit une comparaison des anciennes et nouvelles valeurs des DDD par rapport à la dose quotidienne enregistrée (DQE) moyenne dans la base de données du PMO.
Le tableau A4 présente une comparaison entre les anciennes valeurs des DDD (avant janvier 2009) et les nouvelles valeurs des DDD (en vigueur depuis janvier 2009) pour chaque statine. Les DDD pour l’atorvastatine et la simvastatine ont été augmentées de 100 % et celles de la pravastatine, de la lovastatine et de la fluvastatine, de 50 %. La DDD de la rosuvastatine n’a subi aucune modification.
Tableau A4 : Modifications apportées à la DDD
| Ingrédient |
Ancienne DDD* |
Nouvelle DDD** |
Différence (%) |
DQE 2006-2007 |
DQE 2006-2007 par rapport à l’ancienne DDD |
DQE 2006-2007 par rapport à la nouvelle DDD |
| Atorvastatine |
10 mg |
20 mg |
+100 % |
20,6 mg |
+106 % |
+3 % |
| Simvastatine |
15 mg |
30 mg |
+100 % |
25,1 mg |
+67 % |
-16 % |
| Pravastatine |
20 mg |
30 mg |
+50 % |
26,4 mg |
+32 % |
-12 % |
| Rosuvastatine |
10 mg |
10 mg |
— |
12,6 mg |
+26 % |
+26 % |
| Lovastatine |
30 mg |
45 mg |
+50 % |
27,1 mg |
-10 % |
-40 % |
| Fluvastatine |
40 mg |
60 mg |
+50 % |
35,1 mg |
-12 % |
-41 % |
DQE – Dose quotidienne enregistrée en moyenne dans le PMO
DDD – Dose quotidienne définie par l’OMS
Nota : Sous réverve d’erreurs d’arrondi
*Avant janvier 2009
**En vigueur à compter de janvier 2009
En ce qui concerne des bases de données administratives canadiennes analysées, les modifications récentes constituent un ajustement tant nécessaire de la DDD pour les trois premières statines : l’atorvastatine, la simvastatine et la pravastatine. En 2006-2007, la DQE moyenne pour ces trois médicaments dans le programme de médicaments de l’Ontario était très différente de l’ancienne DDD.
Néanmoins, les modifications apportées à la lovastatine et la fluvastatine ont généré des valeurs de DDD bien éloignées des DQE correspondantes dans le PMO. À l’heure actuelle, on ignore la proportion dans laquelle la dernière DQE moyenne (2008-2009) dans le PMO se compare à la nouvelle DDD pour ces deux médicaments.
Annexe 5 : Sigles et définitions
Sigles
ATC - Anatomique thérapeutique chimique
CEPMB - Conseil d’examen du prix des médicaments brevetés
DDD - Dose quotidienne définie par l’OMS (« Defined Daily Dose »)
DIN - Numéro d’identification du médicament
DQE - Dose quotidienne enregistrée
DQP - Dose quotidienne prescrite
HMG - CoA Hydroxyméthylglutaryl-coenzyme
OMS - Organisation mondiale de la santé
PMO - Programme de médicaments de l’Ontario
SNIUMP - Système national d’information sur l’utilisation des médicaments prescrits
Définitions
DDD
La dose quotidienne définie par l’OMS (« Defined Daily Dose »). Par exemple, la DDD pour l’atorvastatine est de 10 mg.
Dose quotidienne prescrite7
Schéma thérapeutique demandé par le médecin pour le patient et consigné dans l’ordonnance.
Dose quotidienne délivrée
Schéma thérapeutique élaboré et remis au patient par le pharmacien. Schéma thérapeutique élaboré et remis au patient par le pharmacien.
Nombre d’unités
Quantités physiques (p. ex. nombre de pilules) utilisées et déclarées dans la base de données du programme d’assurance-médicaments.
Dose quotidienne enregistrée (DQE)
Le nombre de milligrammes d’un médicament délivrés en moyenne par jour au cours d’un exercice indiqué dans la base de données du Programme des médicaments. Cette mesure est calculée en fonction du nombre de jours d’approvisionnement du médicament délivré et déclaré au champ « Nombre de jours d’approvisionnement » dans la base de données du régime d’assurance-médicaments.
Nombre de DQE
Étant donné que la DQE est calculée en fonction du nombre de jours d’approvisionnement, le nombre de DQE correspond au nombre de jours d’approvisionnement indiqué dans la base de données du régime d’assurance-médicaments. Par exemple, 100 DQE d’atorvastatine correspondent à 100 jours d’approvisionnement.
Nombre de DDD
Représente l’utilisation des médicaments exprimée en DDD. Elle est calculée en convertissant le nombre d’unités déclarées dans les données en nombre de DDD selon la méthodologie de la DDD. Par exemple, 100 DDD d’atorvastatine (DDD = 10) correspond à 1 000 mg.
7 La dose quotidienne prescrite et la dose quotidienne délivrée doivent être pratiquement les mêmes et sont souvent utilisées de façon interchangeable. Néanmoins, elles peuvent varier chez une population, car il se peut que certains médicaments prescrits ne soient pas délivrés. En outre, il est possible que certains médicaments délivrés ne soient pas réellement consommés. La dose quotidienne prescrite et la dose quotidienne délivrée peuvent être déterminées en analysant les ordonnances, les dossiers médicaux ou pharmaceutiques ou encore les dossiers administratifs des demandes de remboursement ou en interrogeant les patients.