Conseil d’examen du prix des médicaments brevetés
L’honorable Jane Philpott
Ministre de la Santé
ISSN : 2292-6291
Table des matières
Message de la présidente
Section I : Vue d’ensemble des dépenses de l’organisation
Section II : Analyse des programmes par résultat stratégique
Section III : Renseignements supplémentaires
Section IV : Coordonnées de l’organisation
Annexe : Définitions
Notes de fin de document
Message de la présidente
J’ai le plaisir de vous présenter le Rapport sur les plans et les priorités 2016-2017 du Conseil d’examen du prix des médicaments brevetés (CEPMB).
Le CEPMB est un organisme indépendant qui détient des pouvoirs quasi judiciaires dont le mandat vise à protéger les consommateurs des médicaments brevetés de prix jugés excessifs et à présenter aux Canadiens des rapports sur les tendances dans les prix de tous les médicaments de même que sur les investissements en recherche et développement (R-D) des titulaires de brevet.
Le CEPMB a été formé en 1987, par l’intermédiaire de modifications apportées à la Loi sur les brevets, dans le contexte d’une réorganisation majeure du régime de brevets sur les médicaments du Canada qui visait à maintenir l’équilibre entre des objectifs stratégiques susceptibles de s’opposer. Ainsi, d’une part, le gouvernement a renforcé la protection que confèrent les brevets aux médicaments afin de stimuler l’investissement en R-D de la part de l’industrie pharmaceutique au pays. D’autre part, le gouvernement a cherché à atténuer les répercussions de ce changement sur les Canadiens en créant le CEPMB, une agence de protection des consommateurs ayant pour mandat de veiller à ce que les prix des médicaments brevetés au Canada ne deviennent pas « excessifs ».
En tant que membre du portefeuille de la Santé, le CEPMB joue un rôle de premier plan dans l’objectif global d’améliorer la santé des Canadiens par l’entremise d’un système de santé responsable, accessible et viable.
Tous les Canadiens qui paient pour un médicament sur ordonnance breveté peuvent profiter des prix plafonds fixés par le CEPMB. Le travail d’analyse et de présentation de rapports du CEPMB appuie les efforts continus des provinces et des territoires visant à mettre en œuvre des politiques d’établissement des prix pour les médicaments génériques et à négocier des prix plus bas pour les médicaments de marque. Par l’intermédiaire de l’initiative du Système national d’information sur l’utilisation des médicaments prescrits (SNIUMP), le CEPMB offre également des renseignements objectifs et à jour sur un éventail de sujets d’intérêt pour les payeurs, les décideurs et les autres intervenants du milieu pharmaceutique, y compris en ce qui touche l’usage et les coûts des médicaments génériques, les médicaments émergents et les inducteurs de coûts dans les régimes publics et privés.
Comme beaucoup d’autres pays, le Canada est confronté à une croissance des coûts des soins de santé et les payeurs de partout au pays peinent à concilier des budgets de soins de santé limités avec l’accès aux nouvelles technologies prometteuses. Tandis que d’autres pays industrialisés ont adopté des réformes ciblées des prix à l’échelle nationale et ont mis en place des mesures visant à régler les problèmes de capacité financière, à optimiser les ressources et à suivre le rythme du marché pharmaceutique, qui évolue rapidement depuis quelques années, le cadre juridique du CEPMBNotes de bas de page i est demeuré essentiellement inchangé depuis la mise sur pied du Conseil.
Ayant récemment célébré son 25e anniversaire, le CEPMB se trouve à une importante croisée des chemins dans son histoire. S’il souhaite demeurer un intervenant pertinent et efficace dans un système pharmaceutique durable en cette période où les prix des médicaments brevetés au Canada dépassent ceux des sept pays avec lesquels il établit des comparaisonsNotes de bas de page ii (à l’exception des États-Unis) ainsi que ceux d’autres pays européens, où la R-D est au niveau le plus bas jamais enregistré et où les payeurs doivent composer avec des prix des médicaments de plus en plus élevés, le CEPMB doit adopter une approche axée davantage sur le consommateur en ce qui touche la façon dont il assume ses fonctions en matière de réglementation et de production de rapportsNotes de bas de page iii.
À cette fin, au cours de la prochaine année, le CEPMB consultera les intervenants afin d’obtenir leurs points de vue sur l’éventuelle nécessité d’apporter des changements à ses pouvoirs de protection des consommateurs – et, le cas échéant, sur la mesure dans laquelle il convient de le faire – pour s’assurer que les médicaments brevetés du Canada demeurent abordables. Le CEPMB devra également intensifier son partenariat avec les payeurs publics pour les aider dans leurs négociations sur des sujets à l’égard desquels il présente des rapports, en plus d’élargir la portée de ces sujets, et ce, afin de mieux servir ces payeurs et les consommateurs.
Le Plan stratégique 2015–2018Notes de bas de page iv du CEPMB, publié récemment, présente les étapes à suivre pour concrétiser la réforme voulue, ce qui permettra au Conseil d’atteindre les objectifs stratégiques établis initialement par le gouvernement en ce qui a trait aux réalités du marché pharmaceutique d’aujourd’hui.
Mary Catherine Lindberg
Section I : Vue d’ensemble des dépenses de l’organisation
Profil organisationnel
Ministre compétent : L’honorable Jane Philpott
Administrateur général : Mary Catherine Lindberg, présidente
Portefeuille ministériel : Santé
Principales autorités législatives : Loi sur les brevetsNotes de bas de page v et Règlements sur les médicaments brevetésNotes de bas de page vi
Année de création : 1987
Autre : La ministre de la Santé est responsable de l’application des dispositions pharmaceutiques de la Loi sur les brevets (la Loi) formulées aux articles 79 à 103. Même s’il fait partie du portefeuille de la Santé, le CEPMB, en raison de ses responsabilités quasi judiciaires, exerce son mandat en toute indépendance à l’égard du ministre. Il fonctionne également d’une façon indépendante de Santé Canada, qui approuve les médicaments sur les plans de l’innocuité, de l’efficacité et de la qualité; d’autres membres du portefeuille de la Santé, comme l’Agence de la santé publique du Canada, les Instituts de recherche en santé du Canada et l’Agence canadienne d’inspection des aliments; des responsables des régimes publics fédéral, provinciaux et territoriaux d’assurance-médicaments, qui autorisent l’inscription des médicaments sur leurs formulaires de médicaments admissibles à un remboursement; et du Programme commun d’évaluation des médicaments, qui est géré par l’Agence canadienne des médicaments et des technologies de la santé (ACMTS), et qui formule des recommandations sur les médicaments qui devraient être admissibles à un remboursement dans le cadre des régimes publics d’assurance-médicaments participants.
Contexte organisationnel
Raison d’être
Créé par le Parlement en 1987, le Conseil d’examen du prix des médicaments brevetés (CEPMB) est un organisme indépendant qui détient des pouvoirs quasi judiciaires. Il est investi d’un double mandat :
- réglementation – veiller à ce que les prix auxquels les titulaires de brevet vendent leurs médicaments au Canada ne soient pas excessifs;
- rapport – faire rapport des tendances des prix de tous les médicaments ainsi que des dépenses des brevetés dans la recherche et le développement au Canada.
Dans l’exécution de son mandat, le CEPMB veille à la protection des Canadiens en s’assurant que les médicaments brevetés ne sont pas vendus au Canada à des prix excessifs et que les intervenants sont tenus informés des tendances relatives aux produits pharmaceutiques.
Responsabilités
Le CEPMB a été créé à la suite des modifications apportées à la Loi sur les brevets (la Loi) en 1987 (projet de loi C-22) et ses pouvoirs de redressement ont été modifiés par d’autres modifications en 1993 (projet de loi C-91). Ces deux ensembles de modifications faisaient partie de réformes politiques visant à équilibrer la nécessité d’établir des mesures plus strictes de protection des brevets à l’égard des produits pharmaceutiques en vue d’encourager les efforts des titulaires de brevets pharmaceutiques en matière de R-D et le besoin de protéger les consommateurs contre les prix potentiellement excessifs en ce qui touche les médicaments brevetés.
Le CEPMB a un double mandat :
Réglementation du prix des médicaments brevetés
Le CEPMB réglemente les prix plafonds départ-usine, à savoir les prix auxquels les brevetés vendent, au Canada, à leurs différents clients (grossistes, hôpitaux, pharmacies et autres), leurs médicaments brevetés pour usage humain ou pour usage vétérinaire distribués sous ordonnance ou en vente libre, et ce, pour veiller à ce que ces prix ne soient pas excessifs. Le mandat du Conseil porte également sur des médicaments qui sont offerts en vertu du Programme d’accès spécial, par l’entremise d’une demande d’essai clinique et en tant que drogues nouvelles de recherche. Les médicaments brevetés en vente libre et les médicaments brevetés à usage vétérinaire sont également réglementés par le Conseil en fonction des plaintes reçues.
Si le personnel détermine que le prix d’un médicament breveté semble excessif, le titulaire du brevet peut se voir offrir la possibilité d’accepter un engagement de conformité volontaire pour remédier à la situation. Si, toutefois, le personnel ne parvient pas à s’entendre avec le breveté pour régler le problème, le président peut tenir une audience sur la question s’il est d’avis que cela sert l’intérêt public.
Les fonctions décisionnelles du CEPMB sont exécutées par les membres. Pendant les audiences, un comité d’étude formé de membres du Conseil agit en tant qu’arbitre neutre entre le personnel du CEPMB et le breveté; le président choisit les membres de ce comité. Les ministres de la Santé provinciaux et territoriaux sont investis, aux termes de la loi, du droit de comparaître devant un tel comité en tant que parties; en outre, d’autres parties ou groupes intéressés peuvent demander l’autorisation de participer en tant qu’intervenants.
Lorsqu’un comité d’étude conclut que le prix d’un médicament breveté est excessif, il peut ordonner une diminution du prix de manière à ce que celui-ci soit ramené à un niveau non excessif. Il peut également ordonner à un breveté de verser au gouvernement du Canada un paiement qui correspond au montant des recettes excessives; en outre, lorsqu’il détermine que le breveté a mis en place une politique de prix excessif, le comité peut doubler le montant à rembourser.
Rapports sur les tendances relatives aux produits pharmaceutiques
Chaque année, le CEPMB rend compte au Parlement, par le truchement du ministre de la Santé, de ses principales activités, de ses analyses du prix des médicaments brevetés et des tendances relatives aux produits pharmaceutiques d’ordonnance ainsi que des dépenses de R–D déclarées par les sociétés pharmaceutiques détentrices de brevets. De plus, par suite de l’établissement du Système national d’information sur l’utilisation des médicaments prescritsNotes de bas de page vii (SNIUMP) par les ministres de la Santé fédéral, provinciaux et territoriaux en septembre 2001, le CEPMB réalise des analyses critiques des tendances relatives aux prix, à l’utilisation et aux coûts pour les médicaments brevetés et non brevetés distribués sous ordonnance pour que les participants clés du système de soins de santé au Canada aient des renseignements plus complets et plus justes sur la façon dont on utilise les médicaments d’ordonnance et sur les facteurs à l’origine des pressions exercées en ce qui a trait aux coûts. Cette fonction vise à aider les gouvernements fédéral, provinciaux et territoriaux et d’autres parties intéressées à se munir d’une source centralisée d’information crédible sur les tendances dans l’industrie pharmaceutique.
Résultat stratégique et Architecture d’alignement des programmes
1. Résultat stratégique : Les médicaments brevetés ne peuvent être vendus au Canada à des prix excessifs, afin de protéger les intérêts de la population canadienne. La population canadienne est également tenue informée.
- 1.1 Programme : Le programme de réglementation du prix des médicaments brevetés
- 1.2 Programme : Le programme sur les tendances relatives aux produits pharmaceutiques
Services internes
Priorités organisationnelles
Priorité :Réglementation et rapports axés sur le consommateur
Description
Si le CEPMB souhaite demeurer le « pilier de la protection des consommateurs », tel qu’il avait été décrit dans le projet de loi C-22, en cette période où les prix des médicaments brevetés au Canada dépassent ceux des sept pays avec lesquels il établit des comparaisons (à l’exception des États Unis) ainsi que ceux d’autres pays européens et où la R-D est au niveau le plus bas jamais enregistré, il doit adopter une approche axée davantage sur le consommateur en ce qui touche la façon dont il assume ses fonctions en matière de réglementation et de production de rapports.
Type de prioritéNote de bas de page 1 – Nouvelle
Principales initiatives à l’appui
| Initiatives prévues |
Début |
Fin |
Lien avec l’Architecture d’alignement des programmes du CEPMB |
Sur le plan réglementaire, le CEPMB :
- orientera ses ressources d’application de la loi vers les cas qui sont les plus pertinents pour les payeurs et qui permettront de soulever des questions susceptibles de clarifier certains aspects de son cadre réglementaire et de lui permettre de devenir un défenseur du consommateur plus efficace;
|
En cours |
|
Le CEPMB n’a qu’un résultat stratégique. Ainsi, toutes les priorités se rapportent à ce résultat. Cette priorité est liée au programme 1.1. |
- envisagera des solutions pour que son processus d’établissement des prix plafonds soit plus précis et conforme à la politique afin veiller à ce que les médicaments brevetés soient abordables pour les Canadiens.
|
Janvier 2016 |
Mars 2017 |
Le CEPMB n’a qu’un résultat stratégique. Ainsi, toutes les priorités se rapportent à ce résultat. Cette priorité est liée au programme 1.1. |
Sur le plan de la production de rapports, le CEPMB :
- collaborera avec les payeurs publics et privés pour tirer parti d’autres occasions de collaboration, notamment en mettant en place des systèmes qui faciliteront et normaliseront la diffusion de données sur les prix, l’utilisation et les coûts, et ce, afin que les assureurs puissent prendre plus rapidement des décisions éclairées.
|
Janvier 2016 |
Mars 2017 |
Le CEPMB n’a qu’un résultat stratégique. Ainsi, toutes les priorités se rapportent à ce résultat. Cette priorité est liée au programme 1.2. |
Priorité :Modernisation du cadre
Description
Depuis quelque temps, les prix des médicaments brevetés au Canada augmentent de façon constante par rapport aux prix observés dans les 7 pays de comparaison du CEPMB. En 2005, le Canada figurait au troisième rang pour ce qui est des prix les plus faibles au sein de ses 7 pays, tandis qu’en 2013, il était au troisième rang en ce qui a trait aux prix les plus élevés, presque à égalité avec l’Allemagne. Parmi les 5 pays – au sein du groupe visé où les prix étaient les plus bas en 2013, il y en a 3, soit le Royaume Uni, la France et l’Italie, qui affichaient des prix de 20 % inférieurs à ceux du Canada. En outre, au pays, tandis que les prix augmentent, la R-D diminue. Depuis 2003, les membres de Médicaments novateurs Canada (anciennement « Les compagnies de recherche pharmaceutique du Canada [Rx&D] ») ne respectent pas leur engagement de maintenir un taux de R-D équivalant à 10 % des ventes; le plus récent ratio déclaré publiquement s’établissait à 5,4 %. Il s’agit du résultat le plus faible depuis 1988, l’année où le CPEMB a commencé à présenter des rapports sur la R-D. Par opposition, le ratio moyen de R-D des 7 pays de comparaison du CEPMB se maintient au dessus de 20 % des ventes. À la lumière de ces constatations, le CEPMB consultera les parties intéressées pour déterminer s’il est nécessaire d’apporter des modifications à ses pouvoirs de protection des consommateurs – et, le cas échéant, la mesure dans laquelle il convient de le faire – pour s’assurer que les médicaments brevetés du Canada sont abordables pour la population.
Type de priorité – Nouvelle
Principales initiatives à l’appui
| Initiatives prévues |
Début |
Fin |
Lien avec l’Architecture d’alignement des programmes du CEPMB |
Le CEPMB :
- consultera les intervenants au sujet d’options de modernisation et de simplification de ses lignes directrices pour l’établissement des prix;
|
Avril 2016 |
Mars 2017 |
Le CEPMB n’a qu’un résultat stratégique. Ainsi, toutes les priorités se rapportent à ce résultat. Cette priorité est liée au programme 1.1. |
- collaborera avec les partenaires fédéraux, provinciaux et territoriaux dans toute discussion au sujet d’une réforme réglementaire et législative plus vaste, en tenant compte des pratiques exemplaires à l’échelle internationale.
|
En cours |
Sans objet |
Le CEPMB n’a qu’un résultat stratégique. Ainsi, toutes les priorités se rapportent à ce résultat. Cette priorité est liée au programme 1.1. |
Priorité :Partenariats stratégiques et sensibilisation du public
Description
Si le CEPMB souhaite réussir à simplifier et à moderniser ses lignes directrices et à réformer globalement la réglementation et la législation fédérales, il doit nouer des relations avec un réseau hétérogène d’intervenants de l’industrie pharmaceutique ayant tous des intérêts et des points de vue uniques quant aux changements envisagés. Afin que ce processus soit efficace, le CEPMB doit accroître la sensibilisation à son mandat de protection des consommateurs et s’appuyer sur sa réputation de courtier honnête auprès des intervenants et du public en général.
Type de priorité – Nouvelle
Principales initiatives à l’appui
| Initiatives prévues |
Début |
Fin |
Lien avec l’Architecture d’alignement des programmes du CEPMB |
Le CEPMB mettra l’accent sur ce qui suit :
- il renforcera son partenariat avec les payeurs publics pour fournir, en temps opportun, encore plus de renseignements pertinents sur le marché;
|
Avril 2016 |
|
Le CEPMB n’a qu’un résultat stratégique. Ainsi, toutes les priorités se rapportent à ce résultat. Cette priorité est liée au programme 1.2. |
- il élargira la portée des sujets sur lesquels il présente des rapports afin de fournir aux payeurs privés et aux consommateurs de l’information qui les aidera à faire des choix plus éclairés et plus bénéfiques;
|
En cours |
|
Le CEPMB n’a qu’un résultat stratégique. Ainsi, toutes les priorités se rapportent à ce résultat. Cette priorité est liée au programme 1.2. |
- il travaillera en étroite collaboration avec ses homologues internationaux en vue de la mise en commun des connaissances et des pratiques exemplaires;
|
En cours |
|
Le CEPMB n’a qu’un résultat stratégique. Ainsi, toutes les priorités se rapportent à ce résultat. Cette priorité est liée aux programmes 1.1 et 1.2. |
- il adoptera une approche plus proactive pour faire connaître ses réalisations en matière de réglementation et de production de rapports aux intervenants et au public.
|
En cours |
|
Le CEPMB n’a qu’un résultat stratégique. Ainsi, toutes les priorités se rapportent à ce résultat. Cette priorité est liée au programme 1.2. |
Priorité :Mobilisation des employés
Description
L’effectif du CEPMB est le plus grand atout de l’organisation. Pour maintenir les normes d’excellence voulues et convaincre les membres de son personnel qu’il est une organisation où l’on peut bel et bien bâtir sa carrière, le Conseil doit attirer, recruter et maintenir en poste des employés très spécialisés, motivés et compétents dont les antécédents sont diversifiés, en plus de rajeunir son effectif.
Type de priorité – Nouvelle
Principales initiatives à l’appui
| Initiatives prévues |
Début |
Fin |
Lien avec l’Architecture d’alignement des programmes du CEPMB |
Le CEPMB :
- continuera d’informer et de mobiliser ses employés dans le cadre de la mise en œuvre du processus de planification stratégique et à mesure que des priorités annuelles sont élaborées et précisées;
|
En cours |
|
Le CEPMB n’a qu’un résultat stratégique. Ainsi, toutes les priorités se rapportent à ce résultat. Cette priorité est liée aux programmes 1.1 et 1.2. |
- fournira aux employés des directives claires sur les objectifs de travail et les comportements attendus afin de promouvoir une culture où le rendement est continuellement élevé;
|
En cours |
|
|
- mettra en œuvre une stratégie de communication interne complète pour permettre des dialogues plus structurés entre les directions, les cadres supérieurs et les employés;
|
En cours |
|
Le CEPMB n’a qu’un résultat stratégique. Ainsi, toutes les priorités se rapportent à ce résultat. Cette priorité est liée au programme 1.2. |
- fournira aux employés un plus large éventail de possibilités d’apprentissage et de perfectionnement;
|
En cours |
|
|
- embauchera de nouveaux employés provenant du gouvernement et de l’externe – qui ont l’expérience et les capacités nécessaires pour lui permettre de réaliser l’ensemble de ses priorités organisationnelles.
|
En cours |
|
|
Pour en savoir davantage sur les priorités organisationnelles, consultez la lettre de mandat de la ministre de la Santé se trouvant sur le site Web du premier ministre du CanadaNotes de bas de page viii.
Analyse des risques
Principaux risques
| Risque |
Stratégie de réaction au risque |
Lien vers l’Architecture d’alignement des programmes |
| À mesure que les provinces et les territoires décident de formaliser l’Alliance pancanadienne pharmaceutiqueNotes de bas de page ix et obtiennent davantage de réductions de prix de la part des fabricants de produits pharmaceutiques, les prix plafonds fixés par le CEPMB courent le risque de devenir moins pertinents pour les payeurs publics. |
- Le CEPMB renforcera son partenariat avec les payeurs publics en leur offrant des renseignements pertinents et à jour sur le marché, et ce, afin de les aider à mettre de l’avant des points de vue éclairés dans leurs négociations avec les fabricants.
- Le CEPMB mènera des interventions réglementaires ciblées qui mettent l’accent sur les cas les plus importants pour les payeurs, comme les cas touchant des médicaments de créneau à prix élevés à l’égard desquels le pouvoir compensateur n’est pas suffisant.
|
Le CEPMB n’a qu’un résultat stratégique. Ainsi, toutes les priorités se rapportent à ce résultat. |
| Les efforts déployés par les responsables du remboursement dans l’UE pour réduire les prix pourraient bientôt faire en sorte que le Canada se classe au deuxième rang des prix des médicaments brevetés les plus élevés, dépassé seulement par les États-Unis, parmi les sept pays de comparaison du CEPMB, et (ou) que les prix soient plus élevés que la médiane internationale. |
- Le CEPMB mènera des consultations pour déterminer s’il est nécessaire d’apporter des modifications à ses pouvoirs de protection des consommateurs – et, le cas échéant, la mesure dans laquelle il convient de le faire – pour s’assurer que les médicaments brevetés demeurent abordables pour les Canadiens.
- Le CEPMB renforcera les liens avec les responsables du remboursement dans d’autres pays afin de partager l’information concernant le marché et de se tenir au courant de l’évolution de la situation concernant la limitation des coûts.
- Le CEPMB envisagera des solutions pour que son processus d’établissement des prix plafonds soit plus précis et conforme à la politique afin de veiller à ce que les médicaments brevetés soient abordables pour les Canadiens
|
Le CEPMB n’a qu’un résultat stratégique. Ainsi, toutes les priorités se rapportent à ce résultat. |
| La mise en œuvre des ententes multilatérales prévues en matière de commerce pourrait lancer un débat sur la question à savoir si le mécanisme actuel visant à maintenir l’équilibre entre les droits de propriété intellectuelle et la protection du consommateur en ce qui a trait aux produits pharmaceutiques fonctionne bel et bien comme on le souhaitait au départ. |
- Le CEPMB mènera des consultations pour déterminer s’il est nécessaire d’apporter des modifications à ses pouvoirs de protection des consommateurs – et, le cas échéant, la mesure dans laquelle il convient de le faire – pour s’assurer que les médicaments brevetés demeurent abordables pour les Canadiens.
|
Le CEPMB n’a qu’un résultat stratégique. Ainsi, toutes les priorités se rapportent à ce résultat. |
| Il y a de multiples affaires judiciaires en cours, à divers niveaux, dans le cadre desquelles on remet en question la compétence du CEPMB ou la constitutionnalité de ses dispositions habilitantes. Il y a un risque que le dénouement de ces affaires limite la compétence du CEPMB et nuise à la capacité de ce dernier de remplir son mandat de protection des consommateurs. |
- Le CEPMB travaille en étroite collaboration avec le procureur général dans le cadre de ces affaires de manière à atténuer tout risque que ses pouvoirs en matière de protection des consommateurs soient restreints par suite d’une décision défavorable de la cour.
|
Le CEPMB n’a qu’un résultat stratégique. Ainsi, toutes les priorités se rapportent à ce résultat. |
| Compte tenu de la nature hautement spécialisée de son mandat de protection des consommateurs, il est possible que le CEPMB ne soit pas en mesure d’attirer et de maintenir en poste les experts en la matière dont il a besoin pour remplir son mandat. |
- Le CEPMB a fait de l’engagement des employés l’une de ses quatre priorités stratégiques et est en voie d’embaucher du personnel possédant les compétences requises pour faire avancer ses priorités.
- Le CEPMB continuera de s’employer à établir une culture où l’on valorise les employés et où l’on sait reconnaître et récompenser leurs efforts de diverses façons.
|
Le CEPMB n’a qu’un résultat stratégique. Ainsi, toutes les priorités se rapportent à ce résultat. |
Le CEPMB avait été décrit comme le « pilier de la protection des consommateurs » dans la législation dont il découle, soit le projet de loi C-22. Cette description a été appuyée en de multiples occasions par les tribunaux, y compris par la Cour suprême du Canada en 2011. L’objectif initial du CEPMB était de veiller à ce que les titulaires de brevet ne fassent pas une utilisation abusive de leurs droits de brevet nouvellement renforcés en imposant aux consommateurs des prix excessifs pendant la période de monopole de droits prévue par la loi. Le projet de loi C-22 était alors un sujet très litigieux; l’on jugeait qu’il était essentiel de voir à la crédibilité et à l’efficacité du CEPMB à titre d’organisme de réglementation pour garantir la viabilité à long terme du compromis stratégique qu’incarnait le Conseil.
Étant donné les prix élevés des médicaments brevetés de même que le creux record de l’investissement en R-D au Canada, nombre de personnes remettent en question l’efficacité du CEPMB pour ce qui est de l’atteinte des objectifs stratégiques fixés au départ par le gouvernement. Cette question a été abordée à nouveau récemment par le Groupe consultatif sur l’innovation des soins de santé dans son rapport du 17 juillet 2015 intitulé Libre cours à l’innovation : Soins de santé excellents pour le Canada; en effet, le Groupe y conclut que le CEPMB doit être renforcé afin de mieux protéger les consommateurs des prix élevés des médicaments brevetés. De même, la plate-forme électorale du Parti libéral comprenait l’engagement suivant : « Nous consulterons les intervenants du secteur et réexaminerons les règles suivies par le Conseil d’examen du prix des médicaments brevetés afin de nous assurer que les gouvernements et les Canadiens paient un juste prix pour les médicaments d’origine ».
Malgré les rajustements qui ont été apportés de temps à autre, le cadre juridique actuel demeure fondé sur une compréhension du secteur pharmaceutique canadien et mondial qui date de 1987. Le Plan stratégique 2015-2018 du CEPMB, publié récemment, présente les étapes à suivre pour concrétiser la réforme voulue, ce qui permettra au Conseil d’atteindre les objectifs stratégiques établis initialement par le gouvernement en ce qui a trait aux réalités du marché pharmaceutique d’aujourd’hui. Ainsi, les priorités et initiatives figurant dans le présent document sont tirées du Plan stratégique 2015-2018, qui est accessible sur le site Web du CEPMB à l’adresse suivante : http://www.pmprb-cepmb.gc.ca/view.asp?ccid=1197.
Dépenses prévues
Ressources financières budgétaires (en dollars)
2016-2017
Budget principal des dépenses |
2016-2017
Dépenses prévues |
2017-2018
Dépenses prévues |
2018-2019
Dépenses prévues |
| 10 965 108 |
10 965 108 |
10 965 108 |
10 965 108 |
Ressources humaines (équivalent temps plein—ETP)
| 2016-2017 |
2017-2018 |
2018-2019 |
| 71 |
71 |
71 |
Sommaire de planification budgétaire pour le Résultat stratégique et les Programmes (en dollars)
| Résultat stratégique, Programmes et Services internes |
2013-2014
Dépenses |
2014-2015
Dépenses |
2015-2016
Dépenses projetées |
2016-2017
Budget principal des dépenses |
2016-2017
Dépenses prévues |
2017-2018
Dépenses prévues |
2018-2019
Dépenses prévues |
| Résultat stratégique 1 : Les médicaments brevetés ne peuvent être vendus au Canada à des prix excessifs, afin de protéger les intérêts de la population canadienne. La population canadienne est également tenue informée. |
| Le programme de réglementation du prix des médicaments brevetés |
Footnote *6 395 602 |
3 543 891 |
5 153 100 |
6 646 758 |
Footnote **6 646 758 |
6 646 758 |
6 646 758 |
| Le programme sur les tendances relatives aux produits pharmaceutiques |
1 146 790 |
1 301 871 |
1 749 520 |
1 704 508 |
1 704 508 |
1 704 508 |
1 704 508 |
| Total partiel |
7 542 392 |
4 845 762 |
6 902 620 |
8 351 266 |
8 351 266 |
8 351 266 |
8 351 266 |
| Total partiel Services internes |
2 998 175 |
3 084 518 |
2 536 900 |
2 613 842 |
2 613 842 |
2 613 842 |
2 613 842 |
| Total |
10 540 567 |
7 930 280 |
9 439 520 |
10 965 108 |
10 965 108 |
10 965 108 |
10 965 108 |
Harmonisation avec les résultats du gouvernement du Canada
Harmonisation des dépenses prévues pour 2016-2017 par Secteur de dépenses du Cadre pangouvernementalNote de bas de page x (en dollars)
| Résultat stratégique |
Programme |
Secteur de dépenses |
Résultat du gouvernement du Canada |
Dépenses prévues 2016-2017 |
| Les médicaments brevetés ne peuvent être vendus au Canada à des prix excessifs, afin de protéger les intérêts de la population canadienne. La population canadienne est également tenue informée. |
Le programme de réglementation du prix des médicaments brevetés |
Affaires sociales |
Des Canadiens en santé |
6 646 758 |
| Le programme sur les tendances relatives aux produits pharmaceutiques |
Affaires sociales |
Des Canadiens en santé |
1 704 508 |
Total des dépenses prévues par Secteurs de dépenses (en dollars)
| Secteur de dépenses |
Total des dépenses prévues |
| Affaires économiques |
|
| Affaires sociales |
8 351 266 |
| Affaires internationales |
|
| Affaires gouvernementales |
|
Tendances relatives aux dépenses du ministère
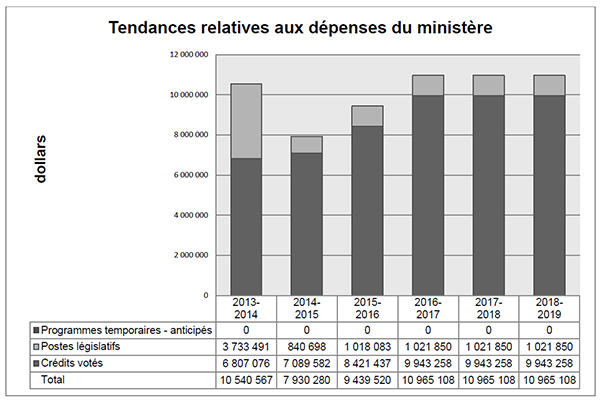
Tendances relatives aux dépenses du ministère
Le graphique à barres illustre les tendances des dépenses prévues et des dépenses réelles du CEPMB au fil du temps. Il présente les dépenses législatives et les dépenses votées réelles pour les exercices 2013-2014, 2014-2015, ainsi que les dépenses législatives et les dépenses votées prévues pour les exercices 2015-2016, 2016-2017, 2017-2018 et 2018-2019.
L’écart entre les dépenses législatives de 2013-2014 et celles de 2014-2015 est en grande partie imputable au financement supplémentaire reçu au moyen d’un mandat de rajustement pour couvrir le montant que la Cour fédérale a ordonné de rembourser à un breveté. La Cour fédérale a annulé une ordonnance du Conseil et ordonné dans sa décision qu’un revenu excédentaire de 2 801 285 $ soit remboursé par le CEPMB au breveté, avec l’intérêt approprié et les frais déterminés.
Le Budget principal des dépenses de 2015-2016 comprend le financement d’une affectation à but spécial (ABS) d’une somme de 2 470 000 $. L’ABS est destinée à la tenue d’audiences publiques et ne peut être utilisée pour couvrir les coûts des conseillers juridiques externes, des témoins experts, etc. Tout montant d’une ABS non utilisé doit être reversé au Fonds du Trésor (le Trésor).
En raison de difficultés rattachées à la prévision du nombre et de la complexité d’audiences tenues, aux fins de la prévision des dépenses anticipées pour 2016-2017 et les années à venir, on suppose que le financement d’ABS sera entièrement utilisé.
Budget des dépenses par crédits votés
Pour plus d’information sur les crédits organisationnels du CEPMB, prière de consulter le Budget principal des dépenses 2016-2017Note de bas de page xi.
Section II : Analyse des programmes par résultat stratégique
Résultat stratégique :
Les médicaments brevetés ne peuvent être vendus au Canada à des prix excessifs, afin de protéger les intérêts de la population canadienne. La population canadienne est également tenue informée.
Programme 1.1 : Le programme de réglementation du prix des médicaments brevetés
Description
Le CEPMB est un organisme indépendant qui détient des pouvoirs quasi judiciaires et qui est responsable de s’assurer que les prix auxquels les brevetés vendent leurs médicaments brevetés au Canada ne sont pas excessifs en vertu des facteurs d’examen du prix prévus à la Loi. Pour décider si un prix est excessif, le Conseil doit tenir compte des facteurs suivants : les prix de vente du médicament et des autres médicaments de la même catégorie thérapeutique au Canada et dans chacun des sept pays mis en comparaison nommés dans le Règlement sur les médicaments brevetés (le Règlement); les variations de l’Indice des prix à la consommation (IPC); et, conformément à la Loi, tous les autres facteurs précisés par les règlements d’application visant l’examen du prixNote de bas de page xii. En vertu de la Loi et du Règlement, les brevetés sont tenus de faire rapport des renseignements sur les prix et les ventes pour chaque médicament breveté vendu au Canada, jusqu’à échéance du brevet ou des brevets. Le personnel du Conseil examine les renseignements soumis par les brevetés au lancement et à chaque période de rapport, et ce, pour tous les médicaments brevetés vendus au Canada. S’il conclut que le prix d’un médicament breveté semble excessif, le personnel du Conseil mène une enquête sur le prix. Une enquête peut se solder par un des résultats suivants : la fermeture de l’enquête lorsqu’il apparaît que le prix n’est pas excessif; un engagement de conformité volontaire par lequel le breveté s’engage à réduire le prix de son produit et à rembourser les recettes excessives qu’il en a tirées au moyen d’un paiement et (ou) d’une réduction du prix d’un autre produit médicamenteux breveté; ou une audience publique dont l’objet est de déterminer si le prix du produit médicamenteux est ou non excessif, y compris une ordonnance corrective rendue par le Conseil. Si le Panel d’audience du Conseil conclut, à l’issue d’une audience publique, qu’un prix est ou était excessif, il peut ordonner au breveté de réduire le prix de son produit et de prendre des mesures qui lui sont dictées pour rembourser les recettes excessives tirées de ce produit. Ce programme assure la protection des Canadiens et de leur système des soins de santé en effectuant l’examen des prix auxquels les brevetés vendent leurs médicaments brevetés au Canada afin d’éviter les prix excessifs.
Ressources financières budgétaires (en dollars)
2016-2017
Budget principal des dépenses |
2016-2017
Dépenses prévues |
2017-2018
Dépenses prévues |
2018-2019
Dépenses prévues |
| 6 646 758 |
6 646 758 |
6 646 758 |
6 646 758 |
Ressources humaines (équivalent temps plein—ETP)
| 2016-2017 |
2017-2018 |
2018-2019 |
| 40 |
40 |
40 |
Mesure de rendement
| Résultats attendus |
Indicateurs de rendement |
Cibles |
Date de réalisation |
| La conformité des brevets à la Loi sur les brevets, à la réglementation et aux Lignes directrices sur les prix excessifs (les Lignes directrices) |
Pourcentage de médicaments brevetés dont les prix sont fixés en conséquence de la conformité volontaire, suivant les Lignes directrices ou ne justifient pas la tenue d’une enquête |
95 % |
Le 31 mars chaque année |
| Taux de conformité aux ordonnances du Conseil relatives au prix ou à la compétence et aux engagements de conformité volontaire |
100 % |
Le 31 mars chaque année |
| Au Canada, les prix des médicaments brevetés se situent dans la fourchette des prix pratiqués dans les sept pays de comparaison nommés dans le Règlement |
Les prix canadiens des médicaments brevetés se trouvent en dessous de la médiane |
50 % |
Le 31 mars chaque année |
Faits saillants de la planification
Le CEPMB orientera ses ressources d’application de la loi vers les cas qui sont les plus pertinents pour les payeurs et qui permettront de soulever des questions susceptibles de clarifier certains aspects de son cadre réglementaire et de lui permettre de devenir un défenseur du consommateur plus efficace. De plus, le CEPMB mènera des consultations pour déterminer s’il est nécessaire d’apporter des modifications à ses pouvoirs de protection des consommateurs – et, le cas échéant, la mesure dans laquelle il convient de le faire – pour s’assurer que les médicaments brevetés demeurent abordables au Canada.
Programme 1.2 : Le programme sur les tendances relatives aux produits pharmaceutiques
Description
Chaque année le CEPMB rend compte au Parlement, par le truchement du ministre de la Santé, de ses activités d’analyse des prix, des prix des médicaments brevetés, des tendances des prix de l’ensemble des médicaments d’ordonnance et des dépenses en recherche et développement (R et D) déclarées par les sociétés pharmaceutiques brevetés. À l’appui de cette exigence en matière de rapport, le Programme sur les tendances relatives aux produits pharmaceutiques fournit des renseignements complets et précis sur les tendances relatives aux prix auxquels les fabricants vendent leurs médicaments brevetés au Canada et sur les dépenses de recherche-développement des brevetés aux intervenants intéressés, notamment l’industrie (c.-à-d. de médicaments de marque, issus de la biotechnologie et générique); les gouvernements fédéral, provinciaux et territoriaux; les groupes de défense de droits des consommateurs et des patients; les tiers payants; et autres. Ces renseignements permettent également de rassurer les Canadiens en leur démontrant que les prix des médicaments brevetés ne sont pas excessifs. De plus, suivant l’établissement du SNIUMP par les ministres de la Santé fédéral, provinciaux et territoriaux, la ministre fédéral de la Santé a demandé au CEPMB d’effectuer des analyses des tendances relatives aux prix, à l’utilisation et aux coûts des médicaments d’ordonnance brevetés et non brevetés afin de s’assurer que le système canadien de soins de santé possède des renseignements plus exhaustifs et précis sur l’utilisation de tout médicaments d’ordonnance et sur les facteurs à l’origine des augmentations de coûts. Cette fonction vise à fournir aux gouvernements fédéral, provinciaux et territoriaux ainsi, qu’aux autres intervenants intéressés, une source d’information centrale et fiable concernant les prix de tout médicament d’ordonnance.
Ressources financières budgétaires (en dollars)
2016-2017
Budget principal des dépenses |
2016-2017
Dépenses prévues |
2017-2018
Dépenses prévues |
2018-2019
Dépenses prévues |
| 1 704 508 |
1 704 508 |
1 704 508 |
1 704 508 |
Ressources humaines (ETP)
| 2016-2017 |
2017-2018 |
2018-2019 |
| 12 |
12 |
12 |
Mesure de rendement
| Résultats attendus |
Indicateurs de rendement |
Cibles |
Date de réalisation |
| Information sur les tendances pharmaceutiques et les facteurs à l’origine des coûts offerte aux intervenants |
Nombre de nouveaux rapports/nouvelles études sur le site Web du CEPMB |
12 rapports et études |
Le 31 mars de chaque année |
| Nombre de présentations du CEPMB à un public externe |
10 séances d’information |
Le 31 mars de chaque année |
Faits saillants de la planification
Le CEPMB adoptera une approche plus proactive pour communiquer ses réalisations en matière de réglementation et de production de rapports au public et s’appuiera sur sa réputation de courtier honnête pour identifier, analyser et faire état des questions d’intérêt pharmaceutique. Cela comprend des efforts pour renforcer son partenariat avec les payeurs publics afin de mieux anticiper leurs exigences en ce qui touche les renseignements sur le marché de même que l’information particulière qui est requise dans le contexte des négociations qui concernent l’Alliance pancanadienne pharmaceutique, ainsi que l’élargissement de la portée des enjeux pharmaceutiques sur lesquels il rend compte pour fournir aux payeurs privés et aux consommateurs de l’information qui les aidera à faire des choix plus éclairés et plus rentables. Le CEPMB renforcera également les liens avec les responsables du remboursement dans d’autres pays afin de partager l’information concernant le marché et de se tenir au courant de l’évolution de la situation dans ce domaine. En outre, comme il l’a fait par le passé, le CEPMB publiera son Programme de recherche du SNIUMP qui reflète les priorités identifiées par le Comité consultatif du SNIUMP et qui énumère les rapports qu’il prévoit terminer et publier au cours de l’exercice.
Services internes
Description
Les services internes sont des groupes d’activités et de ressources connexes qui sont gérés de façon à répondre aux besoins des programmes et des autres obligations générales d’une organisation. Les services internes comprennent uniquement les activités et les ressources qui s’appliquent à l’ensemble d’une organisation, et non celles prévues pour un programme précis. Ces groupes sont les suivants : services de gestion et de surveillance, services des communications, services juridiques, services de gestion des ressources humaines, services de gestion financière, services de gestion de l’information, services des technologies de l’information, services des biens immobiliers, services du matériel et services de gestion des acquisitions.
Ressources financières budgétaires (en dollars)
2016-2017
Budget principal des dépenses |
2016-2017
Dépenses prévues |
2017-2018
Dépenses prévues |
2018-2019
Dépenses prévues |
| 2 613 842 |
2 613 842 |
2 613 842 |
2 613 842 |
Ressources humaines (ETP)
| 2016-2017 |
2017-2018 |
2018-2019 |
| 19 |
19 |
19 |
Faits saillants de la planification
En tant que petit organisme, le CEPMB compte sur un effectif restreint mais polyvalent dont les membres possèdent un large éventail de compétences et sont forts d’antécédents professionnels variés. Pour maintenir les normes d’excellence voulues et convaincre les membres de son personnel qu’il est une organisation où l’on peut bel et bien bâtir sa carrière, le Conseil continuera de donner à ses employés de l’information au sujet du processus de planification stratégique et de mobiliser ceux-ci à l’égard de ce processus, en plus de leur fournir des directives claires sur les objectifs de travail et les comportements attendus afin de promouvoir une culture où le rendement est continuellement élevé. En outre, le CEPMB mettra en œuvre une stratégie de communication interne complète pour permettre des dialogues plus structurés entre les directions, les cadres supérieurs et les employés, et mettra en place des systèmes pour permettre aux employés d’attribuer une cote à leurs gestionnaires afin d’évaluer la mesure dans laquelle ces derniers respectent leurs objectifs en matière de mobilisation. Enfin, le Conseil offrira un accès à une vaste gamme de possibilités d’apprentissage et de développement, y compris, sans s’y limiter, des affectations de mentorat et de perfectionnement.
Section III : Renseignements supplémentaires
État des résultats condensé prospectif
L’état des résultats condensé prospectif donne un aperçu général des opérations du CEPMB. Les prévisions financières concernant les dépenses et les recettes sont préparées sur une base de comptabilité d’exercice pour renforcer la responsabilisation et améliorer la transparence et la gestion financière.
Comme l’état des résultats condensé prospectif est établi sur une base de comptabilité d’exercice et que les prévisions et les dépenses prévues présentées dans d’autres sections du présent rapport sont établies sur la base des dépenses, les montants peuvent différer.
Un état des résultats condensé prospectif plus détaillé et des notes connexesNote de bas de page xiii, y compris un rapprochement des coûts de fonctionnement nets et des autorisations demandées, sont disponibles sur le site Web du CEPMB.
État des résultats condensé prospectif
Pour l’exercice prenant fin le 31 mars 2016
(en dollars)
| Renseignements financiers |
2015-2016
Résultats estimatifs |
2016-2017
Résultats prévus |
Variation
(Résultats prévus pour 2016-2017 moins les résultats estimatifs pour 2015-2016) |
| Total des dépenses |
10 563 636 |
12 157 399 |
1 593 763 |
| Total des revenusNote de bas de page 1 |
48 |
- |
(48) |
| Coût de fonctionnement net avant le financement et les transferts du gouvernement |
10 563 588 |
12 157 399 |
1 593 811 |
Selon le Budget principal des dépenses 2016-2017 et les renseignements sur les obligations, le CEPMB prévoit des dépenses de 12,2 M$. Ce montant ne comprend pas le Budget supplémentaire des dépenses. Cela représente une augmentation de 1,6 M$ par rapport aux prévisions de 2015-2016.
Cette augmentation est principalement attribuable à ce qui suit :
- les dépenses prévues pour 2016-2017 sont fondées sur la supposition voulant que le CEPMB dépensera la totalité des 2,47 millions de dollars reçus sous la forme d’ABS et réservés à la tenue d’audiences publiques. On procède de la sorte puisque ces dépenses se rattachent au nombre d’audiences qui auront lieu de même qu’à la durée et à la complexité de ces audiences, le tout étant difficile à prévoir.
Tableaux de renseignements supplémentaires
Les tableaux de renseignements supplémentaires énumérés dans le Rapport sur les plans et les priorités de 2016-2017 sont affichés sur le site Web du CEPMB.
Dépenses fiscales et évaluations
Il est possible de recourir au régime fiscal pour atteindre des objectifs de la politique publique en appliquant des mesures spéciales, comme de faibles taux d’impôt, des exemptions, des déductions, des reports et des crédits. Le ministère des Finances Canada publie chaque année des estimations et des projections du coût de ces mesures dans sa publication sur les dépenses fiscales et les évaluations connexesNote de bas de page xvi. Les mesures fiscales présentées dans cette publication relèvent de la responsabilité du ministre des Finances.
Section IV : Coordonnées de l’organisation
Le Conseil d’examen du prix des médicaments brevetés
C.P. L40
Centre Standard Life
333, avenue Laurier Ouest
Bureau 1400
Ottawa (Ontario) K1P 1C1
Téléphone : 613-952-7360
Sans frais : 1-877-861-2350
Télécopieur : 613-952-7626
ATS : 613-957-4373
Courriel : pmprb@pmprb-cepmb.gc.ca
Site Web : www.pmprb-cepmb.gc.ca
Annexe : Définitions
Architecture d’alignement des programmes : Inventaire structuré des programmes d’une organisation décrivant la relation hiérarchique entre les programmes et le résultat stratégique auquel ils contribuent.
Cadre pangouvernemental : Présente les contributions financières des organisations fédérales qui reçoivent des crédits en alignant leurs programmes à un ensemble de 16 objectifs de haut niveau définis pour le gouvernement dans son ensemble, groupés en quatre domaines de dépenses.
Cible : Rendement quantifiable ou taux de succès qu’une organisation, qu’un programme ou qu’une initiative prévoit atteindre pour une période donnée. Les cibles peuvent être quantitatives ou qualitatives.
Crédit : Toute autorisation du Parlement de verser une somme d’argent à même le Trésor.
Dépenses budgétaires : Les dépenses de fonctionnement et de capital; les paiements de transfert à d’autres ordres gouvernementaux, organisations ou individus; et les paiements aux sociétés d’État.
Dépenses législatives : Les dépenses approuvées par le Parlement par l’intermédiaire de lois autres que les lois de crédit. Ces lois établissent l’objectif des dépenses ainsi que les modalités applicables à celles-ci.
Dépenses non budgétaires : Les dépenses nettes et les recettes liées aux prêts, placements et avances, qui changent la composition des actifs financiers du gouvernement du Canada.
Dépenses prévues : Aux fins du Rapport sur les plans et les priorités (RPP) et des Rapports ministériels sur le rendement (RMR), les dépenses prévues font référence aux montants qui reçoivent l’approbation du Conseil du Trésor d’ici le 1er février. Par conséquent, les dépenses prévues peuvent comprendre des montants supplémentaires aux dépenses présentées dans le Budget principal des dépenses.
On s’attend à ce qu’un ministère soit informé des pouvoirs qu’il a demandés et reçus. La détermination des dépenses prévues est une responsabilité ministérielle et les ministères doivent être en mesure de défendre les montants des dépenses et de la comptabilité d’exercice présentés dans leurs RPP et RMR.
Dépenses votées : Dépenses approuvées annuellement par le Parlement par l’intermédiaire d’une loi de crédit. Les modalités de la loi en question deviennent les conditions en vertu desquelles ces dépenses peuvent être effectuées.
Équivalent temps plein : Indicateur de la mesure dans laquelle un employé représente une charge complète d’année-personne dans un budget ministériel. Les équivalents temps plein sont calculés selon un taux d’heures de travail assignées en relation aux heures normales de travail. Les heures normales de travail sont établies dans les conventions collectives.
Indicateur de rendement : Moyen quantitatif ou qualitatif de mesurer un extrant ou un résultat en vue de déterminer le rendement d’une organisation, d’un programme, d’une politique ou d’une initiative selon les résultats escomptés.
Plans : Formulation de choix stratégique qui explique comment une organisation prévoit respecter ses priorités et atteindre les résultats connexes. En général, un plan précise la logique derrière les stratégies retenues et met l’accent sur les mesures qui mènent au résultat escompté.
Priorités : plans ou projets sur lesquels une organisation a décidé de se concentrer ou de faire état pendant la période de planification. Les priorités représentent les éléments les plus éléments ou ceux que l’on doit accomplir en premier pour soutenir la réalisation du résultat stratégique souhaité.
Programme : Groupe d’intrant constitué de ressources et d’activités connexes qui est géré pour répondre à un ou des besoins précis et pour réaliser les résultats escomptés, et qui est souvent traité comme une unité budgétaire.
Programme temporarisé : Programme d’une durée limitée qui ne dispose d’aucun pouvoir permanent en matière de financement et de politique. Lorsque le programme arrive à échéance, on doit prendre une décision quant à sa poursuite. Dans le cas d’un renouvellement, la décision doit préciser la portée, le niveau de financement et la durée.
Rapport de rendement : Processus de communication de l’information sur le rendement fondé sur des éléments probants. Le rapport de rendement appuie la prise de décision, l’obligation de rendre compte et la transparence.
Rapport ministériel sur le rendement : Rapports sur les réalisations réelles d’une organisation compétente relativement aux plans, priorités et résultats escomptés énoncés dans le Rapport sur les plans et priorités correspondant. Ces rapports sont déposés au Parlement à l’automne.
Rapport sur les plans et les priorités : Procure de l’information sur les plans et le rendement escompté des organisations compétentes pour une période de trois ans. Ces rapports sont déposés au Parlement au printemps.
Rendement : Ce qu’une organisation fait de ses ressources pour atteindre les résultats escomptés, dans quelle mesure ces résultats se comparent favorablement à ce que l’organisation cherchait à réaliser et dans quelle mesure les leçons à retenir ont été cernées.
Résultat : Conséquence externe attribuable, en partie, à une organisation, à une politique, à un programme ou à une initiative. Les résultats ne sont pas du ressort d’une seule organisation, d’une seule politique, d’un seul programme ou d’une seule initiative, et relèvent plutôt de la sphère d’influence de l’organisation.
Résultat stratégique : Avantage durable et à long terme pour les Canadiens découlant du mandat, de la vision et des fonctions essentielles d’une organisation.
Résultats du gouvernement du Canada : Ensemble de 16 objectifs de haut niveau définis pour tout le gouvernement, groupés en quatre domaines de dépenses : affaires économiques, affaires sociales, affaires internationales et affaires gouvernementales.
Structure de la gestion, des ressources et des résultats : Cadre global qui comprend l’inventaire des programmes, ressources, résultats, indicateurs de performance et informations sur la gouvernance d’une organisation. Les programmes et les résultats sont présentés en relation hiérarchique les uns aux autres et relativement au résultat stratégique auquel ils contribuent. La Structure de la gestion, des ressources et des résultats est établie à partir de l’Architecture d’alignement des programmes.