Rapport Annuel 2012
Le Conseil d'examen du prix des médicaments brevetés a pour mandat de veiller à ce que les brevetés ne vendent pas leurs médicaments brevetés au Canada à des prix excessifs ainsi que de faire rapport des tendances pharmaceutiques de tous les médicaments et des dépenses de R-D des brevetés.
Aperçu statistique de 2012
Mandat de réglementation
Conformité
- Quatre-vingt-deux (82) nouveaux produits médicamenteux brevetés pour usage humain ont fait l’objet d’un rapport au CEPMB.
- Les prix de 57 nouveaux produits médicamenteux brevetés ont été jugés conformes aux Lignes directrices.
- Au total, 1 328 nouveaux produits médicamenteux brevetés pour usage humain relevaient de la compétence du CEPMB.
Application
Jusqu’au 31 mai 2013 :
- Le Conseil a accepté 15 Engagements de conformité volontaire; une réduction des prix et des recettes excessives totalisant 34,5 M$ ont été remboursées au moyen de paiements au gouvernement.
- Le Conseil a mené à terme quatre audiences : Copaxone (réexamen) sur le prix; Pentacel et Quadracel, sur l’ordonnance corrective; Sandoz Canada Inc., pour défaut de soumettre ses rapports; et Tactuo, sur le prix.
- Aucune décision n’est en attente.
- Le Conseil compte deux affaires dans son calendrier d’audiences : Apotex Inc. et Apo-Salvent exempt de CFC.
Mandat de rapport
Tendances observées au niveau des ventes
- Les ventes de produits médicamenteux brevetés ont légèrement diminué de 0,3 %, totalisant 12,8 milliards de dollars.
- La part des ventes de produits médicamenteux brevetés exprimée en pourcentage de la valeur de l’ensemble des ventes a augmenté, passant de 58,6 % en 2011 à 59,3 % en 2012.
- Les agents antinéoplasiques et les agents immunomodulateurs ont le plus contribué à l’accroissement des ventes.
Tendances observées au niveau des prix des produits médicamenteux brevetés
- Les prix départ-usine des produits médicamenteux brevetés, mesurés à l’aide de l’indice des prix des médicaments brevetés, ont augmenté de 0,6 % en moyenne, alors que l’indice des prix à la consommation augmenté de 1,5 %.
- Les prix au Canada des produits médicamenteux brevetés se situaient au quatrième rang des prix les plus élevés par rapport aux prix pratiqués dans les sept pays de comparaison, mais moins élevés que les prix pratiqués en Suisse, en Allemagne et aux États-Unis.
Recherche-développement
- Les brevetés ont fait rapport de dépenses de R-D totalisant 894,8 millions de dollars, ce qui représente un recul de 9,8 % par rapport à 2011.
- Les brevetés membres de Rx&D ont fait rapport de dépenses de R-D totalisant 782,8 millions de dollars, ce qui représente un recul de 13,1 % par rapport à 2011.
- Les ratios des dépenses de R-D par rapport aux recettes tirées des ventes ont enregistré un léger recul en 2012 :
- Chez tous les brevetés, le ratio est passé de 5,6 % en 2011 à 5,3 %.
- Chez les brevetés membres de Rx&D, le ratio est passé de 6,7 % en 2011 à 6,6 %.
Lettre à la ministre
Le 31 mai 2013
L’honorable Leona Aglukkaq, C.P., députée
Ministre de la Santé
Chambre des communes
Ottawa (Ontario)
K1A 0A6
Madame la ministre,
J’ai le plaisir de vous présenter, conformément aux articles 89 et 100 de la Loi sur les brevets, le Rapport annuel du Conseil d’examen du prix des médicaments brevetés pour l’exercice terminé le 31 décembre 2012.
Croyant le tout conforme, je vous prie d’agréer, Madame la ministre, l’assurance de mes sentiments distingués.
Mary Catherine Lindberg
Présidente
Message de la présidente
En décembre 2012, le Conseil d’examen du prix des médicaments brevetés (CEPMB) a célébré son 25e anniversaire. Il y a eu d’importants changements au paysage pharmaceutique au cours des deux dernières décennies. À mesure qu’évolue le milieu, le CEPMB est toujours aussi résolu à protéger les intérêts des consommateurs et à contribuer au système de soins de santé canadien en veillant à ce que les médicaments brevetés ne soient pas vendus au Canada à des prix excessifs, et en faisant rapport des tendances relatives aux produits pharmaceutiques de même qu’aux dépenses des brevetés en matière de recherche et développement.
Au début de l’année, nous avons amorcé une évaluation de programme globale dans le but d’évaluer le rendement et la pertinence du CEPMB, et avons préparé un plan d’action en fonction des résultats. L’évaluation avait pour objet de déterminer si nous atteignons nos objectifs et si les ressources qui nous ont été affectées nous permettent d’exécuter efficacement notre mandat.
Selon le Rapport d’évaluation de programme, les programmes du CEPMB peuvent être appliqués par un organisme fédéral et cadrent bien avec les priorités pangouvernementales de même qu’avec notre résultat stratégique. Le rapport a permis en outre de constater que nous parvenons à atteindre nos résultats escomptés, et que le financement par reconduction reçu en 2008-2009 a été utilisé de façon convenable et a permis d’obtenir les résultats pour lesquels il a été autorisé.
Dans notre Plan d’action de la direction, nous avons abordé les recommandations énoncées dans le Rapport d’évaluation en examinant des façons (i) d’accélérer les processus et de simplifier davantage les Lignes directrices; (ii) de réduire le fardeau réglementaire; et (iii) d’utiliser efficacement les ressources du CEPMB, et ce, sans porter atteinte à son rôle visant à protéger les consommateurs.
À cette fin, nous avons procédé à une consultation auprès des intervenants sur des initiatives proposées se rapportant à la réduction du fardeau réglementaire. De plus, notre Plan de surveillance et d’évaluation des principales modifications aux Lignes directrices s’avère toujours une excellente base de discussion continue avec les brevetés et d’autres intervenants et ainsi nous permet d’apporter des modifications pertinentes aux Lignes directrices en temps opportun.
Nous nous sommes engagés à faire en sorte que nos études et nos rapports soient mis à la disposition des décideurs en temps utile. Grâce à l’initiative du Système national d’information sur l’utilisation des médicaments prescrits (SNIUMP), nous avons été en mesure d’entamer d’autres dialogues avec les intervenants et d’accroître notre participation aux discussions et conférences.
À titre de présidente du Conseil, je tiens à m’assurer que notre cadre continue d’avoir une incidence positive sur les consommateurs tout en reconnaissant la valeur des médicaments novateurs pour les patients. Notre cadre de fixation du prix fait mention des pays dont l’Allemagne et le Royaume-Uni. À mesure que les changements aux politiques en matière de remboursement de médicaments à l’échelle internationale continuent d’évoluer, nous surveillerons ces changements avec intérêt et évaluerons leur importance.
Tout au long de l’année, j’ai eu l’occasion de travailler de concert avec des membres dévoués du personnel et du Conseil. Je tiens à remercier M. Tim Armstrong pour son apport inestimable à la promotion du mandat de l’organisme au cours de ses 10 ans en qualité de membre du Conseil. Je tiens également à remercier Mme Anne Warner La Forest de son engagement à l’égard des principes du CEPMB. Au même titre, j’ai le plaisir d’accueillir deux nouveaux membres du Conseil, M. Normand Tremblay et M. Richard Bogoroch. Experts en leurs domaines respectifs, leur engagement au chapitre du mandat du CEPMB viendra compléter les efforts continus du Conseil visant à servir la population canadienne.
Je profite en outre de cette occasion pour reconnaître le défunt M. Robert G. Elgie, ancien président du CEPMB, qui est décédé en avril dernier. Un homme remarquable, le Dr Elgie croyait en le rôle du CEPMB qui vise à protéger les intérêts des consommateurs et veillait inlassablement à ce que l’organisme respecte ses engagements envers la fonction publique.
Au nom de mes collègues, je réaffirme notre engagement à assurer l’exécution efficace du mandat du CEPMB au service de la population canadienne.
Mary Catherine Lindberg
Le Conseil d’examen du prix des médicaments brevetés
Le Conseil d’examen du prix des médicaments brevetés (CEPMB) est un organisme indépendant qui détient des pouvoirs quasi judiciaires. Il a été créé par le Parlement en 1987 en vertu de la Loi sur les brevets.
Le CEPMB protège les intérêts des Canadiens en s’assurant que les produits médicamenteux brevetés ne sont pas vendus au Canada à des prix excessifs. Il le fait en examinant les prix auxquels les brevetés vendent les produits médicamenteux brevetés sur les marchés canadiens. Si un prix semble être excessif, le Conseil peut tenir des audiences publiques et ordonner la réduction des prix et (ou) le remboursement des recettes excessives. Le CEPMB doit également déclarer les tendances observées au niveau des ventes de produits pharmaceutiques et de l’établissement des prix pour tous les médicaments, ainsi que les dépenses de recherche-développement (R-D) des brevetés.
Le ministre de la Santé est responsable de l’application des dispositions de la Loi sur les brevets (la Loi) formulées aux articles 79 à 103. Le CEPMB fait partie du portefeuille de la Santé, qui est également constitué de Santé Canada, de l’Agence de la santé publique du Canada et des Instituts de recherche en santé du Canada. Le portefeuille de la Santé aide le ministre de la Santé à maintenir et à améliorer la santé des Canadiens.
Même s’il fait partie du portefeuille de la Santé, le CEPMB exerce son mandat en toute indépendance vis-à-vis du ministre de la Santé. Le CEPMB fonctionne également d’une façon indépendante des autres organismes, à savoir Santé Canada, qui autorise la vente des médicaments au Canada après avoir vérifié leur innocuité, leur efficacité et leur qualité; les régimes publics fédéral, provinciaux et territoriaux d’assurance-médicaments qui autorisent l’inscription des médicaments sur leurs formulaires de médicaments admissibles à un remboursement; et le Programme commun d’examen des médicaments, géré par l’Association canadienne des médicaments et des technologies de la santé, qui évalue l’efficience des médicaments avant leur inscription sur les formulaires des régimes publics d’assurance-médicaments participants.
Compétence
Réglementation
Le CEPMB vérifie les prix auxquels les brevetés vendent leurs produits médicamenteux brevetés pour usage humain ou pour usage vétérinaire distribués sous ordonnance ou en vente libre au Canada, afin de s’assurer qu’ils ne sont pas excessifs. Cela comprend les ventes aux grossistes, aux hôpitaux, aux pharmacies et autres clients pour usage humain ou pour usage vétérinaire. Le CEPMB réglemente le prix de chaque médicament breveté. Cela comprend chaque concentration et chaque forme posologique finale de tout médicament.
La compétence du Conseil ne s’applique pas exclusivement aux produits médicamenteux dont le brevet porte sur l’ingrédient actif, mais aussi aux médicaments auxquels un brevet est lié, que ce soit au niveau de son procédé de fabrication, de son mode d’administration, de sa forme posologique, de l’indication/ utilisation, de la préparation ou autre.
Les produits médicamenteux brevetés ne sont pas par définition exclusivement des produits de marque. En effet, certains fabricants de produits génériques sont assujettis à la compétence du Conseil du fait qu’ils vendent en vertu d’une licence d’exploitation le même produit que le produit de marque ou, encore, qu’ils sont titulaires d’un brevet visant le procédé de conditionnement ou de traitement de produits génériques.
Le CEPMB n’est pas habilité à réglementer les prix des médicaments non brevetés et n’a aucun droit de regard sur les prix auxquels les grossistes et les pharmacies vendent les médicaments ni sur les honoraires des pharmaciens. La distribution, l’ordonnance et le remboursement des médicaments échappent aussi à sa compétence.
En vertu de la Loi, les brevetés doivent informer le CEPMB de leur intention de vendre un nouveau produit médicamenteux breveté. Après leur première vente, les brevetés doivent faire rapport au CEPMB du prix de vente de leur produit médicamenteux et de la quantité vendue. Par la suite, à la fin de chaque semestre, ils doivent faire rapport des prix et des ventes au Canada des différentes concentrations de leurs produits médicamenteux.
Même s’ils ne sont pas tenus de faire approuver au préalable les prix de vente de leurs produits médicamenteux, les brevetés doivent respecter à la lettre les dispositions de la Loi pour s’assurer que les prix auxquels ils vendent leurs produits médicamenteux au Canada ne sont excessifs. Lorsque, à l’issue d’une audience publique, il apparaît que le prix d’un produit médicamenteux vendu sur un marché canadien est excessif, le Conseil peut rendre une ordonnance qui oblige le breveté à réduire le prix de son produit médicamenteux et à appliquer les mesures qui lui sont dictées pour rembourser les recettes excessives qu’il a encaissées.
Rapport
Chaque année, le CEPMB rend compte de ses activités au Parlement par le truchement du ministre de la Santé. Le rapport annuel présente une analyse des tendances relatives à la vente et aux prix de tous les produits médicamenteux et fait rapport des dépenses de R-D des brevetés.
Au moyen du programme du Système national d’information sur l’utilisation des médicaments prescrits (SNIUMP), le CEPMB effectue des analyses critiques des tendances des prix des produits médicamenteux d’ordonnance, de l’utilisation faite de ces produits et des coûts en produits médicamenteux au Canada. Les résultats de ces analyses éclairent le processus de décision des régimes d’assurance-médicaments fédéraux, provinciaux et territoriaux participants.
Gouvernance
Le Conseil est composé d’au plus cinq membres siégeant à temps partiel, dont un président et un vice-président. Tous les membres du Conseil sont nommés par le gouverneur en conseil. En vertu de la Loi, le président du Conseil assume également les fonctions de chef de la direction et, en cette qualité, est chargé de la gouverne et de la supervision des activités du CEPMB.
Les membres du Conseil, y compris le président, sont collectivement responsables de la mise en oeuvre des dispositions applicables de la Loi. Ensemble, ils établissent les lignes directrices, les règles, les règlements administratifs et les autres politiques du Conseil, comme le prévoit la Loi et consultent au besoin des intervenants, y compris les ministres provinciaux et territoriaux de la Santé et les représentants de groupes de consommateurs, l’industrie pharmaceutique et d’autres personnes.
En date du 31 mai 2013, il y avait un seul poste vacant au Conseil.
Membres du Conseil
Présidente
Mary Catherine Lindberg, B. Sc. Pharm.
Mary Catherine Lindberg a d’abord été nommée membre et vice-présidente du Conseil d’examen du prix des médicaments brevetés en juin 2006. Le 19 mai 2010, Mme Lindberg assumait les fonctions de la présidence pendant la vacance du poste. Elle a été officiellement nommée présidente du Conseil le 3 mars 2011.
Mme Lindberg a occupé de 2002 à 2009 le poste de directrice exécutive du Ontario Council of Academic Hospitals, un regroupement de 25 hôpitaux universitaires affiliés à une université et à sa faculté de médecine. Avant d’occuper ce poste, Mme Lindberg était sous-ministre adjointe des Services de santé, au ministère de la Santé et des Soins de longue durée de l’Ontario. Elle s’occupait entre autres du Régime d’assurance-maladie de l’Ontario et des Programmes de médicaments.
Mme Lindberg a fait ses études en pharmacie à l’Université de la Saskatchewan et a son permis de pratique en Saskatchewan et en Ontario.
Vice-président
Mitchell Levine, B. Sc., M. Sc., M.D., FRCPC, FISPE
Le Dr Mitchell Levine a été nommé membre et vice-président du Conseil le 3 mars 2011.
Le Dr Levine est professeur au sein des départements de médecine et d’épidémiologie clinique et biostatistiques à la faculté des sciences de la santé de l’Université McMaster à Hamilton, en Ontario. Il est également directeur du Centre for Evaluation of Medicines à St. Joseph’s Healthcare, à Hamilton.
Le Dr Levine a obtenu son diplôme en médecine à l’Université de Calgary en 1979, qui a été suivi d’études supérieures en médecine interne (FRCPC) et en pharmacologie clinique à l’Université de Toronto (de 1981 à 1987). Il a obtenu un diplôme de maîtrise ès sciences en épidémiologie clinique de l’Université McMaster en 1988.
Avant sa nomination au Conseil, le Dr Levine était membre du Groupe consultatif sur les médicaments pour usage humain du CEPMB. Il agit comme consultant spécial en pharmacologie clinique au ministère de la Santé et des Soins de longue durée de l’Ontario. De plus, il est rédacteur en chef du Journal de la thérapeutique des populations et de la pharmacologie clinique et est corédacteur de l’ACP Journal Club: Evidence-Based Medicine.
Normand Tremblay, ASC, M. Sc., Adm. A., C.M.C.
Normand Tremblay a été nommé membre du Conseil en mai 2012.
M. Tremblay est enseignant chargé de cours à l’Université du Québec à Trois-Rivières, dans le domaine de gestion stratégique. Il fait bénéficier le Conseil d’une vaste expérience en planification stratégique et opérationnelle ainsi qu’en développement organisationnel.
M. Tremblay a agi à titre de membre du Conseil national de recherches du Canada de 2007 à 2010. Il est membre de l’Ordre des administrateurs agréés du Québec.
Richard Bogoroch, LL. B.
Richard Bogoroch a été nommé membre du Conseil en décembre 2012.
M. Bogoroch est un avocat chevronné dans le domaine de dommage corporel et de faute médicale qui est engagé activement dans la collectivité juridique. Il est ancien directeur du Ontario Centre for Advocacy Training et de l’Advocates’ Society. Il est également membre de la Toronto Lawyers Association, de la Medico-Legal Society of Toronto, de l’Association of Trial Lawyers of America, de l’American Bar Association, de l’Advocates’ Society et de l’Ontario Trial Lawyers Association. Il a donné de nombreux exposés et écrit bon nombre de documents sur les diverses facettes du contentieux du dommage corporel et de la faute médicale dans le cadre des programmes de formation juridique permanente offerts par le Barreau du Haut-Canada, l’Advocates’ Society, l’Osgoode Hall Law School et autres.
M. Bogoroch a obtenu en 1978 un baccalauréat en droit civil (LL.L.) et en 1979 un baccalauréat en droit (LL. B.) de la Faculté de droit de l’Université McGill. Il a été reçu au Barreau de l’Alberta en 1980 et admis au Barreau de l’Ontario en 1983. En 1993, il a été accrédité par le Barreau du Haut-Canada comme spécialiste en contentieux des affaires civiles.
En 2012, les mandats de M. Thomas (Tim) Armstrong et de Mme Anne Warner La Forest, qui ont siégé au Conseil pendant 10 ans et cinq ans, respectivement, ont pris fin. Ils ont tous les deux apporté au Conseil une contribution inestimable.
Structure organisationnelle et personnel
Directrice exécutive
La directrice exécutive assure la gouverne générale des activités du CEPMB et avise le Conseil. Elle supervise le travail des employés du Conseil et en assume le leadership.
Réglementation et liaison auprès des brevetés
La Direction de la réglementation et de la liaison auprès des brevetés fait l’examen des prix des produits médicamenteux brevetés vendus au Canada pour s’assurer qu’ils ne sont pas excessifs. De plus, elle encourage les brevetés à se conformer volontairement aux Lignes directrices du Conseil, veille à la bonne application des politiques de conformité et fait enquête sur les plaintes reçues concernant les prix de certains produits médicamenteux brevetés. De plus, la Direction sensibilise les brevetés sur les Lignes directrices du Conseil et les informe de leurs obligations en matière de présentation de rapports.
Politiques et analyse économique
La Direction des politiques et de l’analyse économique formule lorsqu’il y a lieu des conseils stratégiques concernant les changements qui pourraient être apportés aux Lignes directrices et à d’autres politiques du Conseil. Elle effectue des recherches, ainsi que des analyses des tendances des prix des produits médicamenteux, et en présente les résultats dans des rapports. Enfin, elle effectue les études à l’appui des activités de conformité et d’application et les études que lui commande le ministre de la Santé.
Services généraux
La Direction des services généraux offre conseils et services en matière de gestion des ressources humaines, des installations, de la santé et sécurité au travail, de la technologie et de la gestion de l’information. Elle s’occupe également de la planification stratégique et financière de même que des rapports, des vérifications, de l’évaluation et de la liaison auprès des agences centrales fédérales compétentes.
Secrétariat du Conseil et communications
Le Secrétariat du Conseil et le groupe des Communications planifient et orchestrent le programme des communications du CEPMB, les relations avec les médias et le suivi aux demandes de renseignements du grand public. Ils gèrent les réunions et les audiences du Conseil, dont les dossiers de procédure. Ils coordonnent les activités du Conseil relatives à l’application de la Loi sur l’accès à l’informationet de la Loi sur la protection des renseignements personnels.
Avocate générale
L’avocate générale fournit des opinions juridiques au CEPMB et dirige l’équipe de la poursuite dans les audiences du Conseil.
Budget
En 2012-2013, le Conseil disposait d’un budget de 11,058 millions de dollars et d’un effectif approuvé de 76 équivalents temps plein (ETP).
Tableau 1 Budget et effectif
| |
2011-2012 |
2012-2013 |
2013-2014 |
| Budget |
11,832 M$ |
11,058 M$ |
10,944 M$ |
| Salaires |
7,034 M$ |
7,034 M$ |
6,920 M$ |
| Opérations |
1,698 M$ |
1,554 M$ |
1,554 M$ |
| Affectation à but spécial* |
3,100 M$ |
2,470 M$ |
2,470 M$ |
| ETP |
76 |
76 |
74 |
* L’affectation à but spécial est réservée aux coûts externes liés à la tenue d’audiences publiques (conseillers juridiques, témoins experts, etc.). Les fonds non dépensés sont retournés au Trésor.
Communications et liaison auprès des brevetés
Le programme des communications est responsable de la planification et de la gestion des communications externes du CEPMB. Il assure également la visibilité de l’organisme et la participation des intervenants. Des renseignements sont échangés sous diverses formes et au moyen de différents supports, avec les consommateurs, les partenaires provinciaux et territoriaux, l’industrie et d’autres intervenants. Les principales activités du programme comprennent, entre autres, les relations avec les médias, répondre aux demandes de renseignements du public, et renseigner le public par la publication de mises à jour sur les audiences devant le Conseil et les décisions du Conseil et de résultats de recherche.
Le groupe des Communications cherche tout particulièrement à adapter le CEPMB aux nouvelles exigences de son environnement opérationnel, en évaluant son efficacité et en envisageant constamment des produits de communication de rechange.
À titre de source fiable et impartiale de renseignements exhaustifs et exacts sur les prix des médicaments, le CEPMB s’engage à développer et à entretenir de façon continue une collaboration avec ses intervenants.
Les intervenants de l’industrie sont consultés et informés de tout changement de l’environnement opérationnel et sont rapidement informés de toute mise à jour au processus de réglementation. Afin de faciliter l’accès à l’information, la Direction de la réglementation et de la liaison auprès des brevetés tient régulièrement des séances d’information à l’intention des brevetés.
Publications
Outre nos publications régulières, dont le Rapport annuel et le bulletin d’information trimestriel La Nouvelle, le CEPMB publie des rapports de recherche du SNIUMP en réponse aux exigences de programme ou de l’organisme.
Le CEPMB continue de publier ses documents uniquement en format électronique dans le but de réduire les coûts et l’incidence environnementale de l’imprimerie. Il compte davantage sur son site Web et les médias sociaux à des fins de collaboration avec ses intervenants.
Le CEPMB est toujours résolu à réaliser son mandat de façon ouverte et transparente.
Rapport d’évaluation de programme et Réponse de la direction
En 2008-2009, le Secrétariat du Conseil du Trésor a approuvé pour le CEPMB une augmentation du financement qui permettrait à ce dernier d’exécuter efficacement son mandat. Corrélativement à la mise en disposition de cette augmentation continue de ressources, le CEPMB s’est engagé à réaliser une évaluation complète de ses programmes en 2011-2012, dont l’objectif était d’évaluer la pertinence, l’efficience et l’efficacité du CEPMB ainsi que d’évaluer la mesure dans laquelle l’augmentation des ressources lui a permis d’atteindre ses objectifs.
En 2012, le CEPMB a amorcé le processus d’évaluation de programme. On a jugé que le Programme de réglementation du prix des médicaments brevetés et le Programme sur les tendances relatives aux produits pharmaceutiques pouvaient être appliqués par un organisme fédéral et cadraient bien avec les priorités pangouvernementales de même qu’avec le résultat stratégique du CEPMB. L’examen a permis de dégager que le CEPMB est parvenu à atteindre ses résultats escomptés.
Selon l’évaluation, le financement par reconduction qui a été reçu en 2008-2009 a été utilisé de façon efficace et a permis d’obtenir les résultats pour lesquels il avait été autorisé.
Tôt en 2013, le CEPMB a publié le rapport d’évaluation de même que la réponse et le plan d’action de la direction pour aborder les dispositions soulevées dans le rapport d’évaluation, notamment :
- accélérer tous les processus du CEPMB;
- simplifier davantage les Lignes directrices;
- encourager l’utilisation d’un langage clair dans toutes les publications du CEPMB;
- élargir le public visé par les efforts de sensibilisation (mettre davantage l’accent sur les payeurs publics, les tiers payeurs et les groupes de défense des droits des patients).
La réponse et le plan d’action de la direction contiennent des renseignements sur les initiatives et les activités que le CEPMB a entreprises ou qu’il entreprendra en vue d’aborder ces dispositions.
À mesure qu’il évolue, le CEPMB continuera d’accroître l’efficacité de ses programmes en surveillant les conséquences de ses Lignes directrices, en les précisant et en les modifiant au besoin. Il restera en outre à l’affût d’occasions de mettre, en temps opportun, ses études et ses rapports à la disposition des décideurs en matière de politique.
Réglementation des prix des médicaments brevetés
Le CEPMB protège les intérêts des consommateurs canadiens en s’assurant que les produits médicamenteux brevetés ne sont pas vendus au Canada à des prix excessifs. Il le fait en examinant les prix auquels les brevetés vendent chaque produit médicamenteux breveté sur les marchés canadiens aux grossistes, aux hôpitaux et aux pharmacies.
Exigences en matière de rapport
Les brevetés sont tenus par la loi de produire des renseignements relatifs à la vente de leurs produits médicamenteux au Canada. La Loi sur les brevets (la Loi) et le Règlement sur les médicaments brevetés (le Règlement) dictent les rapports que doivent soumettre les brevetés, et le personnel du Conseil examine de façon continue les renseignements sur les prix afin de s’assurer que les prix ne sont pas excessifs, et ce, jusqu’à échéance de tous les brevets applicables.
Il existe divers facteurs servant à déterminer si le prix d’un produit médicamenteux est excessif, comme l’énonce l’article 85 de la Loi. Le Compendium des politiques, des Lignes directrices et des procédures (les Lignes directrices) fournit des renseignements sur les tests appliqués aux prix pour décider si le prix auquel un breveté vend son produit est inférieur au prix maximal permis. Les Lignes directrices ont été élaborées en collaboration avec les intervenants, dont les ministres de la Santé provinciaux et territoriaux, les associations de consommateurs et l’industrie pharmaceutique. Lorsqu’à l’issue d’une enquête, on juge qu’il y a un problème relativement au prix d’un produit médicamenteux breveté, on offre au breveté la possibilité de réduire volontairement son prix et (ou) de rembourser ses recettes excessives aux termes des modalités d’un Engagement de conformité volontaire. Si le breveté ne souscrit pas aux résultats de l’enquête et choisit de ne pas présenter un Engagement, le président du Conseil peut émettre un Avis d’audience. Le breveté peut soumettre un Engagement de conformité volontaire après l’émission d’un Avis d’audience, ou bien, l’affaire sera traitée en audience publique. Après l’audition de la preuve, si le Conseil conclut que le prix est en effet excessif, il peut rendre une ordonnance obligeant le breveté à réduire le prix de son produit et (ou) à rembourser les recettes excessives.
Des copies de la Loi, du Règlement, des Lignes directrices et du Guide du brevetésont affichées sur le site Web du CEPMB.
Défaut de présenter ses rapports
Le CEPMB compte sur la ponctualité des brevetés en ce qui a trait à la présentation de leurs rapports sur tous les produits médicamenteux brevetés qu’ils vendent au Canada auxquels un brevet s’applique. En 2012, 12 produits médicamenteux ont été déclarés au CEPMB pour la première fois, même s’ils avaient été brevetés et vendus avant 2012. De plus, neuf produits médicamenteux déclarés antérieurement au CEPMB et dont le brevet était arrivé à échéance, ont de nouveau été déclarés comme ayant un brevet applicable.
Le tableau 2 présente les produits médicamenteux qui étaient brevetés et vendus au Canada avant de faire l’objet d’un rapport au CEPMB.
Tableau 2 Défaut de présenter ses rapports sur les ventes de produits médicamenteux brevetés
| Breveté |
Nom de marque |
Nom générique |
Année où le médicament est devenu assujetti à la compétence du Conseil |
Année où le médicament est devenu assujetti à la compétence du Conseil et dont le brevet était nouveau |
| Baxter Corporation |
Hemofil-M |
Facteur VIII |
|
2005 |
| EMD Serono Canada Inc. |
Gonal-F (10 produits médicamenteux) |
Follitropine alpha |
1999 (2), 2001 (1), 2002 (1), 2003 (3), 2005 (3) |
|
| GlaxoSmithKline Inc. |
Arepanrix |
Virus grippal fragmenté, inactivé, contenant un antigène équivalant à une souche analogue à la variante A/California/7/2009 (H1N1) |
2009 |
|
| Novo Nordisk |
Novolin ge (8 produits médicamenteux) |
Insuline biosynthétique humaine |
|
2009 |
| Orion Corporation |
Simdax |
Levosimendan |
2011 |
|
Défaut de présenter les données sur les prix et sur les ventes (Formulaire 2)
Le défaut de présenter ses rapports fait référence au défaut partiel ou complet d’un breveté de présenter les rapports qu’il est tenu de présenter en vertu de la Loi et du Règlement. Le Conseil n’a pas été appelé à rendre des ordonnances pour défaut de présenter ses rapports en 2012.
Examen scientifique
Groupe consultatif sur les médicaments pour usage humain
Tous les nouveaux produits médicamenteux brevetés ayant fait l’objet d’un rapport au CEPMB sont soumis à une évaluation scientifique dans le cadre du processus d’examen du prix. Le Groupe consultatif sur les médicaments pour usage humain (GCMUH) a été créé par le Conseil dans le but d’offrir une expertise et des conseils indépendants au personnel du Conseil. Le GCMUH entreprend un examen lorsqu’un breveté présente une demande relative à l’amélioration thérapeutique. De plus, il examine et évalue les renseignements scientifiques pertinents et disponibles, notamment toute preuve présentée par le breveté quant au niveau d’amélioration thérapeutique proposé, le choix de produits médicamenteux aux fins de comparaison et les posologies comparables.
Les membres du GCMUH fondent leurs recommandations sur les connaissances médicales et scientifiques et sur les pratiques cliniques actuelles. Le GCMUH est constitué des membres suivants :
- Dre Jean Gray, professeure émérite en enseignement médical, médecine et pharmacologie à l’Université Dalhousie;
- M. Adil Virani, directeur des services pharmaceutiques des basses-terres continentales à Vancouver et chargé de cours à la faculté des sciences pharmaceutiques de l’Université de la Colombie-Britannique;
- Dr Fred Y. Aoki, professeur de médecine, de microbiologie médicale et de pharmacologie et thérapeutique à la faculté de médecine de l’Université du Manitoba;
- Dr Jacques LeLorier, professeur aux départements de médecine et de pharmacologie de l’Université de Montréal et chef du département de pharmacoépidémiologie et de pharmacoéconomie au Centre de recherche du CHUM (CRCHUM);
- M. Muhammad Mamdani, directeur du Centre de recherche appliquée en santé, du Li Ka Shing Knowledge Institute de l’hôpital St. Michael’s de Toronto, ainsi que professeur agrégé au Département des politiques de santé, gestion et évaluation (faculté de médecine) et à la faculté de pharmacie Leslie Dan de l’Université de Toronto
Examen du prix
Le CEPMB examine le prix moyen de chaque concentration et chaque forme posologique d’un médicament breveté. Dans la plupart des cas, cette unité est conforme au numéro d’identification du médicament (DIN) attribué par Santé Canada au moment où la vente au Canada du médicament est approuvée.
Nouveaux produits médicamenteux brevetés ayant fait l’objet d’un rapport au CEPMB en 2012
Aux fins du présent rapport, tout produit médicamenteux breveté lancé ou vendu sur le marché canadien avant l’attribution de son premier brevet entre le 1er décembre 2011 et le 30 novembre 2012 est réputé avoir été breveté en 2012.
Il y avait 82 nouveaux produits médicamenteux brevetés pour usage humain ayant été rapportés comme vendus en 2012. Certains constituent une ou plusieurs concentrations d’une nouvelle substance active et d’autres, de nouvelles présentations de médicaments existants. Dix (12,2 %) des 82 nouveaux produits médicamenteux brevetés ont été commercialisés au Canada avant d’avoir obtenu un premier brevet canadien qui les aurait automatiquement assujettis à la compétence du CEPMB. Le tableau ci-dessous indique l’année de la première commercialisation de ces produits médicamenteux.
Tableau 3 Nombre de nouveaux produits médicamenteux brevetés pour usage humain en 2012 selon l’année de leur première vente
| Année de la première vente |
Nbre de produits médicamenteux |
| 2012 |
72 |
| 2011 |
3 |
| 2010 |
6 |
| 2009 |
1 |
| Total |
82 |
La liste des nouveaux produits médicamenteux brevetés ayant fait l’objet d’un rapport au CEPMB est mise à jour chaque trimestre et affichée sur le site Web sous la rubrique « Réglementation des prix ». Cette liste présente de l’information sur l’état d’avancement de l’examen (p. ex. la question de savoir si le prix du médicament est sous examen, conforme aux Lignes directrices, sous enquête ou assujetti à un Engagement de conformité volontaire ou à un Avis d’audience).
Le graphique 1 illustre le nombre de nouveaux produits médicamenteux brevetés pour usage humain ayant fait l’objet d’un rapport au CEPMB de 1989 à 2012.
Graphique 1
Nouveaux produits médicamenteux brevetés pour usage humain
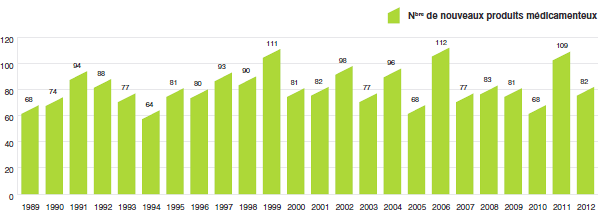
Source : CEPMB
Des 82 nouveaux produits médicamenteux brevetés :
- les prix de 82 produits médicamenteux brevetés avaient été soumis à un examen au 31 mars 2013 :
- les prix de 57 produits médicamenteux brevetés ont été jugés conformes aux Lignes directrices;
- les prix de 9 produits médicamenteux brevetés semblaient excessifs aux termes des Lignes directrices d’un montant ne justifiant pas d’enquête;
- les prix de 15 produits médicamenteux brevetés semblaient excessifs aux termes des Lignes directrices, ayant ainsi des enquêtes ont été lancées;
- le prix d’un seul produit médicamenteux était visé par un Engagement de conformité volontaire.
La liste complète des 82 nouveaux produits médicamenteux brevetés, y compris l’état d’avancement de l’examen de leur prix, est affichée sur le site Web du CEPMB.
Examen des prix des produits médicamenteux brevetés existants pour usage humain en 2012
Aux fins du présent rapport, l’expression « produits médicamenteux brevetés existants » désigne tous les produits médicamenteux brevetés vendus sur le marché canadien et ayant fait l’objet d’un rapport au CEPMB avant le 1er décembre 2012.
Au moment de la rédaction du présent rapport, il y avait 1 246 produits médicamenteux brevetés existants :
- les prix de 1 033 produits médicamenteux brevetés étaient conformes aux Lignes directrices;
- les prix de 130 produits médicamenteux brevetés étaient excessifs aux termes des Lignes directrices d’un montant ne justifiant pas d’enquête;
- les prix de 44 produits médicamenteux brevetés existants faisaient l’objet d’une enquête :
- une seule enquête provoquée par le prix de lancement en 2011;
- 43 enquêtes lancées en fonction des prix annuels.
- les prix de 7 produits médicamenteux étaient sous enquête;
- les prix de 30 produits médicamenteux étaient visés par un Engagement de conformité volontaire;
- 2 produits médicamenteux ont fait l’objet d’une audience sur le prix en vertu de l’article 83 de la Loi (voir la section « Audiences »);
- 1 autre produit médicamenteux fait toujours l’objet d’une audience, même s’il n’était plus breveté en 2012.
Un aperçu de l’état d’avancement de l’examen en 2012 du prix des produits médicamenteux brevetés pour usage humain nouveaux et existants est présenté au tableau 4.
Tableau 4 Produits médicamenteux brevetés pour usage humain vendus au Canada en 2012 – État d’avancement de l’examen du prix en date du 31 mars 2013
| |
Nouveaux produits médicamenteux lancés sur le marché en 2012 |
Produits médicamenteux brevetés existants |
Total |
| Total |
82 |
1 246 |
1 328 |
| Conformes aux Lignes directrices |
57 |
1 033 |
1 090 |
| Sous examen |
0 |
7 |
7 |
| Ne justifient pas d’enquête |
9 |
130 |
139 |
| Sous enquête |
15 |
44 |
59 |
| Engagements de conformité volontaire |
1 |
30 |
31 |
| Audiences sur le prix |
0 |
2 |
2 |
Mise à jour : Rapport annuel 2011
- L’examen de l’ensemble des produits médicamenteux brevetés pour usage humain ayant fait l’objet d’un rapport sous examen dans le rapport annuel de 2011 est terminé.
- Des 68 enquêtes mentionnées dans le rapport annuel de 2011, 64 d’entre elles se sont soldées par les résultats suivants :
- la fermeture de l’enquête lorsqu’il apparaît que le prix est conforme aux Lignes directrices;
- un Engagement de conformité volontaire par lequel le breveté s’engage à réduire le prix de son produit et à rembourser les recettes excessives au moyen d’un paiement et (ou) d’une réduction du prix d’un autre produit médicamenteux breveté (voir la section « Engagements de conformité volontaire »);
- une audience publique dont l’objet est de déterminer si le prix du produit médicamenteux est ou non excessif, y compris une ordonnance corrective rendue par le Conseil (voir la section « Audiences »).
Produits médicamenteux brevetés en vente libre et produits médicamenteux brevetés pour usage vétérinaire
Le personnel du Conseil ne fait l’examen des prix des produits médicamenteux brevetés en vente libre et des produits médicamenteux brevetés pour usage vétérinaire qu’à la suite de la réception d’une plainte. Le Conseil n’a reçu aucune plainte en 2012.
Engagements de conformité volontaire et audiences
Le personnel du Conseil fait l’examen des prix de tous les produits médicamenteux brevetés offerts sur le marché canadien. Lorsque le prix d’un produit semble excessif aux termes des Lignes directrices et que les circonstances justifient une enquête, le personnel du Conseil mène une enquête aux fins de déterminer si le prix du produit est ou non conforme aux Lignes directrices. L’enquête peut mener à l’un ou l’autre des résultats suivants :
- la fermeture de l’enquête lorsqu’il apparaît que le prix est conforme aux Lignes directrices;
- la fermeture de l’enquête lorsque le président est d’avis que l’émission d’un Avis d’audience ne servirait pas l’intérêt public;
- un Engagement de conformité volontaire par lequel le breveté s’engage à réduire le prix de son produit à un niveau non excessif et à rembourser les recettes excessives qu’il a encaissées – les recettes excessives peuvent être remboursées au moyen d’un paiement et (ou) de la réduction supplémentaire du prix du produit médicamenteux breveté, ou d’une réduction du prix d’un autre produit médicamenteux breveté;
- l’émission par le président d’un Avis d’audience pour la tenue d’une audience publique sur le prix d’un médicament breveté.
Engagements de conformité volontaire
L’Engagement de conformité volontaire est un engagement écrit par lequel le breveté s’engage à rendre le prix de son produit médicamenteux conforme aux Lignes directrices du Conseil. En vertu des Lignes directrices, les brevetés peuvent soumettre un Engagement de conformité volontaire lorsque le personnel du Conseil détermine, à la suite d’une enquête, que le prix auquel le produit médicamenteux breveté est vendu au Canada est excessif aux termes des Lignes directrices. Il peut également soumettre un Engagement de conformité volontaire après l’émission d’un Avis d’audience.
En 2012, la présidente a approuvé 11 Engagements de conformité volontaire visant 24 produits médicamenteux. En plus de la réduction du prix de certains produits médicamenteux, des recettes excessives totalisant 27 264 768,52 $ ont été remboursées au moyen de paiements versés au gouvernement du Canada.
En 2013, à ce jour, la présidente a accepté 3 Engagements de conformité volontaire visant 10 produits médicamenteux. Des recettes excessives totalisant 6 828 178,77 $ ont été remboursées au moyen de paiements versés à la Couronne.
Les brevetés doivent s’assurer que les prix de leurs produits médicamenteux brevetés demeurent conformes aux Lignes directrices du Conseil et ce, pour toutes les périodes où le produit médicamenteux relève de la compétence du CEPMB.
Tableau 5 Engagements de conformité volontaire en 2012 jusqu’au 31 mai 2013
| Produit médicamenteux breveté |
Usage thérapeutique |
Breveté |
Date d’approbation |
Remboursement des recettes excessives |
| Réduction de prix |
Paiement à la Couronne |
| Engagements de conformité volontaire en 2012 |
| Thalomid (3 produits médicamenteux) |
Myélome multiple |
Celgene Corporation |
Janv. |
|
10 000 000 $ |
| Dovobet (1 produit médicamenteux) |
Psoriasis |
LEO Pharma Inc. |
Janv. |
|
32 019,98 $ |
| Precedex (1 produit médicamenteux) |
Sédation |
Corporation de soins de la santé Hospira (Canada) |
Mars |
Réduction du prix du médicament Docetaxel –
807 490 $ |
|
| Diflucan (1 produit médicamenteux) |
Antibiotique antifongique |
Pfizer Canada Inc. |
Avril |
✓ |
30 951,51 $ |
| Trileptal® (3 produits médicamenteux) |
Crises épileptiques |
Novartis Pharmaceuticals Canada Inc. |
Mai |
✓
2 471 084,02 $ |
1 000 000,00 $ |
| Pariet (1 produit médicamenteux) |
Sécrétions d’acide gastrique |
Janssen Inc. |
Mai |
✓ |
225 122,07 $ |
| Avonex PS (1 produit médicamenteux) |
Sclérose en plaques |
Biogen Idec Canada Inc. |
Juillet |
|
76 347,23 $ |
| Banzel (2 produits médicamenteux) |
Crises épileptiques |
Eisai Limited |
Oct. |
|
30 905,80 $ |
| Lyrica (1 produit médicamenteux) |
Fibromyalgie; douleur névralgique diabétique; douleur après un épisode du zona; crises partielles |
Pfizer Canada Inc. |
Nov. |
|
63 981,64 $ |
| Halaven (1 produit médicamenteux) |
Cancer du sein |
Eisai Limited |
Déc. |
|
47 822,80 $ |
|
Procytox (6 produits médicamenteux)
Uromitexan (1 produit médicamenteux)
Ifex (2 produits médicamenteux)
|
Troubles lymphoprolifé-ratifs et néoplasmes
Toxicité des voies urinaires
Sarcome des tissus mous, cancers du pancréas et du col de l’utérus
|
Corporation Baxter
|
Déc.
|
|
6 520 381,87 $
5 834 001,29 $
3 403 234,33 $
|
| Engagements de conformité volontaire en 2013 jusqu’au 31 mai |
| Novolin® (8 produits médicamenteux) |
Diabète sucré |
Novo Nordisk Canada Inc. |
Avril |
✓ |
6 503 426,81 $ |
| Mavik (1 produit médicamenteux) |
Hypertension artérielle |
Abbott Laboratories Limited |
Avril |
✓ |
118 168,48 $ |
| Airomir (1 produit médicamenteux) |
Asthme |
Graceway Canada Inc. |
Avril |
|
206 583,48 $ |
| Tactuo (1 produit médicamenteux) |
Acné |
Galderma Canada Inc. |
Avril |
|
Ordonnance du Conseil : 419 468,12 $ |
| Total |
|
|
|
|
34 512 415,41 $ |
Audiences
Lorsque le prix d’un médicament breveté semble excessif, le Conseil peut tenir une audience publique et, s’il est démontré que le prix du produit médicamenteux est excessif, rendre une ordonnance obligeant le breveté à réduire le prix de son produit et à rembourser les recettes excessives qu’il a tirées de la vente de son produit à un prix excessif. Les décisions du Conseil peuvent être assujetties à une révision judiciaire devant la Cour fédérale du Canada.
En 2012, le Conseil a rendu des décisions et (ou) des ordonnances pour mener à terme trois affaires : Copaxone (réexamen), sur le prix; Pentacel et Quadracel, sur l’ordonnance corrective; et Sandoz Canada Inc., pour défaut de soumettre ses rapports.
En 2012, le Conseil a émis un Avis d’audience dans l’affaire de Galderma Canada Inc. et de son médicament Tactuo. Le Panel d’audience a accepté un Engagement de conformité volontaire visant à rembourser les recettes excessives totalisant 419 468,12 $ par l’entremise d’un paiement versé au gouvernement du Canada. Par ailleurs, le Panel a rendu une ordonnance du Conseil en avril 2013, menant ainsi à terme l’affaire.
Le Conseil compte deux affaires dans son calendrier d’audiences : Apotex Inc. et Apo-Salvent exempt de CFC.
Sommaire
En 2012 et jusqu’au 31 mai 2013, des recettes excessives totalisant 39 826 577,68 $ ont été remboursées au moyen de paiements versés au gouvernement du Canada en vertu d’Engagements de conformité volontaire et d’ordonnances du Conseil.
Depuis 1993, 93 Engagements de conformité volontaire ont été approuvés et 26 audiences publiques ont été entamées. Ces mesures ont donné lieu à des réductions de prix et au remboursement des recettes excessives au moyen de réductions supplémentaires de prix ou de paiements versés au gouvernement du Canada. Un montant d’environ 146 millions de dollars a été recueilli en vertu d’Engagements de conformité volontaire et d’ordonnances du Conseil par l’entremise de paiements versés au gouvernement du Canada et (ou) aux clients, dont les hôpitaux et les cliniques médicales.
Tableau 6 État d’avancement des audiences devant le Conseil en 2012 jusqu’au 31 mai 2013
| Produit médicamenteux breveté |
Usage thérapeutique |
Breveté |
Date de l’Avis d’audience |
État d’avancement |
| Apo-Salvent exempt de CFC |
Asthme |
Apotex Inc. |
Le 8 juillet 2008 |
En cours |
| Copaxone – Réexamen |
Sclérose en plaques |
Teva Canada |
Nouveau panel nommé en février 2010 |
Ordonnance du Conseil rendue le 23 février 2012
Recettes excessives remboursées : 2 801 285,00 $
Décision de la Cour fédérale rendue le 30 avril 2013 – Demande accueillie; décision du Conseil annulée; affaire renvoyée au nouveau panel pour réexamen
|
| Pentacel et Quadracel |
Immunisation |
sanofi pasteur Limited |
Le 27 mars 2007 |
Ordonnance du Conseil rendue le 14 juin 2012, par suite du nouvel examen de l’ordonnance corrective suivant les directives de la Cour fédérale
Recettes excessives remboursées : 2 512 877,74 $
|
| ratio- Salbutamol HFA |
Asthme |
ratiopharm Inc. (actuellement Teva Canada) |
Le 18 juillet 2008 |
Décision du Conseil rendue le 27 mai 2011
Ordonnance du Conseil rendue le 17 octobre 2011
Demandes de révision judiciaire présentées à la Cour fédérale le 27 juin 2011; tenue de l’audience prévue du 4 au 6 novembre 2013
|
| Tactuo |
Acné |
Galderma Canada Inc. |
Le 26 septembre 2012 |
Ordonnance du Conseil rendue le 24 avril 2013
Engagement de conformité volontaire – recettes excessives remboursées : 419 468,12 $
|
| Breveté |
Objet de l’audience |
Date de l’Avis de demande |
État d’avancement |
| Apotex Inc. |
Défaut de soumettre ses rapports (questions relatives à la compétence du Conseil) |
Le 3 mars 2008 |
En cours |
| ratiopharm Inc. (actuellement Teva Canada) |
Défaut de soumettre ses rapports (questions relatives à la compétence du Conseil) |
Le 28 août 2008 |
Ordonnance du Conseil rendue le 30 juin 2011; modifiée le 17 octobre 2011
Demande de révision judiciaire présentée à la Cour fédérale le 29 juillet 2011; tenue de l’audience prévue du 4 au 6 novembre 2013
|
| Sandoz Canada Inc. |
Défaut de soumettre ses rapports (questions relatives à la compétence du Conseil) |
Le 8 mars 2010 |
Ordonnance du Conseil rendue le 1er août 2012; rendue de nouveau le 1er octobre 2012
Demande de révision judiciaire présentée à la Cour fédérale le 31 août 2012; date de l’audience à communiquer
|
Affaires interjetées auprès de la Cour fédérale
Trois décisions du Conseil font actuellement l’objet d’une révision judiciaire par la Cour fédérale dans les affaires suivantes : ratio-Salbutamol HFA (T-1058-11; T-1825-11); ratiopharm Inc. (actuellement Teva Canada) (T-1252-11); et Sandoz Canada Inc. (T-1616-12). L’audience des affaires relatives à ratiopharm devant la Cour est prévue du 4 au 6 novembre 2013, tandis que la date d’audience de l’affaire de Sandoz n’a toujours pas été fixée.
L’audition de l’affaire de Copaxone (réexamen) (T-586-12) devant la Cour fédérale a eu lieu le 5 février 2013. La Cour a rendu sa décision le 30 avril 2013; elle a accueilli la demande de Teva, a annulé la décision du Conseil rendue le 23 février 2012 et a renvoyé l’affaire à un différent panel d’audience du Conseil pour réexamen.
Mise en oeuvre des Lignes directrices
Le CEPMB s’engage à rendre le processus d’examen du prix ouvert et plus transparent pour tous les intervenants. Le Compendium des politiques, des Lignes directrices et des procédures (les Lignes directrices) fournit une orientation aux brevetés et au personnel du Conseil concernant l’application des différents facteurs prévus dans la Loi sur les brevets et le Règlement sur les médicaments brevetés afin de déterminer si le prix d’un produit médicamenteux breveté vendu au Canada est excessif.
En 2010, le CEPMB a mis en oeuvre de nouvelles Lignes directrices. Depuis, le CEPMB surveille et évalue de façon continue l’application et l’incidence des modifications apportées. En juin 2011, le CEPMB a publié le Plan de surveillance et d’évaluation des principales modifications aux Lignes directrices. La deuxième évaluation annuelle en vertu de ce plan a été présentée au Conseil en décembre 2012 et un tableau sommaire des résultats a été publié en janvier 2013.
Au fur et à mesure que le brevetés et le personnel du Conseil acquièrent de l’expérience relativement à l’application des nouvelles Lignes directrices, et à la lumière des résultats de la surveillance et du processus d’évaluation, de nouvelles questions d’intérêt continueront d’être cernées. Toute précision est communiquée de façon opportune au moyen du bulletin d’information trimestriel La Nouvelle, et les intervenants sont consultés sur les modifications proposées aux Lignes directrices par l’entremise du processus d’Avis et commentaires. Une version révisée des Lignes directrices, qui tient compte de toutes les modifications est publiée chaque année au mois de juin.
Réduction du fardeau réglementaire
En cadrant avec le Plan d’action pour la réduction du fardeau administratif et le Plan d’action économique du gouvernement, et pour donner suite aux dispositions énoncées dans l’Évaluation des programmes du CEPMB de 2012, le CEPMB s’est engagé à revoir son processus d’examen des prix afin de cerner des moyens possibles de réduire le fardeau réglementaire imposé aux brevetés et d’en accroître l’efficience sans porter atteinte à son mandat visant à protéger les consommateurs.
À ce jour, l’examen interne du CEPMB s’est focalisé sur deux initiatives prioritaires relatives à la réduction du fardeau réglementaire afin de revoir, notamment :
- la méthodologie de rajustement du prix du médicament pour tenir compte des variations de l’indice des prix à la consommation (IPC);
- la faisabilité d’une transition vers le dépôt annuel par les brevetés des données relatives aux médicaments brevetés existants et modifier l’exigence des brevetés de fournir des données sur les ventes faites le premier jour de mise en marché de nouveaux produits médicamenteux.
Ces initiatives ont été jugées prioritaires par le Conseil dans son Rapport sur les plans et les priorités de même que dans le document Réponse de la direction et plan d’action publié récemment en réponse à l’Évaluation des programmes du CEPMB de 2012.
Le CEPMB demande actuellement conseil sur ces initiatives puisqu’il souhaite trouver le juste milieu entre la certitude, la flexibilité, la faisabilité et l’efficience du processus d’examen du prix tout en réduisant le fardeau réglementaire imposé aux brevetés.
En outre, le CEPMB a élaboré de nouvelles normes de service qui viennent préciser les attentes et accroître la constance du système fédéral de réglementation du prix des médicaments, plus précisément à l’égard de l’examen scientifique des nouveaux médicaments brevetés et de l’examen du prix des nouveaux médicaments et des médicaments existants.
Par ces initiatives, on cherche à réduire le fardeau de la réglementation imposée aux brevetés et à accroître l’efficience du processus d’examen du prix tout en veillant au mandat fondamental du CEPMB visant à protéger les intérêts des consommateurs.
Tendances relatives aux ventes des produits médicamenteux brevetés
Le CEPMB fait rapport des tendances observées au chapitre des ventes de produits pharmaceutiques et des prix de tous les médicaments, ainsi que des dépenses de recherche-développement des brevetés. De plus, il dirige des études et mène des analyses portant sur diverses questions relatives aux prix et aux coûts des produits pharmaceutiques.
En vertu du Règlement sur les médicaments brevetés (le Règlement), les brevetés doivent faire rapport au CEPMB de leurs ventes de produits médicamenteux brevetés au Canada, à savoir les quantités vendues et les recettes nettes tirées des ventes de chaque produit médicamenteux, par catégorie de clients et par province et territoire. Le CEPMB utilise ces éléments d’information dans ses analyses des tendances aux niveaux des ventes, des prix et de l’utilisation faite des produits médicamenteux brevetés1. La présente section donne les résultats statistiques clés de cette analyse.
Ventes et prix
La population canadienne consacre aujourd’hui une partie beaucoup plus grande de son budget à l’achat de produits médicamenteux qu’elle ne le faisait il y a une dizaine d’années; toutefois, il est important de préciser qu’une augmentation des dépenses en produits médicamenteux n’est pas nécessairement attribuable à une augmentation des prix. Selon les rapports annuels des années 1995 à 2003, la valeur des ventes de produits médicamenteux brevetés a augmenté de plus de 10 % par année, alors que les taux moyens de variation des prix n’atteignaient même pas 1 %. Dans ces cas, ce sont le volume et la composition de l’utilisation faite des produits médicamenteux qui sont à l’origine de la croissance de la valeur des ventes.
Différents facteurs peuvent être à l’origine de tels changements, dont les suivants :
- augmentation de la population totale;
- variations de la composition démographique de la population (p. ex. vieillissement de la population et, partant, une plus grande incidence de problèmes de santé);
- augmentation de l’incidence des problèmes de santé nécessitant une pharmacothérapie;
- nouvelles pratiques d’ordonnance des médecins (p. ex. tendance à prescrire des nouveaux produits médicamenteux plus onéreux pour traiter une condition qui était jusque-là traitée avec des produits existants souvent vendus à moindre prix, ou ordonnance de concentrations plus fortes ou plus fréquentes);
- recours plus régulier à des pharmacothérapies en remplacement d’autres formes de traitement;
- recours à de nouveaux produits médicamenteux pour traiter des conditions pour lesquelles il n’existait pas encore un traitement efficace.
Tendances observées au niveau des ventes
Le tableau 7 présente la valeur des ventes par les brevetés au Canada des produits médicamenteux brevetés pour les années 1990 à 2012. Les ventes de produits médicamenteux brevetés ont totalisé 12,8 milliards de dollars en 2012, soit 0,3 % de moins qu’en 2011, où ce montant totalisait 12,9 milliards de dollars. En guise de comparaison, la croissance annuelle des ventes de produits médicamenteux brevetés était de 27,0 % en 1999, et s’est maintenue à deux chiffres jusqu’en 2003.
La dernière colonne du tableau 7 présente la valeur des ventes des produits médicamenteux brevetés exprimée en pourcentage de la valeur des ventes de tous les produits médicamenteux brevetés et non brevetés. Entre 1990 et 2003, le pourcentage de la valeur des ventes est passé respectivement de 43,2 % à un sommet de 72,7 %. Ce pourcentage a reculé, en général, depuis 2003, ce qui signifie que les ventes des produits médicamenteux de marque non brevetés et des produits médicamenteux génériques ont augmenté davantage au cours de cette période que celles de produits médicamenteux brevetés.
Tableau 7 Ventes de produits médicamenteux brevetés, 1990-2012
| Année |
Produits médicamenteux brevetés |
Valeur des ventes de produits médicamenteux brevetés exprimée en pourcentage de la valeur des ventes de tous les médicaments (%)* |
| Ventes (milliards $) |
Variation (%) |
| 2012 |
12,8 |
-0,3 |
59,3 |
| 2011 |
12,9 |
4,0 |
58,6 |
| 2010 |
12,4 |
-3,8 |
56,0 |
| 2009 |
12,9 |
2,4 |
59,2 |
| 2008 |
12,6 |
2,4 |
61,7 |
| 2007 |
12,3 |
3,4 |
63,2 |
| 2006 |
11,9 |
3,5 |
67,8 |
| 2005 |
11,5 |
4,5 |
70,6 |
| 2004 |
11,0 |
7,8 |
72,2 |
| 2003 |
10,2 |
14,3 |
72,7 |
| 2002 |
8,9 |
17,5 |
67,4 |
| 2001 |
7,6 |
18,9 |
65,0 |
| 2000 |
6,3 |
16,7 |
63,0 |
| 1999 |
5,4 |
27,0 |
61,0 |
| 1998 |
4,3 |
18,9 |
55,1 |
| 1997 |
3,7 |
22,6 |
52,3 |
| 1996 |
3,0 |
12,8 |
45,0 |
| 1995 |
2,6 |
10,8 |
43,9 |
| 1994 |
2,4 |
-2,1 |
40,7 |
| 1993 |
2,4 |
9,4 |
44,4 |
| 1992 |
2,2 |
14,0 |
43,8 |
| 1991 |
2,0 |
13,1 |
43,2 |
| 1990 |
1,7 |
— |
43,2 |
* Le dénominateur dans ce ratio comprend la valeur des ventes des produits médicamenteux de marque brevetés, des produits médicamenteux de marque non brevetés, et des produits médicamenteux génériques. L’estimation de la valeur totale des ventes utilisée pour calculer le ratio à compter de 2005 se fonde sur les données tirées de la base de données MIDAS d’IMS Health. Pour les années antérieures, les données d’IMS Health n’ont été utilisées que pour calculer la valeur des ventes des produits médicamenteux génériques. Quant à la valeur des ventes des produits médicamenteux de marque non brevetés, elle était alors estimée à l’aide des données fournies par les brevetés. Pour mettre un terme aux anomalies attribuables aux variations annuelles de la liste des brevetés, le CEPMB n’utilise plus cette approche. Les ratios rapportés pour les années avant 2005 gonflaient légèrement la part des produits médicamenteux brevetés. Ce léger écart n’invalide toutefois pas la forte tendance à la hausse observée pour les années 1990 à 2003.
Sources : CEPMB et MIDAS©, 2005-2012, IMS Health Incorporated ou sociétés affiliées. Tous droits réservés2.
Facteurs à la source de la croissance des ventes
Le tableau 8 décompose en différents éléments la croissance des ventes enregistrée entre 2011 et 2012. Ces éléments sont les suivants :
- produits médicamenteux brevetés dont le brevet est arrivé à échéance ou dont le brevet a été cédé au domaine public (« effet du retrait du médicament »);
- produits médicamenteux brevetés lancés sur le marché canadien en 2012 (« effet du nouveau médicament »);
- variations des prix des produits médicamenteux brevetés vendus au Canada en 2011 et en 2012 (« effet du prix »);
- écarts de quantités vendues de ces produits médicamenteux en 2011 et en 2012 (« effet du volume »);
- interactions des variations de prix et de quantité (« effets croisés »).
La première rangée du tableau 8 présente les incidences d’après leur valeur monétaire et la deuxième rangée les présente au moyen de la variation des ventes en 2012 par rapport à 2011. Pour des fins de comparaison, la troisième rangée présente les incidences avec les taux moyens annuels de variation des ventes pour la période de 2007 à 20113.
Les résultats de ce tableau révèlent que la diminution des ventes observée en 2012 par rapport à 2011 découlait du nombre de produits médicamenteux dont le brevet arrivait à échéance; toutes les autres composantes ont eu une incidence négative sur la diminution totale des ventes. L’augmentation des ventes de nouveaux médicaments ainsi que les effets de prix et de volume n’étaient pas assez importants pour atténuer l’incidence négative des médicaments existants sur l’ensemble des ventes.
Tableau 8 Décomposition des variations au chapitre des ventes de produits médicamenteux brevetés
| |
Variation totale |
Effet du retrait du médicament |
Effet du nouveau médicament |
Effet du prix |
Effet du volume |
Effets croisés |
| Incidence sur les ventes, 2012/2011 (millions $) |
-27,25 |
-317,82 |
282,07 |
43,22 |
37,68 |
-72,40 |
| Proportion de la variation totale, 2012/2011 (%) |
100,00 |
1 166,33 |
-1 035,13 |
-158,60 |
-138,29 |
265,69 |
| Proportion moyenne de la variation totale, 2007-2011 (%) |
100,00 |
168,02 |
-122,76 |
-21,94 |
38,27 |
38,40 |
Source : CEPMB
Le recul marqué des taux de croissance des ventes au cours des dernières années est pour le moins surprenant. Le graphique 2 présente pour 2012 une ventilation des ventes de produits médicamenteux brevetés selon l’année de leur première vente au Canada. Au cours de la dernière partie des années 1990 et du début des années 2000, la croissance des ventes a été associée à une succession de nouveaux médicaments « vedettes » qui ont donné lieu à des volumes de ventes très élevés. Malgré l’expiration récente des brevets (chute des brevets), ces produits représentent toujours un pourcentage important des ventes en 2012. Depuis le début des années 2000, les changements observés dans l’environnement pharmaceutique canadien ainsi que la diminution du taux de mise en marché de nouveaux produits médicamenteux de grande vente ont entraîné une plus faible croissance.
Graphique 2
Pourcentage des ventes de produits médicamenteux brevetés selon l’année de lancement, 2012
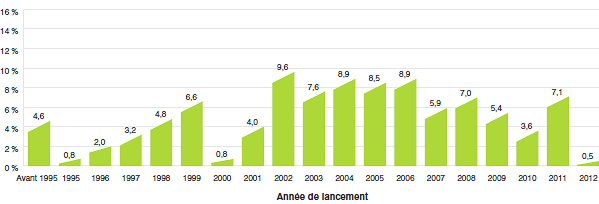
Source : CEPMB
Ventes selon la catégorie thérapeutique
Pour ses analyses de prix au niveau du groupe thérapeutique, le CEPMB classe généralement les produits médicamenteux à l’aide du Système de classification anatomique, thérapeutique, chimique (ATC) de l’Organisation mondiale de la Santé (OMS). Ce système hiérarchique classe les produits médicamenteux selon leur utilisation thérapeutique principale et leur composition chimique. Au premier niveau de ce système, à savoir au niveau 1, le système ATC classe les médicaments selon la partie de l’anatomie à laquelle ils sont principalement associés.
Le tableau 9 ventile les ventes des produits médicamenteux brevetés effectuées au Canada en 2012 selon le groupe thérapeutique principal, à savoir le premier niveau de la classification ATC. Il présente les ventes effectuées en 2012 dans les différents groupes de produits médicamenteux, leur part de l’ensemble des ventes, ainsi que le taux d’augmentation de la valeur de leurs ventes par rapport à 2011. Les valeurs présentées dans la dernière colonne correspondent à la composante de la croissance de l’ensemble des ventes attribuables aux produits médicamenteux du groupe4. La mesure ainsi obtenue permet de dégager que ce sont les agents antinéoplasiques et immunomodulateurs qui ont le plus contribué à la croissance de la valeur des ventes. Cette contribution a été plus que compensée par le recul des ventes des produits médicamenteux brevetés agissant sur le système cardiovasculaire et, accessoirement, sur le sang et les organes sanguinoformateurs.
Tableau 9 Ventes des produits médicamenteux brevetés selon leur groupe thérapeutique principal, 2012
| Groupe thérapeutique |
Ventes en 2012 (millions $) |
Part de ventes en 2012 (%) |
Croissance : 2012/2011 (millions $) |
Croissance : 2012/2011 (%) |
Incidence sur la variation des dépenses (%) |
| A : Tube digestif et métabolisme |
1 263,7 |
9,8 |
148,5 |
13,3 |
-390,1 |
| B : Sang et organes sanguinoformateurs |
826,9 |
6,4 |
-126,7 |
-13,3 |
332,8 |
| C : Système cardiovasculaire |
1 342,8 |
10,5 |
-682,4 |
-33,7 |
1 792,4 |
| D : Produits dermatologiques |
108,1 |
0,8 |
25,4 |
30,7 |
-66,6 |
| G : Système génito-urinaire et hormones sexuelles |
555,8 |
4,3 |
8,0 |
1,5 |
-21,1 |
| H : Préparations hormonales systémiques |
55,3 |
0,4 |
-18,2 |
-24,7 |
47,7 |
| J : Antiinfectieux généraux pour usage systémique; P : Produits antiparasitaires* |
1 446,5 |
11,3 |
80,9 |
5,9 |
-212,5 |
| L : Agents antinéoplastiques et agents immunomodulateurs |
3 259,4 |
25,4 |
451,7 |
16,1 |
-1 186,5 |
| M : Système musculo-squelettaire |
420,4 |
3,3 |
-11,6 |
-2,7 |
30,5 |
| N : Système nerveux |
1 941,1 |
15,1 |
134,2 |
7,4 |
-352,3 |
| R : Système respiratoire |
1 062,8 |
8,3 |
-101,0 |
-8,7 |
265,4 |
| S : Organes sensoriels |
505,2 |
3,9 |
57,6 |
12,9 |
-151,2 |
| V : Divers |
54,8 |
0,4 |
-4,4 |
-7,5 |
11,6 |
| Tous les groupes thérapeutiques |
12 842,9 |
100,0 |
-38,1 |
-0,3 |
100,0 |
* Pour des raisons de confidentialité, les données sur ces deux groupes ont été combinées.
Source : CEPMB
Notes
1 Les résultats statistiques présentés dans le présent chapitre se fondent sur les données que les brevetés ont soumises au CEPMB en date d’avril 2013. Il arrive que des brevetés soumettent un nouveau rapport révisant les données présentées ou contenant des données qui n’avaient pas été présentées dans un rapport antérieur. Ces données peuvent modifier d’une façon assez importante les statistiques utilisées pour la préparation du présent chapitre du rapport annuel. Pour tenir compte d’une telle éventualité, le CEPMB révise le calcul des données sur les ventes (voir la section « Ventes des produits médicamenteux brevetés »). Il fait aussi rapport du calcul révisé des indices de prix et de quantité (voir la section « Tendances observées au niveau des prix », ainsi que la section « Utilisation faite des produits médicamenteux brevetés ») et des ratios des prix pratiqués dans les pays de comparaison par rapport aux prix pratiqués au Canada (voir la section « Comparaison des prix pratiqués au Canada avec ceux pratiqués dans les pays de comparaison ») pour les cinq années précédant l’année sur laquelle porte le présent rapport annuel. Les nouvelles valeurs ainsi obtenues reflètent les données courantes disponibles. En conséquence, lorsque la révision des données a été faite, les valeurs rapportées dans le présent rapport peuvent être différentes de celles présentées dans des rapports annuels antérieurs.
2 Bien que fondés en partie sur les données obtenues en vertu d’une licence de la base de données MIDAS d’IMS, les énoncés, les constatations, les observations, les avis et les opinions exprimés dans le présent rapport annuel peuvent seulement être attribués au CEPMB et ne doivent pas être interprétés comme appartenant à IMS AG.
3 Dans le présent cas, l’« effet du retrait du médicament » correspond au montant des ventes générées en 2012 par les produits médicamenteux qui étaient assujettis à la compétence du CEPMB en 2011, mais non en 2012. L’« effet du nouveau médicament » correspond au montant des ventes générées en 2012 par les produits médicamenteux qui relevaient de la compétence du CEPMB en 2012, mais non en 2011. Les autres effets sont calculés au moyen de la relation suivante :
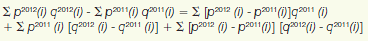
où py(i) correspond au prix du produit médicamenteux « i » l’année « y », qy(i) au volume physique du produit médicamenteux « i » vendu l’année « y » et Ó à la somme des produits médicamenteux qui relevaient de la compétence du CEPMB en matière d’examen du prix pour les années 2011 et 2012. La partie gauche de l’équation représente la variation des ventes de ces produits médicamenteux en 2012 par rapport à 2011. Du côté droit de l’équation, les trois termes définissent respectivement l’effet de volume, l’effet de prix et les effets croisés. Ces effets sont présentés dans le tableau 8.
4 Ratio annuel de la variation de la valeur monétaire des ventes des produits médicamenteux de cette catégorie thérapeutique par rapport à la variation de la valeur des ventes de tous les produits médicamenteux brevetés.
Tendances observées au niveau des prix
Le CEPMB utilise l’indice des prix des médicaments brevetés (IPMB) pour faire le suivi des tendances des prix des produits médicamenteux brevetés. L’IPMB mesure la variation moyenne des prix auxquels les brevetés vendent leurs produits médicamenteux brevetés sur le marché canadien (prix départ-usine) par rapport à l’année précédente. L’indice est calculé à l’aide de la formule qui correspond à la moyenne de la variation des prix au niveau du produit médicamenteux pondérée en fonction des ventes5. La méthodologie utilisée rappelle celle qu’utilise Statistique Canada pour compiler l’indice des prix à la consommation (IPC). L’IPMB est mis à jour tous les six mois à la lumière de l’information sur les prix et sur les ventes dont les brevetés font rapport au Conseil.
Il est important de bien comprendre la relation théorique qui existe entre l’IPMB et les coûts des produits médicamenteux. L’IPMB ne mesure pas les effets des changements de l’utilisation faite des produits médicamenteux. Cette mesure est prise par un autre indice appelé l’indice du volume des ventes de médicaments brevetés – l’IVVMB (voir la section « Utilisation faite des produits médicamenteux brevetés »). L’IPMB ne mesure pas non plus l’incidence sur les coûts des nouvelles habitudes d’ordonnance des médecins ou de l’arrivée sur le marché de nouveaux produits médicamenteux. L’IPMB est conçu pour isoler la composante de variation des ventes attribuable aux variations des prix des produits médicamenteux brevetés.
Le graphique 3 présente le taux annuel de variation de l’IPMB pour les années 1988 à 2012. Selon la mesure prise par l’IPMB, les prix départ-usine des produits médicamenteux brevetés ont légèrement augmenté (0,6 %), en moyenne, en 2012 par rapport à 2011.
Graphique 3
Taux annuel de variation de l’indice des prix des médicaments brevetés (IPMB), 1988-2012
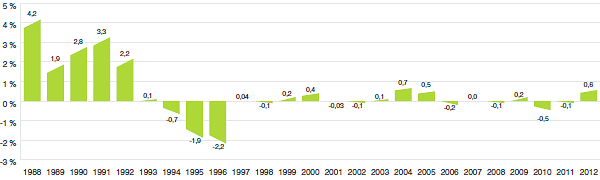
Source : CEPMB
La Loi sur les brevets prévoit que le CEPMB doit tenir compte des variations de l’indice des prix à la consommation (IPC), entre autres facteurs, lorsqu’il est appelé à déterminer si le prix d’un médicament breveté est ou non excessif. Le graphique 4 présente les variations annuelles de l’IPMB par rapport aux variations de l’IPC pour les mêmes années. L’inflation générale des prix, mesurée au moyen de l’IPC, a été supérieure à l’augmentation moyenne des prix des produits médicamenteux brevetés presque chaque année depuis 1988. En 2012, l’IPC a augmenté de 1,5 %, alors que l’IPMB a légèrement augmenté, en moyenne, de 0,6 %.
Graphique 4
Taux annuel de variation de l’indice des prix des médicaments brevetés (IPMB) et de l’indice des prix à la consommation (IPC), 1988-2012
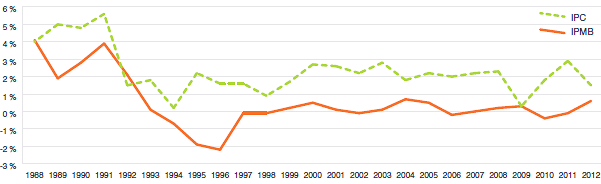
Source : CEPMB, Statistique Canada
Il n’est pas surprenant que l’IPMB ait rarement augmenté au même rythme que l’IPC. Les Lignes directrices du CEPMB prévoient en effet que les prix des produits médicamenteux brevetés ne peuvent augmenter davantage que le taux moyen d’augmentation de l’indice des prix à la consommation calculé sur une période de trois ans. (Les Lignes directrices limitent également les augmentations annuelles de prix à une fois et demie le taux d’inflation calculé à l’aide de l’IPC.) Cette exigence a pour effet de limiter les augmentations des prix des produits à celles de l’IPC sur une période de trois années6. En pratique, les variations de l’IPC n’atteignent jamais cette limite, étant donné qu’un grand nombre de brevetés n’augmentent pas les prix de leurs médicaments dans toute la mesure autorisée par les Lignes directrices, ou lorsqu’ils ne les réduisent pas.
Variation du prix selon le groupe thérapeutique
Le tableau 10 présente les taux moyens de variation des prix des produits médicamenteux brevetés selon leur groupe thérapeutique principal. Ce tableau a été établi en appliquant la méthode de calcul de l’IPMB aux données sur les prix des différents produits médicamenteux brevetés ventilées selon le groupe thérapeutique principal (niveau 1 de la classification ATC). La dernière colonne du tableau présente le résultat de la décomposition de la variation globale de l’IPMB où chaque entrée représente la composante attribuable aux produits médicamenteux du groupe thérapeutique correspondant. Selon cette mesure, une légère augmentation de l’IPMB (0,6 %) indique que les prix des produits médicamenteux des différentes catégories thérapeutiques sont relativement stables. Veuillez noter que toutes les catégories thérapeutiques, à l’exception des groupes concernant le tube digestif et le métabolisme, le système musculo-squelettaire et les préparations hormonales systémiques ont affiché, en moyenne, un taux de variation des prix inférieur au taux d’inflation de l’IPC7.
Tableau 10 Variation de l’indice des prix des médicaments brevetés (IPMB) selon le groupe thérapeutique principal, 2012
| Groupe thérapeutique |
Pourcentage des ventes en 2012 (%) |
Variation des prix de 2011 à 2012 (%) |
Contribution : variation de l’IPMB (%) |
| A : Tube digestif et métabolisme |
9,8 |
3,8 |
0,4 |
| B : Sang et organes sanguinoformateurs |
6,4 |
0,0 |
0,0 |
| C : Système cardiovasculaire |
10,5 |
0,5 |
0,1 |
| D : Produits dermatologiques |
0,8 |
0,3 |
0,0 |
| G : Système génito-urinaire et hormones sexuelles |
4,3 |
0,9 |
0,0 |
| H : Préparations hormonales systémiques |
0,4 |
1,6 |
0,0 |
| J : Antiinfectieux généraux pour usage systémique; P : Produits antiparasitaires* |
11,3 |
-0,7 |
-0,1 |
| L : Agents antinéoplastiques et agents immunomodulateurs |
25,4 |
-0,4 |
-0,1 |
| M : Système musculo-squelettaire |
3,3 |
2,0 |
0,1 |
| N : Système nerveux |
15,1 |
0,9 |
0,1 |
| R : Système respiratoire |
8,3 |
0,8 |
0,1 |
| S : Organes sensoriels |
3,9 |
0,4 |
0,0 |
| V : Divers |
0,4 |
-0,9 |
0,0 |
| Tous les groupes thérapeutiques |
100,0 |
0,6 |
0,6 |
* Pour des raisons de confidentialité, les données sur ces deux groupes ont été combinées.
Source : CEPMB
Variation des prix selon la catégorie de clients
Le graphique 5 présente les taux moyens de variation des prix selon la catégorie de clients8. Ces taux ont été obtenus en appliquant la méthodologie de l’IPMB aux données sur les ventes faites aux hôpitaux, aux pharmacies et aux grossistes9. Pour 2012, les taux de variation des prix étaient de - 1,2 %, 0,8 % et 1,3 %, respectivement.
Graphique 5
Taux annuel de variation de l’indice des prix des médicaments brevetés (IPMB) selon la catégorie de clients, 2009-2012
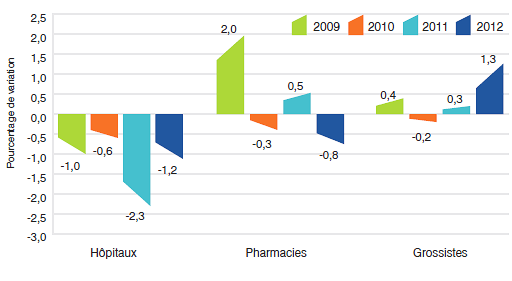
Source : CEPMB
Variation des prix selon la province ou le territoire
Le graphique 6 présente les taux moyens de variation des prix des produits médicamenteux brevetés selon la province ou le territoire. Ces taux ont été obtenus en appliquant la méthodologie du calcul de l’IPMB aux données sur les prix ventilées selon la province ou le territoire dans lequel les ventes ont été effectuées. Ces résultats révèlent que, entre 2011 et 2012, les prix des produits médicamenteux brevetés au Yukon ont reculé, en moyenne. Le Nouveau-Brunswick a connu la plus importante augmentation moyenne des prix (2,0 %).
Graphique 6
Taux annuel de variation des prix par province ou territoire*, par catégorie de clients**, 2012
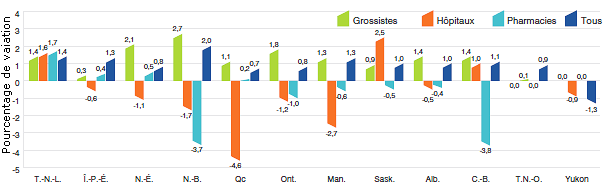
* Les valeurs pour le Nunavut sont comprises dans celles des Territoires du Nord-Ouest (T.N.-O.).
** Les résultats pour la catégorie « Tous » dans le graphique 6 comprennent ceux de la catégorie « Autre ».
Source : CEPMB
Variation du prix d’un produit médicamenteux breveté dans les années qui suivent son lancement sur le marché canadien
Le prix d’un produit médicamenteux breveté varie-t-il beaucoup au cours des années qui suivent son lancement sur le marché canadien? Le graphique 7 répond à cette question en présentant le ratio moyen des prix de vente des produits médicamenteux en 2012 par rapport aux prix auxquels ils ont été offerts au moment de leur lancement sur le marché canadien.
Graphique 7
Ratio moyen du prix de 2012 par rapport au prix de lancement, par année de lancement
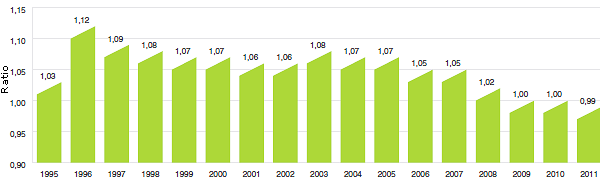
Source : CEPMB
Les résultats présentés dans le graphique 7 ne révèlent pas une tendance à la hausse ou à la baisse des prix après la période de lancement des produits médicamenteux sur le marché canadien. En 2012, le prix d’un produit médicamenteux breveté se situait dans une marge de quelques points de son prix de lancement, et ce, pour toute année de lancement du produit médicamenteux sur le marché canadien10.
Variation des prix selon le pays
La Loi et son Règlement obligent les brevetés à faire rapport au CEPMB des prix de leurs produits médicamenteux brevetés accessibles au public qu’ils pratiquent dans les sept pays de comparaison nommés dans le Règlement. Ces pays sont la France, l’Allemagne, l’Italie, la Suède, la Suisse, le Royaume-Uni et les États-Unis.
Le CEPMB utilise ces données aux fins suivantes :
- pour effectuer ses comparaisons des prix internationaux prévues dans les Lignes directrices;
- pour comparer les prix des produits médicamenteux pratiqués au Canada avec les prix pratiqués dans d’autres pays.
Le graphique 8 présente les taux moyens annuels de variation des prix pour le Canada et pour les sept pays de comparaison. Ces valeurs ont été obtenues en appliquant la méthodologie de l’IPMB (avec pondération pour tenir compte des tendances des ventes au Canada) aux données sur les prix pratiqués dans les différents pays de comparaison soumises par les brevetés. À titre d’information, les résultats pour les États Unis sont fondés sur des prix moyens qui tiennent compte des prix de la Classification fédérale des approvisionnements (US Federal Supply Schedule ou FSS)11.
Graphique 8
Taux moyens annuels de variation des prix pratiqués au Canada et dans les pays de comparaison, 2012
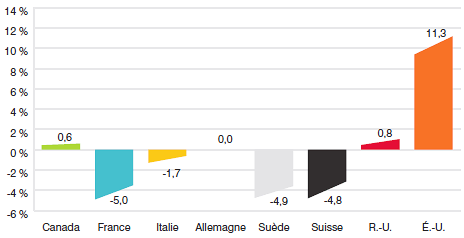
Source : CEPMB
Selon le graphique 8, les prix des produits médicamenteux brevetés aux États-Unis ont augmenté en 2012 d’un taux moyen de 11,3 %. Les augmentations de prix ont été beaucoup plus modestes en Allemagne et au Royaume-Uni, alors que les prix en France, en Italie, en Suisse et en Suède ont enregistré un recul.
Notes
5 Ces calculs sont effectués au niveau défini par le numéro d’identification du médicament (DIN) attribué par Santé Canada. Chaque DIN représente une combinaison unique d’ingrédients actifs, de forme(s) posologique(s) et de concentration(s).
6 Les prix des produits médicamenteux (et donc l’IPMB) peuvent au cours d’une année donnée augmenter davantage que l’IPC. Cette situation peut se produire lorsque les brevetés n’ont pas appliqué les dernières augmentations annuelles de prix auxquelles ils avaient droit. Elle peut également se produire lorsque le taux prévu d’inflation/IPC se révèle plus élevé que le taux réel. Pour permettre aux brevetés d’établir des prix au préalable, la méthodologie de rajustement du prix selon l’IPC permet de calculer les facteurs de rajustement du prix selon l’IPC selon les changements de l’IPC prévus. Lorsque le taux d’inflation de l’IPC prévu se révèle plus élevé que le taux d’inflation de l’IPC réel, les augmentations de prix peuvent être plus élevées que le taux d’inflation de l’IPC. Toutefois, le gain du breveté ne sera pas permanent, du fait qu’il est tenu de se conformer à l’IPC réel pour toute période de rapport ultérieure.
7 R représente le taux général de variation de l’IPMB et N, les groupes thérapeutiques nommés 1,2… N. R(i) représente le taux moyen de variation du prix du groupe thérapeutique principal i obtenu avec la méthodologie de l’IPMB. R étant une moyenne des variations des prix de tous les produits médicamenteux pondérée en fonction de la valeur des ventes, il devient facile de calculer la relation suivante :
R = w(1) x R(1) + w(2) x R(2) + … + w(N) x R(N),
où w(i) représente la part du groupe thérapeutique principal de l’ensemble des ventes. Cette dernière équation constitue la base de la décomposition par groupe thérapeutique présentée à la dernière colonne du tableau 10. Chaque terme du côté droit multiplie le taux moyen de variation du prix pour un groupe thérapeutique donné par sa part de l’ensemble des ventes. La valeur ainsi obtenue peut facilement être considérée comme la contribution du groupe thérapeutique correspondant à la variation de l’IPMB. L’envergure de cette contribution dépend du taux de variation du prix pour le groupe thérapeutique en question et de son importance relative, mesurée par sa part de l’ensemble des ventes.
La décomposition dans le tableau 10 est approximative, étant donné que les pondérations utilisées pour calculer la contribution de chaque groupe thérapeutique sont tirées des données sur les ventes annuelles, alors que le taux de variation du prix (pour l’ensemble des groupes thérapeutiques ou pour chaque groupe thérapeutique) est calculé avec des données couvrant des périodes de six mois. L’écart obtenu est généralement minime.
8 Ce sont les grossistes qui accaparent la part de lion des ventes de produits médicamenteux brevetés avec 78,2 % de l’ensemble des ventes effectuées en 2012. Les hôpitaux sont à l’origine de 8,4 % des ventes et les ventes directes aux pharmacies de 5,1 %. Depuis 2001, les ventes aux pharmacies de produits médicamenteux brevetés ont subi une baisse draconienne, passant de 20,1 % qu’elles étaient en 2001 à 5,1 % en 2012.
9 Les résultats pour une quatrième catégorie de clients, la catégorie « autres », ne sont pas fournis. En 2012, cette catégorie a été à la source d’environ 8,3 % de toutes les ventes de produits médicamenteux brevetés. Les acheteurs de la catégorie « autres » sont essentiellement des établissements de soins de santé autres que les hôpitaux, notamment des cliniques médicales et des centres de soins infirmiers, mais également les gouvernements. Étant donné que la composition de cette catégorie de clients varie beaucoup d’année en année, son analyse des variations de prix apparaît plus ou moins utile.
10 Ce constat fait référence au comportement des prix en général. Il peut y avoir des cas où le prix d’un produit médicamenteux a augmenté ou baissé d’une façon marquée depuis son lancement sur le marché canadien.
11 L’industrie pharmaceutique des États-Unis a fait valoir que les prix accessibles au public dans ce pays ne reflètent pas vraiment les prix réels en raison des remises et des rabais consentis sur une base confidentielle. Depuis janvier 2000, dans la foulée d’une consultation publique, le CEPMB inclut les prix figurant dans la Classification fédérale des approvisionnements (Federal Supply Schedule ou FSS) des États-Unis dans son calcul des prix moyens des médicaments brevetés pratiqués aux États-Unis. Les prix de la Classification fédérale sont négociés entre les fabricants et le département des Anciens combattants des États-Unis. Ils sont généralement moins élevés que les autres prix accessibles au public pratiqués aux États-Unis et divulgués dans les rapports des brevetés au CEPMB.
Comparaison des prix pratiqués au Canada avec ceux pratiqués dans les pays de comparaison
Les tableaux 11 et 12 présentent des statistiques détaillées qui permettent de comparer les prix des produits médicamenteux pratiqués dans les sept pays de comparaison avec ceux pratiqués au Canada. Chaque tableau présente deux séries de ratios de prix moyens. Ils sont différents l’un de l’autre selon la méthode de conversion en équivalents dollars canadiens des prix exprimés dans la devise des différents pays. Les deux tableaux présentent également le nombre de produits médicamenteux (DIN) et le volume des ventes couverts par chaque ratio de prix12.
Les ratios de prix moyens donnés dans les tableaux 11 et 12 sont des moyennes d’arithmétique pondérées de ratios de prix obtenues pour des produits médicamenteux individuels, dont la pondération est fondée sur les tendances des ventes au Canada. Les ratios moyens de prix interprétés de cette façon donnent des réponses exactes aux questions comme celle-ci :
« Combien les Canadiens auraient-ils payé, en plus ou en moins, leurs produits médicamenteux brevetés en 2012 s’ils les avaient achetés aux prix pratiqués dans le pays X? »
Par exemple, vous pouvez voir dans le tableau 11 que le ratio du prix moyen en France par rapport au prix moyen au Canada calculé avec la moyenne arithmétique est de 0,76 pour 2012. Ce ratio signifie que les Canadiens auraient payé leurs médicaments brevetés 24 % de moins en 2012 s’ils avaient pu les acheter aux prix pratiqués en France.
Pendant bon nombre d’années, le CEPMB a fait rapport des ratios des prix moyens pratiqués dans les pays de comparaison par rapport aux prix moyens pratiqués au Canada après avoir converti les prix pratiqués dans les pays de comparaison en équivalents dollars canadiens à l’aide des moyennes des taux de change (ou, plus précisément, les moyennes mobiles des taux de change sur une période de 36 mois que le CEPMB utilise généralement lorsqu’il applique ses Lignes directrices). Le tableau 11 compare également les ratios des prix des produits médicamenteux dans les pays de comparaison par rapport à leurs prix au Canada, après avoir converti la devise à l’aide de la parité des pouvoirs d’achat. Le taux de parité des pouvoirs d’achat représente le coût de la vie relatif dans ces deux pays exprimé dans leurs devises respectives. En pratique, le coût de la vie est calculé à l’aide d’un panier de produits et de services aux prix courants. Étant donné que les taux de parité des pouvoirs d’achat représentent le coût de la vie dans chacun des pays, ils constituent un moyen simple de rajuster les prix pour tenir compte des différences de revenus et d’autres valeurs monétaires dans la comparaison des prix pratiqués dans les différents pays. Appliqués au calcul des ratios moyens des prix pratiqués dans les pays de comparaison par rapport aux prix pratiqués au Canada, les taux de parité des pouvoirs d’achat fournissent des statistiques qui nous permettent de répondre à des questions comme celle-ci :
« Dans quelle mesure les Canadiens auraient-ils dû sabrer dans leur consommation de biens et de services pour acheter des produits médicamenteux brevetés, ou encore, auraient-ils pu augmenter leur consommation de biens et de services si, en 2012, ils avaient vécu et acheté leurs produits médicamenteux brevetés dans le pays X? »
On ne peut répondre à telle question en limitant la comparaison aux prix des produits médicamenteux. Il faut en effet calculer ce que chaque prix représente en termes de biens et de services sacrifiés. C’est précisément ce que permettent de faire les parités des pouvoirs d’achat.
Comparaisons bilatérales des prix
Le tableau 11 compare les prix pratiqués dans les sept pays de comparaison avec ceux pratiqués au Canada. D’après les résultats de la conversion des différentes devises aux taux de change du marché, les prix des produits médicamenteux brevetés pratiqués au Canada se situent habituellement dans la gamme des prix observés parmi les pays de comparaison. Les prix canadiens étaient plus ou moins semblables aux prix de la Suisse. Les prix en France, en Italie, au Royaume-Uni et en Suède étaient beaucoup moins élevés qu’au Canada, alors que ceux en Allemagne étaient beaucoup plus élevés. Comme dans les années antérieures, les prix déclarés étaient beaucoup plus élevés aux États Unis qu’au Canada, et que dans les autres pays de comparaison.
Tableau 11 Ratios moyens des prix pratiqués dans les pays de comparaison par rapport aux prix pratiqués au Canada, comparaisons bilatérales, 2012
| |
Canada |
France |
Italie |
Allemagne |
Suède |
Suisse |
Royaume-Uni |
États-Unis |
| Taux de change du marché |
| Ratio moyen des prix en 2012 |
1,00 |
0,76 |
0,80 |
1,11 |
0,90 |
1,01 |
0,80 |
2,02 |
| Ratio moyen des prix en 2011 |
1,00 |
0,84 |
0,84 |
1,20 |
0,95 |
1,03 |
0,82 |
1,98 |
| Parités des pouvoirs d’achat |
| Ratio moyen des prix en 2012 |
1,00 |
0,79 |
0,91 |
1,24 |
0,84 |
0,82 |
0,89 |
2,42 |
| Ratio moyen des prix en 2011 |
1,00 |
0,81 |
0,89 |
1,27 |
0,88 |
0,81 |
0,91 |
2,28 |
| Nombre de produits médicamenteux brevetés |
1 264 |
736 |
809 |
903 |
870 |
842 |
864 |
1 070 |
| Ventes (millions $) |
12 842,9 |
10 262,5 |
10 389,8 |
11 023,8 |
10 828,0 |
10 768,9 |
10 794,9 |
12 083,6 |
Source : CEPMB
Les ratios moyens de prix obtenus suite à la conversion des devises aux taux de parité des pouvoirs d’achat présentent des différences entre le Canada et les pays de comparaison. Si l’on tient compte des différences du coût de la vie dans les sept pays de comparaison, le Canada se présente comme le pays où les coûts de consommation pour les produits médicamenteux brevetés sont les plus élevés. En effet, ils donnent à penser que les Canadiens ont dû sacrifier en 2012 un taux beaucoup plus élevé de leur pouvoir d’achat pour se procurer des médicaments brevetés que n’ont dû le faire les consommateurs des pays de comparaison, exclusion faite des consommateurs de l’Allemagne et des États-Unis.
Le graphique 9 présente ces résultats dans une perspective historique. En 2005, les prix au Canada étaient généralement égaux ou inférieurs aux prix correspondants pratiqués dans tous les pays de comparaison, sauf en Italie. En 2012, les prix au Canada étaient généralement plus élevés que ceux pratiqués au Royaume-Uni, en France et en Italie, et un peu plus élevés que ceux pratiqués en Suède.
Graphique 9
Ratios moyens des prix pratiqués dans les pays de comparaison par rapport aux prix pratiqués au Canada : 2005, 2012
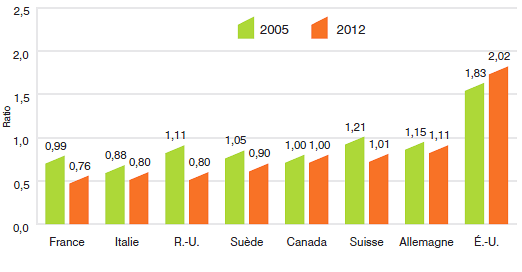
Source : CEPMB
Comparaisons multilatérales des prix
Le tableau 12 présente les ratios moyens des prix pratiqués dans les pays de comparaison par rapport aux prix pratiqués au Canada, et ce, pour plusieurs mesures multilatérales des prix pratiqués dans les pays de comparaison. Le « prix international médian » correspond à la médiane des prix de vente des produits médicamenteux dans les sept pays de comparaison. D’autres ratios de prix multilatéraux comparent la moyenne minimale, maximale et simple des prix pratiqués dans les pays de comparaison par rapport aux prix au Canada.
Tableau 12 Ratios moyens des prix pratiqués dans les pays de comparaison par rapport aux prix pratiqués au Canada, comparaisons multilatérales, 2012
| |
Médiane |
Minimum |
Maximum |
Moyenne |
| Ratio moyen des prix aux taux de change du marché |
1,07 |
0,78 |
2,07 |
1,19 |
| Ratio moyen des prix aux parités de pouvoir d’achat |
1,09 |
0,84 |
2,44 |
1,29 |
| Nombre de produits médicamenteux brevetés |
1 204 |
1 204 |
1 204 |
1 204 |
| Ventes (millions $) |
12 614,0 |
12 614,0 |
12 614,0 |
12 614,0 |
Source : CEPMB
Sous l’angle des résultats obtenus avec les taux de change du marché, le ratio de la médiane des prix pratiqués dans les pays de comparaison par rapport aux prix pratiqués au Canada s’est maintenu à 1,07 en 2012 (la valeur correspondante pour 2010 était de 1,05). Il convient de noter que la moyenne des prix pratiqués dans les pays de comparaison semble produire des ratios des prix pratiqués dans les pays de comparaison par rapport aux prix pratiqués au Canada plus élevés que les médianes des prix pratiqués dans les pays de comparaison. Cette situation s’explique par l’influence des prix pratiqués aux États-Unis où les prix sont beaucoup plus élevés que dans tous les autres pays. Même si les prix pratiqués aux États-Unis sont presque toujours pris en compte dans la détermination du prix moyen pratiqué dans les pays de comparaison, ils ne sont presque jamais dans la médiane des prix pratiqués dans les pays de comparaison.
Le graphique 10 replace ces résultats dans leur contexte historique, présentant l’historique des ratios moyens des prix pratiqués dans les pays de comparaison par rapport aux prix pratiqués au Canada de 2001 à 2012. Même si le ratio a beaucoup changé au cours de cette période, il est demeuré au-dessus de la parité.
Graphique 10
Ratio moyen du prix international médian pratiqué dans les pays de comparaison par rapport aux prix pratiqués au Canada, aux taux de change du marché, 2001-2012
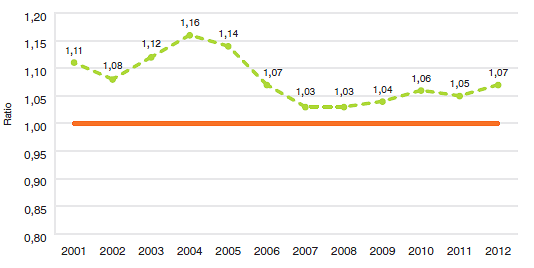
Source : CEPMB
Le graphique 11 présente avec encore plus de détails les ratios des prix des produits médicamenteux brevetés pratiqués dans les pays de comparaison par rapport aux prix pratiqués au Canada, ventilés selon la moyenne de la valeur des ventes présentée dans le tableau 12. Ce graphique ventile les ventes de médicaments brevetés effectuées en 2012 selon le ratio de la médiane des prix des produits médicamenteux brevetés pratiqués dans les pays de comparaison par rapport aux prix pratiqués au Canada (ou, pour être plus précis, selon la fourchette dans laquelle s’inscrit le ratio)13. Ces résultats révèlent une dispersion importante des ratios de prix au niveau du produit. Alors que les produits dont les ratios du prix international médian par rapport au prix au Canada se situent entre 0,90 et 1,10 accaparaient 26,2 % des ventes, ceux dont les ratios se situent sous 0,90 en accaparaient 47,4 % et ceux dont les ratios dépassaient 1,10 en accaparaient 26,4 %.
Graphique 11
Distribution d’intervalle des ventes selon le ratio du prix international médian pratiqué dans les pays de comparaison par rapport aux prix pratiqués au Canada, 2012
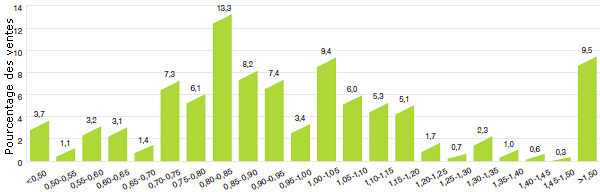
Notes
12 Le nombre de produits médicamenteux et la valeur des ventes visés par ces ratios varient, étant donné qu’il n’est pas toujours possible de trouver pour chaque produit médicamenteux breveté vendu au Canada les prix auxquels le médicament est vendu dans les pays de comparaison. Il convient de noter que tous les ratios moyens de prix bilatéraux indiqués au tableau 11 combinés représentent au moins 80 % des ventes effectuées au Canada en 2012, alors que les ratios multilatéraux du tableau 12 couvrent plus de 98 %.
13 Pour obtenir ces résultats, les prix dans les pays de comparaison ont été convertis en équivalents dollars canadiens à l’aide des taux de change du marché.
Utilisation faite des produits médicamenteux brevetés
Les données sur les prix et sur la valeur des ventes utilisées pour calculer l’IPMB servent également à déterminer les tendances des quantités de médicaments brevetés vendus au Canada. Le CEPMB calcule à cette fin l’indice de volume des ventes de médicaments brevetés (IVVMB). Le graphique 12 présente pour les années 1988 à 2012 les taux moyens de croissance de l’utilisation des produits médicamenteux brevetés, mesurée à l’aide de l’IVVMB. Les résultats obtenus confirment que la croissance de l’utilisation faite des produits médicamenteux brevetés est le principal facteur d’augmentation de la valeur des ventes. Les taux de croissance de l’utilisation des dernières années talonnent en effet de près les taux de croissance de la valeur des ventes. Cette tendance de suivi s’est maintenue en 2012, le taux d’utilisation des produits médicamenteux brevetés n’ayant pas varié, en moyenne (0,0 %) entre 2011 et 2012 et les ventes ayant reculé de 0,3 %.
Graphique 12
Taux annuel de variation de l’indice du volume des ventes de médicaments brevetés (IVVMB), 1988-2012
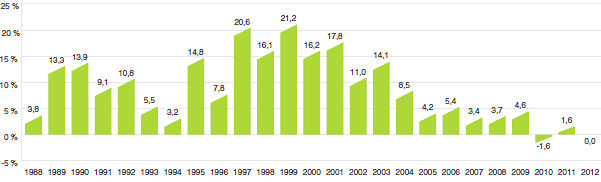
Source : CEPMB
Croissance de l’utilisation faite des produits médicamenteux brevetés selon le groupe thérapeutique principal
Le tableau 13 présente les taux moyens de croissance de l’utilisation faite des produits médicamenteux brevetés. Ces taux sont ventilés selon les groupes thérapeutiques principaux. Les résultats présentés dans ce tableau ont été obtenus en appliquant la méthodologie de l’IVVMB aux données du niveau 1 de la classification ATC. Comme dans le tableau 10, la dernière colonne donne un aperçu de la contribution des différents groupes thérapeutiques à la variation de l’IVVMB.
En 2012, les niveaux d’utilisation n’ont pas augmenté dans six groupes thérapeutiques. La croissance modérée de l’utilisation des produits agissant sur le tube digestif et le métabolisme, sur les agents antinéoplastiques et immunomodulateurs ainsi que sur le système nerveux représente la plus grande partie de la croissance de l’utilisation faite de l’ensemble des produits. Quant aux produits médicamenteux agissant sur le système cardiovasculaire et accessoirement, sur le sang et les organes sanguinoformateurs, leur taux d’utilisation a reculé.
Tableau 13 Variation de l’indice du volume des ventes des médicaments brevetés (IVVMB) selon le groupe thérapeutique principal, 2012
| Groupe thérapeutique |
Pourcentage des ventes en 2012 (%) |
Variation du volume de 2011 à 2012 (%) |
Contribution : Variation de l’IVVMB (%) |
| A : Tube digestif et métabolisme |
9,8 |
20,8 |
2,0 |
| B : Sang et organes sanguinoformateurs |
6,4 |
-13,8 |
-0,9 |
| C : Système cardiovasculaire |
10,5 |
-34,7 |
-3,6 |
| D : Produits dermatologiques |
0,8 |
12,4 |
0,1 |
| G : Système génito-urinaire et hormones sexuelles |
4,3 |
3,5 |
0,2 |
| H : Préparations hormonales systémiques |
0,4 |
2,9 |
0,0 |
J : Antiinfectieux généraux pour usage systémique;
P : Produits antiparasitaires* |
11,3 |
9,7 |
1,1 |
| L : Agents antinéoplastiques et agents immunomodulateurs |
25,4 |
8,4 |
2,1 |
| M : Système musculo-squelettaire |
3,3 |
2,4 |
0,1 |
| N : Système nerveux |
15,1 |
11,0 |
1,7 |
| R : Système respiratoire |
8,3 |
-2,6 |
-0,2 |
| S : Organes sensoriels |
3,9 |
12,2 |
0,5 |
| V : Divers |
0,4 |
9,9 |
0,0 |
| Tous les groupes thérapeutiques |
100,0 |
0,0 |
0,0 |
* Pour des raisons de confidentialité, les données sur ces deux groupes ont été combinées.
Source : CEPMB
Dépenses en produits médicamenteux au Canada par rapport aux marchés mondiaux
IMS Health14 fait régulièrement rapport des ventes médicaments dans différents pays. Selon les données sur les ventes provenant de cette source, le graphique 13 illustre la répartition de ces ventes entre le Canada et les sept pays que le CEPMB utilise dans ses examens de prix15. En ce qui concerne le Canada, les ventes de produits médicamenteux en 2012 ont représenté 2,6 % de l’ensemble des ventes sur les principaux marchés mondiaux.
Graphique 13
Distribution des ventes de produits médicamenteux entre les grands marchés mondiaux, 2012
Source : MIDAS©, 2005-2012, IMS Health Incorporated ou sociétés affiliées. Tous droits réservés16.
Le graphique 14 présente la part des ventes du Canada sur les principaux marchés canadiens pour les années 2005 à 2012. Pendant toutes ces années, la part des ventes du Canada s’est maintenue entre 2,4 % et 2,7 %.
Graphique 14
Pourcentage des ventes de produits médicamenteux du Canada sur les principaux marchés mondiaux, 2005-2012
Source : MIDAS©, 2005-2012, IMS Health Incorporated ou sociétés affiliées. Tous droits réservés16.
Le graphique 15 compare la croissance des ventes de produits médicamenteux au Canada à celle des sept pays de comparaison, ensemble et séparément. Entre 2005 et 2012, les ventes au Canada de produits médicamenteux ont augmenté à un taux annuel moyen de près de 4,0 %. Pour la même période, les ventes dans les sept pays de comparaison ont augmenté à un taux annuel moyen de 3,3 %.
Graphique 15
Taux moyen de croissance des ventes de produits médicamenteux aux taux de change constants du marché de 2012, par pays, 2005-2012
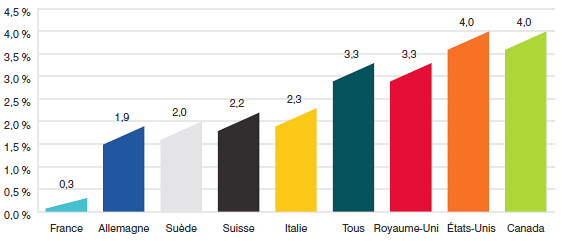
Source : MIDAS©, 2005-2012, IMS Health Incorporated ou sociétés affiliées. Tous droits réservés16.
Le graphique 16 compare les taux annuels de croissance des ventes de produits médicamenteux au Canada et dans l’ensemble des pays de comparaison. En 2012, pour la deuxième année consécutive, les ventes ont augmenté à un taux plus lent au Canada que dans les pays de comparaison.
Graphique 16
Taux moyen annuel de variation des ventes de produits médicamenteux aux taux de change constants du marché de 2012, au Canada et dans les pays de comparaison, 2006-2012
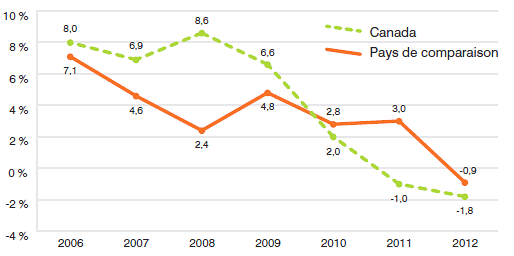
Source : MIDAS©, 2005-2012, IMS Health Incorporated ou sociétés affiliées. Tous droits réservés16.
La comparaison des dépenses en produits médicamenteux peut aussi être faite à l’aide de la proportion du produit intérieur brut consacrée à l’achat de médicaments17. Le graphique 17 présente les dépenses en produits médicamenteux exprimées en pourcentage du produit intérieur brut (PIB) du Canada et des sept pays de comparaison (données de 2010). Dans les sept pays de comparaison, les dépenses en produits médicamenteux ont accaparé entre 1,1 % et 2,1 % du PIB. Sur cette échelle, la valeur du Canada (1,9 %) s’inscrit près de la limite supérieure.
Graphique 17
Dépenses en produits médicamenteux, exprimées en pourcentage du PIB, 2010
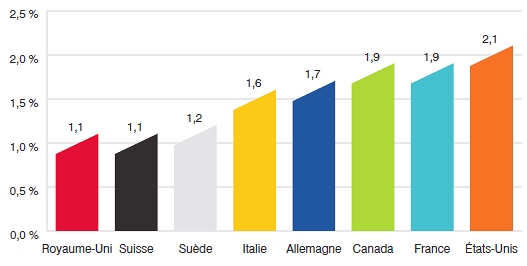
Source: OECD
Le tableau 14 présente le ratio des dépenses par rapport au PIB dans son contexte historique. Entre 2000 et 2010, les dépenses en produits médicamenteux au Canada ont augmenté d’environ le double du taux de croissance du PIB.
Tableau 14 Dépenses en produits médicamenteux, exprimées en pourcentage du PIB , 2010
| |
Part des ventes en produits médicamenteux, 2010 (% du PIB) |
Part des ventes en produits médicamenteux, 2000 (% du PIB) |
Croissance des dépenses en produits médicamenteux, 2000-2010 (%) |
Croissance du PIB, 2000-2010 (%) |
| Canada |
1,90 |
1,42 |
150,30 |
86,69 |
| France |
1,86 |
1,81 |
74,74 |
70,41 |
| Allemagne |
1,72 |
1,43 |
97,65 |
64,63 |
| Italie |
1,60 |
1,74 |
65,98 |
80,55 |
| Suède |
1,21 |
1,18 |
57,35 |
53,50 |
| Suisse |
1,11 |
1,11 |
58,17 |
58,77 |
| Royaume-Uni |
1,13 |
1,14 |
51,87 |
52,84 |
| États-Unis |
2,09 |
1,46 |
111,89 |
47,71 |
Source: OECD
Le tableau 15 présente la distribution des ventes de produits médicamenteux selon le groupe thérapeutique au Canada, dans chaque pays de comparaison ainsi que dans l’ensemble des pays de comparaison18. Ces résultats font ressortir un degré d’uniformité remarquable entre les différents pays.
Tableau 15 Distribution des ventes de produits médicamenteux brevetés (exprimée en pourcentage) selon le groupe thérapeutique principal, au Canada et dans les pays de comparaison, 2012
| Groupe thérapeutique |
Canada |
Pays de comparaison |
France |
Italie |
Allemagne |
Suède |
Suisse |
Royaume-Uni |
États-Unis |
| A : Tube digestif et métabolisme |
12,6 |
12,2 |
10,6 |
11,2 |
11,5 |
10,1 |
11,1 |
11,0 |
12,6 |
| B : Sang et organes sanguinoformateurs |
4,1 |
5,6 |
7,4 |
8,2 |
5,6 |
7,7 |
4,8 |
4,2 |
5,3 |
| C : Système cardiovasculaire |
14,3 |
10,7 |
12,9 |
13,6 |
9,6 |
6,2 |
12,1 |
8,9 |
10,5 |
| D : Produits dermatologiques |
3,1 |
2,7 |
2,4 |
2,1 |
2,7 |
2,5 |
3,6 |
3,2 |
2,8 |
| G : Système génito-urinaire et hormones sexuelles |
5,2 |
5,0 |
3,3 |
3,9 |
3,8 |
5,0 |
4,6 |
4,7 |
5,4 |
| H : Préparations hormonales systémiques |
1,1 |
1,8 |
1,8 |
1,9 |
2,0 |
2,5 |
1,5 |
2,1 |
1,7 |
| J : Antiinfectieux généraux pour usage systémique |
7,3 |
10,6 |
12,2 |
12,9 |
10,1 |
7,8 |
10,7 |
10,4 |
10,4 |
| L : Agents antinéoplastiques et agents immunomodulateurs |
15,6 |
16,7 |
16,7 |
16,6 |
20,6 |
21,4 |
19,8 |
16,4 |
16,1 |
| M : Système musculo-squelettaire |
3,7 |
3,1 |
3,4 |
3,9 |
3,7 |
3,0 |
5,0 |
2,6 |
3,0 |
| N : Système nerveux |
18,6 |
17,5 |
14,7 |
12,1 |
15,2 |
18,4 |
15,7 |
18,5 |
18,5 |
| P : Produits antiparasitaires |
0,2 |
0,2 |
0,2 |
0,0 |
0,1 |
0,2 |
0,1 |
0,3 |
0,2 |
| R : Système respiratoire |
7,2 |
7,8 |
6,3 |
5,8 |
6,7 |
9,1 |
6,2 |
10,2 |
8,1 |
| S : Organes sensoriels |
3,1 |
2,5 |
2,9 |
1,9 |
2,6 |
2,6 |
3,1 |
3,3 |
2,4 |
| V : Divers |
4,0 |
3,6 |
5,2 |
5,8 |
5,9 |
3,5 |
1,8 |
4,2 |
3,0 |
| Tous les groupes thérapeutiques* |
100,0 |
100,0 |
100,0 |
100,0 |
100,0 |
100,0 |
100,0 |
100,0 |
100,0 |
* Il se peut que les valeurs n’équivaillent pas à 100,0 en raison de l’arrondissement.
Source : MIDAS©, 2005-2012, IMS Health Incorporated ou sociétés affiliées. Tous droits réservés16.
Notes
14 La plupart des résultats statistiques présentés dans cette section sont fondés sur les données sur les ventes provenant de MIDAS©, 2005-2012, IMS Health Incorporated ou sociétés affiliées. Tous droits réservés. Ces données couvrent les pharmacies et les hôpitaux.
15 Les résultats présentés dans les graphiques 13 à 16 sont fondés sur les estimations des recettes de ventes à l’usine qui comprennent les produits médicamenteux de marque brevetés et non brevetés et des produits médicamenteux génériques. Ces estimations ont été converties en équivalents dollars canadiens à l’aide des taux de change annuels moyens du marché. Les fluctuations de ces taux peuvent exercer une influence importante sur la distribution entre les grands marchés mondiaux.
16 Bien que fondés en partie sur les données obtenues en vertu d’une licence de la base de données MIDAS d’IMS, les énoncés, les constatations, les observations, les avis et les opinions exprimés dans le présent rapport annuel peuvent seulement être attribués au CEPMB et ne doivent pas être interprétés comme appartenant à IMS AG.
17 Les comparaisons faites sur cette base tiennent compte des différences aux niveaux des prix des produits médicamenteux, de l’utilisation faite des produits médicamenteux, des choix thérapeutiques habituellement faits et du revenu national.
18 Les données utilisées pour produire le tableau 15 couvrent les produits médicamenteux de marque et génériques tant brevetés que non brevetés. Par conséquent, les résultats présentés dans le tableau 15 ne peuvent être directement comparés à ceux présentés dans le tableau 9 qui, lui, couvre exclusivement les produits médicamenteux brevetés.
Analyse des dépenses de recherche-développement
La Loi sur les brevets (la Loi) confère au CEPMB le mandat de faire le suivi des dépenses des brevetés en recherche-développement (R-D) et de faire rapport des tendances observées (la Loi ne confère toutefois pas au CEPMB un droit de regard sur le montant des dépenses des brevetés dans la recherche-développement ni sur le type de recherche-développement). Le présent chapitre fournit les statistiques clés sur la situation actuelle des investissements dans la recherche-développement pharmaceutique au Canada.
Sources des données
Les résultats statistiques présentés dans cette section ont été tirés des rapports semestriels que les brevetés ont soumis au CEPMB.
La Loi oblige les brevetés à faire rapport au CEPMB des recettes qu’ils tirent des ventes de tous leurs produits médicamenteux pour usage humain et pour usage vétérinaire (y compris les recettes tirées des ventes de produits médicamenteux non brevetés et les recettes découlant d’ententes de production sous licence) ainsi que de leurs dépenses de R-D au Canada pour leurs différents produits médicamenteux (brevetés et non brevetés, pour usage humain et pour usage vétérinaire). Les brevetés transmettent ces renseignements au CEPMB au moyen de son formulaire 3 (Recettes et dépenses en recherche et développement fournies en application du paragraphe 88(1) de la Loi sur les brevets).
Le Règlement sur les médicaments brevetés (le Règlement) exige que chaque formulaire 3 déposé soit accompagné d’un document certifiant l’exactitude et la conformité des données fournies. Le CEPMB ne vérifie pas systématiquement l’information qui lui est présentée sur le formulaire 3, mais cherche plutôt les anomalies et les contradictions et, lorsqu’il y a lieu, demande aux brevetés de corriger leurs données ou de les étoffer. Pour garantir la juste interprétation des données, chaque breveté est invité à confirmer, avant la publication du ratio, l’exactitude du ratio de ses dépenses de R-D par rapport aux recettes tirées des ventes calculé par le CEPMB.
Défaut de soumettre son rapport sur ses dépenses de R-D
Les brevetés sont tenus de soumettre un formulaire 3 complet et exact dans les délais mentionnés dans le Règlement. Lorsqu’un breveté omet de respecter les exigences en matière de présentation de rapport, le Conseil peut rendre une ordonnance exigeant le respect de ces exigences. Le Conseil n’a pas été appelé à rendre des ordonnances pour la période de déclaration de 2012.
Couverture
Notons que les sociétés pharmaceutiques qui n’ont fait aucune vente au Canada ne sont pas tenues de présenter un rapport sur leurs dépenses de R-D au CEPMB. Cela a deux conséquences.
D’abord, les résultats statistiques indiqués ci-dessous ne devraient pas être utilisés afin de couvrir toute la recherche pharmaceutique réalisée au Canada. Par exemple, une société peut vendre uniquement des produits médicamenteux non brevetés, mais elle peut toujours effectuer beaucoup de recherche au Canada. De même, une société peut effectuer de la recherche et ne vendre aucun produit19. Les résultats présentés ci-dessous ne tiendront pas compte des dépenses de R-D des entreprises dans l’une ou l’autre situation.
Ensuite, comme de nouveaux produits médicamenteux non brevetés sont entrés dans le marché canadien et que les brevets existants expirent, le nombre et l’identité des sociétés pharmaceutiques ayant soumis un rapport sur leurs dépenses de R-D varie d’année en année. Au total, 85 sociétés ont déclaré leurs activités de R-D en 2012. Parmi elles, 35 étaient membres des Compagnies de recherche pharmaceutique du Canada (Rx&D).
Définition de recettes tirées des ventes
Aux fins du présent rapport, les recettes tirées des ventes s’entendent du produit brut des ventes de produits médicamenteux au Canada ainsi que des recettes découlant d’ententes de vente sous licence (p. ex. redevances et droits versés au breveté liés au ventes effectuées au Canada par des titulaires de licence).
Définition de dépenses de R-D
En vertu de l’article 6 du Règlement, les brevetés ne doivent inclure dans leurs rapports que leurs dépenses de R-D qui auraient été admissibles au crédit d’impôt à l’investissement pour la recherche scientifique et le développement expérimental (RS-DE) aux termes de la Loi de l’impôt sur le revenu, dans sa version qui est entrée en vigueur le 1er décembre 198720. Ainsi, les dépenses de R-D peuvent inclure les dépenses courantes, les coûts d’immobilisation et l’amortissement autorisé. Les frais engagés pour les études de marché, la promotion des ventes, le contrôle de la qualité ou les essais systématiques de matériel, de dispositifs ou de produits ainsi que pour la collecte de données n’étant pas admissibles au crédit d’impôt à l’investissement, ils ne doivent pas figurer dans les dépenses de R-D déclarées par les brevetés.
Recettes tirées des ventes et dépenses de R-D totales
Le tableau 16 donne un aperçu des recettes tirées des ventes et des dépenses de R-D déclarées au cours de la période de 1988 à 2012.
La valeur des recettes brutes tirées des ventes de produits médicamenteux au Canada déclarées par les brevetés a totalisé 16,8 milliards de dollars en 2012, ce qui représente un recul de 5,8 % par rapport à 2011. Les recettes tirées des ventes déclarées par les brevetés membres de Rx&D ont totalisé 11,9 milliards de dollars pour la même période, soit 71,0 % de l’ensemble des recettes tirées des ventes. (De ce montant, moins de 1 % des recettes découlent d’ententes de vente sous licence.)
Les dépenses de R-D déclarées par les brevetés ont totalisé 894,8 millions de dollars en 2012, ce qui représente un recul de 9,8 % par rapport à 2011. Les dépenses de R-D déclarées par les brevetés membres de Rx&D ont totalisé 782,8 millions de dollars en 2012, ce qui représente un recul de 13,1 % par rapport à l’année précédente. Les brevetés membres de Rx&D ont effectué 87,5 % de toutes les dépenses de R-D déclarées pour 2012.
Tableau 16 Dépenses de R-D déclarées par les brevetés et ratio des dépenses de R-D par rapport aux recettes tirées des ventes des brevetés, 1988-2012
| Année |
Tous les brevetés |
Rx&D |
Ratio des dépenses de R-D par rapport aux recettes tirées des ventes : Tous les brevetés (%) |
Ratio des dépenses de R-D par rapport aux recettes tirées des ventes : Brevetés membres de Rx&D (%) |
| Nbre de brevetés |
Dépenses de R-D de tous les brevetés (millions $) |
Variation par rapport à l’année précédente (%) |
Recettes tirées des ventes (millions $) |
Variation par rapport à l’année précédente (%) |
Dépenses de R-D de tous les brevetés membres de Rx&D (millions $) |
Variation par rapport à l’année précédente (%) |
Recettes tirées des ventes des brevetés membres de Rx&D (millions $) |
Variation par rapport à l’année précédente (%) |
| 2012 |
85 |
894,8 |
-9,8 |
16 754,4 |
-5,8 |
782,8 |
-13,1 |
11 896,1 |
-11,5 |
5,3 |
6,6 |
| 2011 |
79 |
991,7 |
-15,8 |
17 798,8 |
4,7 |
901,2 |
-9,9 |
13 446,1 |
10,7 |
5,6 |
6,7 |
| 2010 |
82 |
1 178,2 |
-7,4 |
17 000,0 |
-0,3 |
1 000,2 |
-11,7 |
12 149,0 |
-11,8 |
6,9 |
8,2 |
| 2009 |
81 |
1 272,0 |
-2,9 |
17 051,9 |
4,5 |
1 132,9 |
-3,4 |
13 780,0 |
4,6 |
7,5 |
8,2 |
| 2008 |
82 |
1 310,7 |
-1,1 |
16 316,7 |
2,0 |
1 172,2 |
-1,0 |
13 178,2 |
-1,4 |
8,1 |
8,9 |
| 2007 |
82 |
1 325,0 |
9,5 |
15 991,0 |
7,3 |
1 184,4 |
24,8 |
13 359,8 |
20,0 |
8,3 |
8,9 |
| 2006 |
72 |
1 210,0 |
-1,9 |
14 902,0 |
4,7 |
949,0 |
-8,8 |
11 131,2 |
-5,8 |
8,1 |
8,5 |
| 2005 |
80 |
1 234,3 |
5,5 |
14 231,3 |
0,5 |
1 040,1 |
3,9 |
11 821,4 |
0,0 |
8,7 |
8,8 |
| 2004 |
84 |
1 170,0 |
-2,0 |
14 168,3 |
4,0 |
1 000,8 |
0,8 |
11 819,0 |
8,8 |
8,3 |
8,5 |
| 2003 |
83 |
1 194,3 |
-0,4 |
13 631,1 |
12,8 |
992,9 |
-3,6 |
10 865,7 |
5,2 |
8,8 |
9,1 |
| 2002 |
79 |
1 198,7 |
13,0 |
12 081,2 |
12,5 |
1 029,6 |
10,1 |
10 323,8 |
16,8 |
9,9 |
10,0 |
| 2001 |
74 |
1 060,1 |
12,6 |
10 732,1 |
15,3 |
935,2 |
14,7 |
8 835,4 |
14,3 |
9,9 |
10,6 |
| 2000 |
79 |
941,8 |
5,3 |
9 309,6 |
12,0 |
815,5 |
4,0 |
7 728,8 |
11,6 |
10,1 |
10,6 |
| 1999 |
78 |
894,6 |
12,0 |
8 315,5 |
19,2 |
784,3 |
9,9 |
6 923,4 |
22,8 |
10,8 |
11,3 |
| 1998 |
74 |
798,9 |
10,2 |
6 975,2 |
10,9 |
713,7 |
8,6 |
5 640,2 |
10,6 |
11,5 |
12,7 |
| 1997 |
75 |
725,1 |
9,0 |
6 288,4 |
7,4 |
657,4 |
10,3 |
5 098,2 |
4,9 |
11,5 |
12,9 |
| 1996 |
72 |
665,3 |
6,4 |
5 857,4 |
9,9 |
595,8 |
6,5 |
4 859,5 |
8,7 |
11,4 |
12,3 |
| 1995 |
71 |
625,5 |
11,5 |
5 330,2 |
7,5 |
559,5 |
9,8 |
4 468,8 |
1,4 |
11,7 |
12,5 |
| 1994 |
73 |
561,1 |
11,4 |
4 957,4 |
4,4 |
509,5 |
10,4 |
4 407,2 |
2,0 |
11,3 |
11,6 |
| 1993 |
70 |
503,5 |
22,1 |
4 747,6 |
14,0 |
461,4 |
24,0 |
4 321,4 |
14,4 |
10,6 |
10,7 |
| 1992 |
71 |
412,4 |
9,6 |
4 164,4 |
6,9 |
372,1 |
9,0 |
3 778,4 |
6,5 |
9,9 |
9,8 |
| 1991 |
65 |
376,4 |
23,2 |
3 894,8 |
18,1 |
341,4 |
24,7 |
3 546,9 |
19,5 |
9,7 |
9,6 |
| 1990 |
65 |
305,5 |
24,8 |
3 298,8 |
11,0 |
273,8 |
25,8 |
2 967,9 |
10,5 |
9,3 |
9,2 |
| 1989 |
66 |
244,8 |
47,4 |
2 973,0 |
9,4 |
217,6 |
34,7 |
2 685,5 |
7,3 |
8,2 |
8,1 |
| 1988 |
66 |
165,7 |
— |
2 718,0 |
— |
161,5 |
— |
2 502,3 |
— |
6,1 |
6,5 |
Source : CEPMB
Ratio des dépenses de R-D par rapport aux recettes tirées des ventes
Le tableau 16 présente également les ratios des dépenses de R-D par rapport aux recettes tirées des ventes. Il convient de noter que dans ce contexte, en contrepartie de l’adoption des modifications apportées à la Loi en 1987, Rx&D s’était engagé à augmenter graduellement ses dépenses annuelles de R-D pour qu’elles totalisent en 1996 au moins 10 % des recettes tirées des ventes21. Ce niveau de dépenses de R-D a été atteint en 1993, ayant même dépassé 10 % certaines années. Toutefois, depuis 2003, le ratio des dépenses de R-D de tous les brevetés et des membres de Rx&D a diminué.
Le ratio de 2012 des dépenses de R-D par rapport aux recettes tirées des ventes parmi les brevetés était de 5,3 %, un recul par rapport au ratio de 5,6 % en 2011. Ces valeurs se situent près des données observées en 1988. Le ratio global des dépenses de R-D par rapport aux recettes tirées des ventes a été inférieur à 10 % pendant les 12 dernières années consécutives.
Le ratio correspondant des dépenses de R-D par rapport aux recettes tirées des ventes des brevetés membres de R&D était de 6,6 % en 2012, un recul par rapport au ratio de 6,7 % en 201122. Ces valeurs se situent également près des données observées en 1988. Le ratio des brevetés membres de R&D a été inférieur à 10 % pendant les 10 dernières années consécutives.
Le tableau 21 à l’annexe 2 présente les ratios des dépenses de R-D par rapport aux recettes tirées des ventes de 2012. Des 85 brevetés ayant présenté des rapports sur leurs dépenses de R-D au CEPMB en 2012, 83,5 % ont présenté un ratio de R-D par rapport aux recettes tirées des ventes de moins de 10 %.
Graphique 18
Ratio des dépenses de R-D par rapport aux recettes tirées des ventes chez les brevetés, 1988-2012
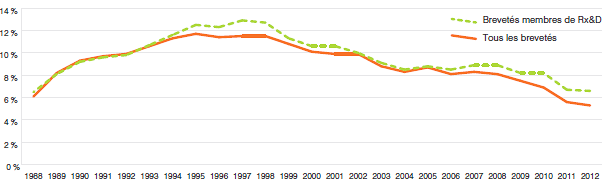
Source : CEPMB
Faits nouveaux
En 2012, Rx&D ont chargé KPMG de mettre à jour les résultats du rapport de 2010 intitulé Résumé des conclusions du sondage du secteur pharmaceutique sur les dépenses en R&D et autres investissements de la part des membres de Rx&D. Le sondage actualisé suivait la même méthodologie que celle utilisée pour le sondage financé conjointement par Rx&D et les Instituts de recherche en santé du Canada (IRSC). Le sondage original, qui utilisait des données de 2010, a été réalisé au titre d’un comité directeur dirigé par Industrie Canada dont l’objectif était d’étudier les niveaux actuels d’investissement en R-D des membres de Rx&D à l’échelle nationale. Le CEPMB ainsi que les IRSC ont collaboré au sein du comité directeur.
Dépenses courantes selon le type de recherche
Le tableau 17 et le graphique 19 (ainsi que le graphique 21 de l’annexe 2) présentent des données sur la ventilation des dépenses courantes de R-D23 engagées en 2012 selon le type de recherche, à savoir la recherche fondamentale, la recherche appliquée et autres types de R-D admissible24. Pour 2012, les brevetés ont fait état de dépenses de recherche fondamentale totalisant 114,6 millions de dollars ou 13,2 % du total des dépenses courantes de R-D, soit un recul de 30,5 % par rapport à l’année antérieure. Les brevetés ont déclaré des dépenses de recherche appliquée totalisant 520,9 millions de dollars, ou, encore, 60,2 % des dépenses courantes de R-D. Les essais cliniques ont accaparé 69,4 % des dépenses de recherche appliquée.
Tableau 17 Dépenses courantes de R-D selon le type de recherche, 2012 et 2011
| Type de recherche |
Dépenses en 2012 (millions $) |
Part en 2012 (%) |
Dépenses en 2011 (millions $) |
Part en 2011 (%) |
Variation annuelle des dépenses (%) |
| Recherche fondamentale |
114,6 |
13,2 |
164,9 |
17,3 |
-30,5 |
| Chimique |
78,3 |
9,1 |
99,4 |
10,4 |
-21,2 |
| Biologique |
36,3 |
4,2 |
65,5 |
6,9 |
-44,6 |
| Recherche appliquée |
520,9 |
60,2 |
525,1 |
55,0 |
-0,8 |
| Processus de fabrication |
82,2 |
9,5 |
77,4 |
8,1 |
6,2 |
| Essais précliniques I |
35,7 |
4,1 |
16,9 |
1,8 |
111,2 |
| Essais précliniques II |
41,5 |
4,8 |
35,7 |
3,8 |
16,2 |
| Essais cliniques de phase I |
34,3 |
4,0 |
29,8 |
3,1 |
15,1 |
| Essais cliniques de phase II |
82,1 |
9,5 |
83,0 |
8,7 |
-1,1 |
| Essais cliniques de phase III |
245,1 |
28,3 |
282,3 |
29,5 |
-13,2 |
| Autre R-D admissible |
230,1 |
26,6 |
265,2 |
27,8 |
-13,2 |
| Total |
865,6 |
100,0* |
955,3 |
100,0* |
-9,4 |
* Il se peut que les valeurs n’équivaillent pas à 100,0 en raison de l’arrondissement.
Source : CEPMB
Graphique 19
Dépenses courantes de R-D selon le type de recherche, 1988-2012
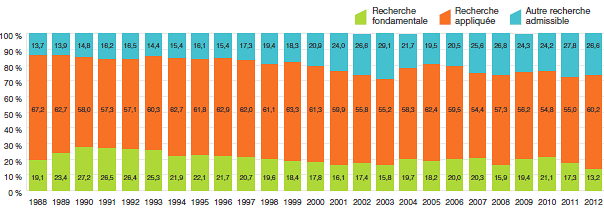
Source : CEPMB
Dépenses courantes de R-D selon le milieu de recherche
Les brevetés peuvent inclure dans leurs dépenses de R-D la recherche qu’ils effectuent eux-mêmes à l’interne ainsi que la recherche qu’ils font faire à l’externe, notamment par des universités, des hôpitaux et d’autres sociétés pharmaceutiques. Le tableau 18 révèle que, en 2012, 49,1 % des dépenses courantes de recherche ont été effectuées à l’interne. La proportion de la R-D effectuée à l’externe pour le compte des brevetés a représenté 25,3 % de l’ensemble des dépenses courantes de R-D. Quant à la recherche effectuée par les universités et par les hôpitaux, sa valeur a représenté 15,1 % des dépenses courantes de R-D.
Tableau 18 Dépenses courantes de R-D selon le milieu de recherche, 2012 et 2011
| Milieu de recherche |
Dépenses en 2012 (millions $) |
Part en 2012 (%) |
Dépenses en 2011 (millions $) |
Part en 2011 (%) |
Variation annuelle des dépenses (%) |
| À l’interne |
| Brevetés |
425,3 |
49,1 |
496,1 |
51,9 |
-14,3 |
| À l’externe |
| Universités et hôpitaux |
131,0 |
15,1 |
151,7 |
15,9 |
-13,7 |
| Autres sociétés |
218,6 |
25,3 |
196,9 |
20,6 |
11,1 |
| Autres |
90,7 |
10,5 |
110,6 |
11,6 |
-18,0 |
| Total |
865,6 |
100,0* |
955,3 |
100,0* |
-9,4 |
* Il se peut que les valeurs n’équivaillent pas à 100,0 en raison de l’arrondissement.
Source : CEPMB
Dépenses courantes de R-D selon la provenance des fonds
Le tableau 19 présente des renseignements sur la provenance des fonds que les brevetés ont investis pour financer leurs activités de R-D. En 2012, les brevetés ont financé à même leurs propres fonds la majeure partie de leur R-D (86,8 % des dépenses courantes de R-D). Les fonds provenant du gouvernement n’ont servi à financer que 2,7 % de l’ensemble des dépenses courantes de R-D.
Tableau 19 Ensemble des dépenses de R-D selon la provenance des fonds, 2012 et 2011
| Provenance des fonds |
Dépenses en 2012 (millions $) |
Part en 2012 (%) |
Expenditures: 2011 ($millions) |
Part en 2011 (%) |
Variation annuelle des dépenses (%) |
| Brevetés |
777,1 |
86,8 |
879,2 |
88,6 |
-11,6 |
| Gouvernements fédéral et provinciaux |
23,8 |
2,7 |
28,7 |
2,9 |
-17,1 |
| Autres |
93,9 |
10,5 |
83,8 |
8,5 |
12,1 |
| Total |
894,8 |
100,0* |
991,7 |
100,0 |
-9,8 |
* Il se peut que les valeurs n’équivaillent pas à 100,0 en raison de l’arrondissement.
Source : CEPMB
Dépenses courantes de R-D selon la région géographique
Le tableau 20 (ainsi que le tableau 23 et le tableau 24 de l’annexe 2) ventile les dépenses courantes de R-D selon la région géographique dans laquelle elles ont été engagées.
Cette année encore, les dépenses ont été surtout engagées en Ontario et au Québec, qui ont accaparé 83,6 % de la valeur totale des dépenses courantes de R-D au Canada. La valeur des dépenses de R-D a reculé d’un taux annuel de 1,8 % dans l’Ouest du pays; elle a également reculé de 8,5 % en Ontario et de 13,8 % au Québec.
Tableau 20 Dépenses courantes de R-D selon la région géographique, 2012 et 2011
| Région géographique |
Dépenses en 2012 (millions $) |
Part en 2012 (%) |
Dépenses en 2011 (millions $) |
Part en 2011 (%) |
Augmentation annuelle des dépenses (%) |
| Provinces de l’Atlantique |
21,9 |
2,5 |
17,9 |
1,9 |
22,1 |
| Québec |
354,8 |
41,0 |
411,8 |
43,1 |
-13,8 |
| Ontario |
368,6 |
42,6 |
403,0 |
42,2 |
-8,5 |
| Provinces de l’Ouest |
120,3 |
13,9 |
122,5 |
12,8 |
-1,8 |
| Territoires |
0,0 |
0,0 |
0,0 |
0,0 |
-100,0 |
| Total |
865,6 |
100,0* |
955,3 |
100,0* |
-9,4 |
* Il se peut que les valeurs n’équivaillent pas à 100,0 en raison de l’arrondissement.
Source : CEPMB
Le contexte mondial
Le graphique 20 compare pour les années 2000 et 2010 les ratios des dépenses de R-D pharmaceutiques par rapport aux recettes tirées des ventes du Canada aux mêmes ratios des sept pays de comparaison du CEPMB25. Le ratio du Canada était de 10,1 % en 2000. Cette année-là, seule l’Italie présentait un ratio plus bas (6,2 %), tandis que la Suisse présentait le ratio le plus élevé (102,5 %).
La même tendance a été observée pour 2010. L’Italie présentait le ratio le moins élevé (6,2 %) suivi du Canada (6,9 %). Le ratio de tous les autres pays de comparaison est demeuré bien supérieur à celui du Canada. Le ratio obtenu avec l’ensemble des dépenses de R-D et des ventes de tous les pays de comparaison était de 22,5 %, soit trois fois et demie celui du Canada.
On peut comparer les ratios des dépenses de R-D par rapport aux recettes tirées des ventes présentés dans le graphique 20 avec les ratios des prix moyens bilatéraux mentionnés au tableau 11 (voir la section « Comparaison des prix pratiqués au Canada avec ceux pratiqués dans les pays de comparaison »). Plusieurs pays de comparaison, dont les prix des produits médicamenteux brevetés sont, en moyenne, beaucoup moins élevés qu’au Canada, ont obtenu des ratios des dépenses de R-D par rapport aux recettes tirées des ventes bien supérieurs à ceux au Canada. De plus en plus, l’incidence du prix des médicaments sur les décisions des sociétés quant au repérage d’investissements ou à l’exécution de recherches est remise en question. D’autres facteurs tels que ceux qui se rapportent au lieu où les sociétés peuvent trouver le meilleur fondement scientifique à un coût raisonnable, aux incitations fiscales, aux marchés du travail flexibles et à la stabilité économique sont jugés importants26.
Graphique 20
Ratios des dépenses de R-D par rapport aux recettes tirées des ventes, au Canada et dans les pays de comparaison
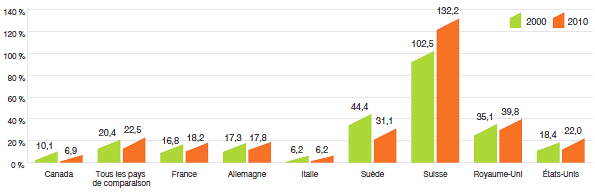
Notes
19 Il s’agit probablement de la situation d’une majeure partie du secteur de biotechnologie du Canada. Cependant, il convient de noter que si un breveté requiert de la recherche d’une autre société spécialisée dans la recherche en biotechnologie, le breveté devrait normalement inclure cela dans les dépenses de recherche qu’il déclare au CEPMB.
20 Le budget de 2012 a proposé des réductions au crédit d’impôt pour la recherche scientifique et le développement expérimental (RS-DE) et de nouvelles restrictions relatives aux déductions. Il a également introduit de nouvelles mesures visant à appuyer l’innovation et la R-D. Aux termes du Règlement, le CEPMB définit la R-D selon la définition de RS-DE de 1987.
21 Selon le Résumé de l’étude d’impact de la réglementation (REIR) du Règlement sur les médicaments brevetés, 1988, publié dans la partie II de la Gazette du Canada, vol. 122, no 20 – SOR/DORS/88-474.
22 Dans le tableau 16, les ratios des dépenses de R-D par rapport aux ventes tiennent compte des dépenses de recherche financées par le gouvernement au moyen de subventions. Si l’on exclut le financement accordé par le gouvernement, le ratio pour tous les brevetés en 2012 est de 5,2 % et celui pour les brevetés membres de Rx&D est de 6,4 %.
23 Les dépenses courantes de R-D comprennent les dépenses autres qu’en capital directement associées à la recherche, dont (a) les salaires; (b) le matériel direct, (c) les honoraires des entrepreneurs et des sous-traitants, (d) d’autres coûts directs de production tels que les frais généraux, (e) les paiements aux institutions désignées, (f) les paiements aux organismes subventionnaires et (g) les paiements à d’autres organismes. Ces éléments sont décrits plus en détail dans le formulaire 3 (Recettes et dépenses en recherche et développement), affiché sur le site Web du CEPMB sous la rubrique « Loi, Règlement et Lignes directrices/Guide du breveté ». Les dépenses courantes de R-D représentent 96,7 % de l’ensemble des dépenses de R-D de 2012. Les coûts en immobilisations représentent 1,4 % des dépenses courantes de R-D et les frais d’amortissement admissibles, 1,8 %.
24 La « recherche fondamentale » désigne les travaux qui contribuent à faire avancer la connaissance scientifique et qui sont entrepris sans aucune application scientifique en vue. La « recherche appliquée » désigne les travaux qui contribuent à faire avancer la connaissance scientifique et qui sont entrepris avec une application pratique en vue. Elle peut viser à créer de nouveaux produits ou procédés, à améliorer ceux qui existent à l’aide de procédés de fabrication ou, encore, d’études précliniques ou cliniques. Enfin, l’expression « Autre R-D admissible » désigne les présentations sur la réglementation des médicaments, les études de biodisponibilité ainsi que les essais cliniques de phase IV.
25 Dans le graphique 20, les ventes sont celles effectuées exclusivement au pays. Elles ne comprennent pas les ventes à l’exportation.
26 NERA ECONOMIC CONSULTING. Key Factors in Attracting Internationally Mobile Investments by the Research-Based Pharmaceutical Industry. Cité par le Medicines, Pharmacy & Industry Group. Réponse du gouvernement à la consultation, Département de la santé, Londres (Royaume-Uni), 2011.
Système national d’information sur l’utilisation des médicaments prescrits
Le Système national d’information sur l’utilisation des médicaments prescrits (SNIUMP) est une initiative de recherche établie en septembre 2001 par les ministres fédéral, provinciaux et territoriaux de la Santé.
Le SNIUMP vise à fournir aux décideurs et aux gestionnaires des régimes publics d’assurance-médicaments des analyses critiques des tendances relatives aux prix, à l’utilisation et aux coûts des médicaments, afin de s’assurer que le système canadien des soins de santé a accès à des renseignements exhaustifs et précis sur l’utilisation des médicaments d’ordonnance et sur les facteurs à l’origine des augmentations des coûts.
Le CEPMB est autorisé à réaliser des activités en vertu de l’initiative du SNIUMP suite à une demande officielle du ministre fédéral de la Santé en vertu de l’article 90 de la Loi sur les brevets, et conformément au mandat du CEPMB qui consiste à faire rapport sur les tendances relatives aux produits pharmaceutiques.
Le Comité directeur du SNIUMP conseille le CEPMB sur son programme de recherche et lui suggère des sujets d’étude. Le comité est constitué de représentants des régimes publics d’assurance-médicaments de la Colombie-Britannique, de l’Alberta, de la Saskatchewan, du Manitoba, de l’Ontario, du Nouveau-Brunswick, de la Nouvelle-Écosse, de l’Île-du- Prince-Édouard, de Terre-Neuve-et-Labrador, du Yukon et de Santé Canada.
Publications
Les plus récents rapports du SNIUMP publiés par le CEPMB sont les suivants : L’Observateur des médicaments émergents – quatrième livraison et L’utilisation de bandelettes de test glycémique dans certains régimes publics d’assurance-médicaments, 2008.
Le CEPMB prévoit publier divers rapports du SNIUMP plus tard en 2013, alors que d’autres études sont actuellement en cours d’élaboration. Le programme d’activités à long terme du SNIUMP fournit des renseignements détaillés sur les études analytiques courantes.
Annexe 1 : Glossaire
Pour de plus amples explications et définitions, veuillez consulter la Loi sur les brevets, le Règlement sur les médicaments brevetés, le Compendium des politiques, des Lignes directrices, et des procédures du CEPMB et le Règlement sur les aliments et drogues ou, encore, communiquer avec le CEPMB.
ATC : Système de classification anatomique, thérapeutique, chimique (ATC) conçu et tenu à jour par le Centre collaborateur de l’OMS pour la méthodologie sur l’établissement des statistiques concernant les produits médicamenteux de l’Organisation mondiale de la santé (OMS). Ce système distingue les médicaments selon leur site d’action et leurs caractéristiques thérapeutiques et chimiques. Le CEPMB utilise ce système pour sélectionner les médicaments qui seront utilisés dans les comparaisons des prix.
AVIS DE CONFORMITÉ : Avis donné par la Direction générale des produits de santé et des aliments de Santé Canada en vertu de l’article C.08.004 du Règlement sur les aliments et drogues. Cet avis confirme que le produit médicamenteux respecte les normes prescrites par Santé Canada pour une administration à des humains ou à des animaux, selon le cas, et que sa vente est autorisée au Canada.
BREVET : Instrument émis par le Commissaire aux brevets sous forme de lettres patentes. Le brevet confère à son titulaire un monopole d’une durée limitée pour les allégations formulées. Le brevet confère également à son titulaire et à ses représentants légaux le droit exclusif de fabriquer, de construire, d’exploiter ou de vendre son invention.
BREVET EN INSTANCE : Demande pour un brevet qui n’a pas encore été attribué
BREVETÉ : Aux termes du paragraphe 79(1) de la Loi sur les brevets, le mot « breveté » désigne « la personne ayant droit aux retombées d’un brevet pour une invention liée à un médicament, ainsi que quiconque était titulaire d’un brevet pour une telle invention ou qui exerce ou a exercé les droits d’un titulaire dans un cadre autre qu’une licence prorogée en vertu du paragraphe 11(1) de la Loi de 1992 modifiant la Loi sur les brevets ».
CERTIFICAT DE DÉCISION PRÉALABLE : Certificat révocable émis à la demande du breveté en vertu du paragraphe 98(4) de la Loi sur les brevets lorsque le Conseil estime que le prix pratiqué ou proposé du produit médicamenteux ne dépasse pas le prix moyen maximal potentiel qu’autorisent ses Lignes directrices.
CESSION DE BREVET : Avis donné par le breveté au Commissaire aux brevets l’informant qu’il renonce irrévocablement à ses droits de propriété à l’égard du brevet en cause et qu’il les cède au domaine public. Nota : Depuis le 30 janvier 1995, le Conseil ne reconnaît pas la cession d’un brevet lorsque le breveté utilise cette mesure pour se soustraire à sa compétence en matière d’examen du prix.
DÉFAUT DE PRÉSENTER SES RAPPORTS : Défaut partiel ou complet d’un breveté de déclarer un produit médicamenteux breveté vendu conformément aux exigences réglementaires en matière de présentation de rapport prévues à la Loi sur les brevets et au Règlement sur les médicaments brevetés.
DÉFAUT DE SOUMETTRE SES RAPPORTS : Défaut partiel ou complet d’un breveté de respecter les exigences réglementaires en matière de soumission de rapport prévues à la Loi sur les brevets et au Règlement sur les médicaments brevetés.
DÉPENSES DE RECHERCHE ET DÉVELOPPEMENT : Aux termes du Règlement sur les médicaments brevetés, et plus particulièrement de ses articles 5 et 6, la recherche-développement s’entend des activités qui auraient été considérées admissibles au crédit d’impôt à l’investissement pour la recherche scientifique et le développement expérimental en vertu de la Loi de l’impôt sur le revenu dans sa version du 1er décembre 1987.
DÉPENSES COURANTES DE RECHERCHE-DÉVELOPPEMENT : Désigne les dépenses autres qu’en capital directement liées à la recherche, dont : (a) salaires, (b) matériel direct, (c) entrepreneurs et sous-traitants, (d) autres coûts directs tels que les frais généraux de production, (e) paiements aux institutions désignées, (f) paiements aux organismes subventionnaires et (g) paiements aux autres organismes. Ces éléments sont décrits plus amplement dans le formulaire 3 présenté dans le Guide du breveté que vous trouverez dans sur notre site Web sous « Loi, Règlement et Lignes directrices ».
ENGAGEMENT DE CONFORMITÉ VOLONTAIRE : Engagement écrit pris par le breveté de réduire le prix de son produit médicamenteux pour le rendre conforme aux Lignes directrices du Conseil. Le président du Conseil peut, en lieu d’un Avis d’audience, accepter un Engagement de conformité volontaire s’il sert les meilleurs intérêts du grand public. La politique du Conseil sur la conformité et l’application autorise le breveté à soumettre un Engagement de conformité volontaire même si un Avis d’audience a été émis. Toutefois, l’Engagement soumis à ce point doit être approuvé par le panel d’audience et non seulement par le président du Conseil. Le Conseil publie tous les Engagements acceptés par le président ou le Conseil.
INDICE DES PRIX DES MÉDICAMENTS BREVETÉS (IPMB) : Indice établi par le CEPMB pour mesurer la variation annuelle des prix de transaction des médicaments brevetés vendus au Canada. Cet indice est établi à partir des données sur les prix et sur les ventes déclarées par les brevetés.
LICENCE VOLONTAIRE : Engagement contractuel entre un breveté et un titulaire de licence permettant à ce dernier de bénéficier des retombées d’un brevet ou d’exercer des droits à l’égard de celui-ci moyennant une contrepartie financière (comme redevances sous forme de pourcentage des recettes tirées des ventes).
MÉDICAMENT : Toute substance ou tout mélange de substances produites biologiquement, chimiquement ou autrement qui est appliqué ou administré in vivo pour faciliter le diagnostic, le traitement, l’atténuation ou la prévention d’une maladie, de symptômes, de troubles ou d’états physiques anormaux ou, encore, pour modifier des fonctions organiques chez les humains ou chez les animaux. Cette définition comprend les vaccins, les préparations topiques, les anesthésiques et les produits de diagnostic in vivo quel que soit le mode d’administration (p. ex. préparations transdermiques, gélules, solutions injectables, solutions pour inhalation, etc.). Cette définition exclut les appareils médicaux, les produits de diagnostic in vitro et les désinfectants qui ne sont pas utilisés in vivo.
NUMÉRO D’IDENTIFICATION DU MÉDICAMENT (DIN) : Numéro d’identification que la Direction générale de la protection de la santé de Santé Canada attribue à chaque produit médicamenteux vendu sous ordonnance ou en vente libre et commercialisé en vertu du Règlement sur les aliments et drogues. Le DIN est assigné en tenant compte des éléments suivants : le fabricant du produit, le ou les ingrédients actifs, la concentration de ou des ingrédients actifs, la forme posologique, le nom de marque du produit et son mode d’administration.
PRODUIT GÉNÉRIQUE : Produit médicamenteux contenant le même ingrédient actif, la même concentration et la même forme posologique que son équivalent de marque.
PRODUIT MÉDICAMENTEUX : Présentation d’un médicament qui se distingue par sa forme pharmaceutique et la concentration de son ingrédient actif.
PROGRAMME D’ACCÈS SPÉCIAL : Programme en vertu duquel Santé Canada permet à des praticiens d’avoir accès à des produits médicamenteux n’ayant pas encore reçu l’Avis de conformité et qui ne seraient autrement pas disponibles sur le marché canadien.
RECHERCHE ET DÉVELOPPEMENT (R-D) : Recherche fondamentale ou appliquée visant à créer de nouveaux matériaux, dispositifs, produits ou procédés ou à améliorer ceux qui existent (p. ex. procédés de fabrication).
RECHERCHE ET DÉVELOPPEMENT – AUTRE R-D ADMISSIBLE : Comprend les dépenses de recherche-développement qui ne correspondent à aucune des catégories de R-D susmentionnées. Elle comprend les présentations sur la réglementation des produits médicamenteux, les études de biodisponibilité ainsi que les essais cliniques de phase IV.
RECHERCHE ET DÉVELOPPEMENT – RECHERCHE APPLIQUÉE : Travaux qui sont entrepris avec une application pratique en vue. Ils peuvent viser à créer de nouveaux produits ou procédés, à améliorer ceux qui existent à l’aide de procédés de fabrication ou, encore, d’études précliniques et cliniques.
RECHERCHE ET DÉVELOPPEMENT – RECHERCHE FONDAMENTALE : Travaux qui contribuent à faire avancer la connaissance scientifique et qui sont entrepris sans aucune application pratique en vue.
SUBSTANCE ACTIVE : Substance chimique ou biologique responsable de l’effet pharmacologique d’un produit médicamenteux.
Annexe 2 : Recherche et développement
Tableau 21 Intervalle des ratios des dépenses de R-D par rapport aux recettes tirées des ventes, selon le nombre de brevetés ayant présenté des rapports et la valeur des recettes tirées des ventes
| Intervalle : Ratio des dépenses de R-D par rapport aux recettes tirées des ventes |
Nbre de brevetés ayant présenté un rapport en 2012 |
Recettes tirées des ventes en 2012 (millions $) |
Part en 2012 (%) |
Nbre de brevetés ayant présenté un rapport en 2011 |
Recettes tirées des ventes en 2011 (millions $) |
Part en 2011 (%) |
| 0% |
32 |
1 596,4 |
9,5 |
30 |
1 625,4 |
9,1 |
| ≤10% |
39 |
11 794,4 |
73,2 |
37 |
12 995,1 |
73,0 |
| > 10% |
14 |
3 363,6 |
17,3 |
12 |
3 178,3 |
17,9 |
| Total |
85 |
16 754,4 |
100,0* |
79 |
17 798,8 |
100,0* |
* Il se peut que les valeurs n’équivaillent pas à 100,0 en raison de l’arrondissement.
Source : CEPMB
Graphique 21
Dépenses courantes de R-D selon le type de recherche, 1988-2012
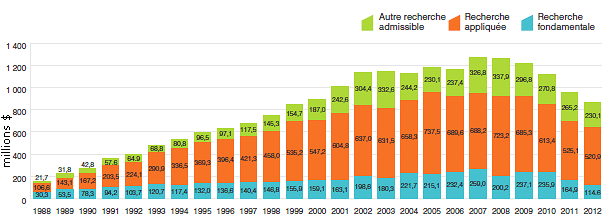
Source : CEPMB
Tableau 22 Ratios des dépenses de R-D par rapport aux recettes tirées des ventes selon les brevetés1 qui ont présenté un rapport, 2012 et 2011
| Breveté |
Ratio des dépenses de R-D par rapport aux recettes tirées des ventes (%) |
| 2012 |
2011 |
| Abbott Laboratories, Ltd.2,3 |
1,0 |
1,1 |
| AbbVie Corporation.2,4 |
1,9 |
— |
| Actelion Pharmaceutiques Canada Inc.2 |
4,4 |
5,9 |
| Alcon Canada Inc. |
0,1 |
0,1 |
| Alexion Pharmaceuticals Inc.3 |
0,0 |
0,0 |
| Allergan Inc. |
5,3 |
6,1 |
| Alveda Pharmaceuticals Inc.5 |
0,0 |
— |
| Amgen Canada Inc.2,3 |
6,9 |
6,8 |
| Astellas Pharma Canada Inc.2,6 |
5,3 |
6,3 |
| AstraZeneca Canada Inc.2,3 |
1,8 |
3,5 |
| Bausch & Lomb Canada Inc. |
0,0 |
0,0 |
| Baxter Corporation3 |
0,2 |
0,3 |
| Bayer Inc., Healthcare Division2 |
4,4 |
3,3 |
| Biogen Idec Canada Inc.3 |
12,5 |
10,2 |
| BioMarin Canada Inc.3 |
52,3 |
27,9 |
| Biovitrum AB |
0,0 |
0,0 |
| Boehringer Ingelheim (Canada) Ltd.2 |
11,3 |
12,6 |
| Bracco Diagnostics Canada Inc.2 |
0,0 |
0,0 |
| Bristol-Myers Squibb Pharmaceutical Group2,3 |
13,1 |
8,0 |
| Celgene Canada3 |
1,8 |
2,9 |
| CSL Behring Canada Inc.5 |
0,8 |
— |
| Duchesnay Inc. |
6,4 |
3,2 |
| Eisai Limited2,3 |
172,5 |
0,0 |
| Eli Lilly Canada Inc. (includes Provel Animal Health Division)2,3 |
10,0 |
11,1 |
| EMD Serono Canada Inc.2 |
7,2 |
9,7 |
| Ferring Inc. |
3,1 |
1,0 |
| Fresenius Medical Care Canada |
0,0 |
0,0 |
| Galderma Canada Inc. |
0,0 |
0,0 |
| GE Healthcare Inc. (Amersham Health Inc.) |
0,0 |
0,0 |
| Genzyme Canada Inc.2,3 |
0,9 |
1,3 |
| Gilead Sciences Inc.2,3 |
24,6 |
19,8 |
| GlaxoSmithKline Inc.2 |
10,6 |
10,6 |
| Grifols Canada Ltd (Talecris Biotherapeutics Ltd.)3 |
0,9 |
0,9 |
| Hoffmann-La Roche Ltd. Canada2 |
3,9 |
3,7 |
| Hospira Healthcare Corp. |
0,0 |
0,0 |
| INO Therapeutics2 |
0,0 |
0,0 |
| Intermune Canada Inc. |
0,0 |
0,0 |
| Iroko International LP |
0,0 |
0,0 |
| Janssen Inc.2,3 |
3,3 |
5,1 |
| Johnson & Johnson Inc. |
0,0 |
0,0 |
| Johnson & Johnson Medical Products |
0,8 |
0,0 |
| Lantheus MI Canada Inc. |
0,0 |
0,0 |
| LEO Pharma Inc.2 |
2,1 |
1,8 |
| Lundbeck Canada Inc.2 |
0,7 |
0,6 |
| Lundbeck Inc. (Ovation Pharmaceuticals Inc.) |
0,0 |
0,0 |
| McNeil Consumer Healthcare Canada |
2,6 |
2,6 |
| Medical Futures Inc.5 |
0,0 |
— |
| Medicis Canada Ltd.5 |
0,0 |
— |
| Merck Canada Inc.2,3 |
3,3 |
1,7 |
| Merz Pharma Canada Ltd. |
6,5 |
19,6 |
| Novartis Pharmaceuticals Canada Inc.2,3 |
12,6 |
11,4 |
| Novo Nordisk Canada Inc.3 |
1,7 |
2,2 |
| Optimer Pharmaceuticals Canada Inc.5 |
0,0 |
— |
| Osiris Therapeutics Inc.5 |
372,2 |
— |
| Otsuka America Pharmaceuticals2 |
17,4 |
0,0 |
| Paladin Laboratories Inc.2 |
0,02 |
0,2 |
| Pfizer Canada Inc.2,3 |
6,0 |
7,6 |
| Pharmascience Inc. |
8,6 |
7,4 |
| Purdue Pharma2 |
2,4 |
2,0 |
| Ranbaxy Pharmaceuticals Canada Inc. |
0,0 |
0,0 |
| Rare Disease Therapeutics Inc. |
0,0 |
0,0 |
| sanofi pasteur Ltd.2,3,7 |
53,4 |
46,0 |
| sanofi-aventis Pharma Inc.2,3,8 |
5,8 |
8,2 |
| Santhera Pharmaceuticals Canada Inc.3 |
2,7 |
2,5 |
| Sandoz Canada Inc. |
1,2 |
— |
| Seattle Genetics Inc.5 |
14,7 |
— |
| Sunovion (Sepracor Pharmaceuticals Canada Inc.)2 |
0,0 |
0,01 |
| Servier Canada Inc.2 |
5,4 |
3,8 |
| Shire Canada Inc.2 |
0,1 |
0,2 |
| Shire Human Genetic Therapies3 |
2,5 |
1,7 |
| Sigma Tau Pharmaceuticals Inc. |
0,0 |
0,0 |
| Sopherion Therapeutics Canada Inc. |
0,0 |
0,0 |
| Takeda Canada Inc.2,3 |
0,0 |
5,9 |
| Teva Canada Ltd. (Ratiopharm) |
0,0 |
0,0 |
| Teva Canada Innovation GP3 |
2,1 |
7,4 |
| Tribute Pharma Canada Inc.5 |
0,0 |
— |
| Triton Pharma Inc. |
0,0 |
0,0 |
| Tyco Healthcare Group Canada Inc. |
0,0 |
0,0 |
| UCB Pharma Canada Inc.3 |
9,7 |
12,3 |
| Unither Biotech Inc. |
0,0 |
0,0 |
| Valeant Canada Ltd.3,9 |
0,0 |
0,0 |
| Vertex Pharma Canada Inc.5 |
26,4 |
— |
| VIIV Healthcare ULC.2 |
0,0 |
0,0 |
| Warner Chilcott Canada Inc.2 |
0,1 |
0,3 |
| Watson Pharma Co.5 |
0,0 |
— |
1 Pour éviter la double comptabilisation des recettes tirées des ventes, les recettes tirées des redevances sont incluses dans le calcul du ratio de chaque société mais non dans le calcul du ratio à l’échelle de l’industrie. Les subventions des gouvernements fédéral et provinciaux ne sont pas comptabilisées dans les dépenses utilisées pour le calcul des ratios des dépenses de R-D de chaque société, mais elles le sont dans les statistiques pour l’ensemble des brevetés. La liste des brevetés ayant soumis un rapport sur les prix n’est pas identique à celle des brevetés ayant soumis un rapport sur leurs activités de R-D en raison des différences des modalités de rapport entre les brevetés et leurs sociétés affiliées ou les détenteurs d’une licence d’exploitation. Il convient également de noter que les titulaires d’un brevet pour un médicament vétérinaire sont tenus de présenter chaque année un rapport sur leurs dépenses de R-D, mais pas nécessairement sur les prix qu’ils pratiquent et la valeur de leurs ventes.
2 Membre de Rx&D.
3 Membre de BIOTECanada.
4 Société dérivée de la division des produits de marque déposée d’Abbott en entité juridique distincte à partir du 31 octobre 2012.
5 N’était pas un breveté en 2011.
6 Anciennement connu sous le nom Fujisawa Canada Inc.
7 Anciennement connu sous le nom Aventis Pasteur Ltd.
8 Anciennement connu sous le nom Aventis Pharma Inc.
9 Anciennement connu sous le nom ICN Canada Ltd.
Tableau 23 Dépenses courantes de R-D selon la province ou le territoire, 2012
| Province |
Dépenses par tous les brevetés (000 $) |
Part régionale (%) |
Dépenses par tous les brevetés membres de Rx&D (000 $) |
Part régionale (%) |
| Terre-Neuve |
5 308,33 |
0,613 |
4 379,63 |
0,577 |
| Île-du-Prince-Édouard |
51,32 |
0,006 |
51,32 |
0,007 |
| Nouvelle-Écosse |
14 673,60 |
1,695 |
13 643,39 |
1,798 |
| Nouveau-Brunswick |
1 882,83 |
0,218 |
1 354,22 |
0,178 |
| Québec |
354 793,95 |
40,986 |
297 461,97 |
39,198 |
| Ontario |
368 642,16 |
42,586 |
331 874,77 |
43,733 |
| Manitoba |
6 491,07 |
0,750 |
5 361,41 |
0,707 |
| Saskatchewan |
2 413,53 |
0,279 |
1 701,77 |
0,224 |
| Alberta |
70 108,02 |
8,099 |
66 082,45 |
8,708 |
| Colombie-Britannique |
41 278,92 |
4,769 |
36 954,88 |
4,870 |
| Territoires |
0 |
0,000 |
0 |
0,000 |
| Canada |
865 643,73 |
100,0* |
758 865,81 |
100,0* |
* Il se peut que les valeurs n’équivaillent pas à 100,0 en raison de l’arrondissement.
Source : CEPMB
Tableau 24 Dépenses courantes R-D selon le milieu de recherche et la province ou le territoire, 2012
| Province |
|
Brevetés |
Autres sociétés |
Universités |
Hôpitaux |
Autres |
| Terre-Neuve |
000 $ |
644,0 |
2 326,3 |
1 208,1 |
131,0 |
998,9 |
| % |
12,1 |
43,8 |
22,8 |
2,5 |
18,8 |
| Île-du-Prince-Édouard |
000 $ |
0,0 |
30,4 |
0,0 |
21,2 |
0,0 |
| % |
0,0 |
59,3 |
0,0 |
41,3 |
0,0 |
| Nouvelle-Écosse |
000 $ |
1 628,4 |
2 632,5 |
6 787,4 |
1 441,2 |
2 184,1 |
| % |
11,1 |
17,9 |
46,3 |
9,8 |
14,9 |
| Nouveau-Brunswick |
000 $ |
208,3 |
903,9 |
0,0 |
388,6 |
382,0 |
| % |
11,1 |
48,0 |
0,0 |
20,6 |
20,3 |
| Québec |
000 $ |
186 355,5 |
96 333,7 |
10 953,7 |
16 577,7 |
44 573,2 |
| % |
53,8 |
25,4 |
3,2 |
4,8 |
12,9 |
| Ontario |
000 $ |
168 651,7 |
90 854,3 |
20 607,3 |
56 746,1 |
31 782,7 |
| % |
45,7 |
24,6 |
5,6 |
15,4 |
8,6 |
| Manitoba |
000 $ |
1 863,7 |
1 828,7 |
107,6 |
1 341,1 |
1 350,0 |
| % |
28,7 |
28,2 |
1,7 |
20,7 |
20,8 |
| Saskatchewan |
000 $ |
477,1 |
845,5 |
671,9 |
278,9 |
140,2 |
| % |
19,8 |
35,0 |
27,8 |
11,6 |
5,8 |
| Alberta |
000 $ |
49 488,7 |
8 612,2 |
4 192,0 |
3 544,9 |
4 270,2 |
| % |
70,6 |
12,3 |
6,0 |
5,1 |
6,1 |
| Colombie-Britannique |
000 $ |
15 953,3 |
14 265,4 |
2 229,2 |
3 782,9 |
5 048,1 |
| % |
38,6 |
34,6 |
5,4 |
9,2 |
12,2 |
| Territories |
000 $ |
0,0 |
0,0 |
0,0 |
0,0 |
0,0 |
| % |
0,0 |
0,0 |
0,0 |
0,0 |
0,0 |
| Canada |
000 $ |
425 270,3 |
218 632,9 |
46 757,64 |
84 253,6 |
90 729,5 |
| % |
49,1 |
25,3 |
5,4 |
9,7 |
10,5 |
Remarques :
- Le pourcentage figurant sous chaque catégorie de R-D correspond au pourcentage de toutes les dépenses engagées dans cette catégorie dans la province ou le territoire.
- Les dépenses présentées sous forme de pourcentage du total correspondent au pourcentage des dépenses de R-D par rapport à l’ensemble des dépenses de R-D faites au Canada.
- Le total des colonnes et des rangées ne correspond pas nécessairement, car certains chiffres ont été arrondis.
- Dépenses courantes plus dépenses en immobilisations (équipement + amortissement) = total des dépenses de R-D.
Source : CEPMB